Abstract
Descending necrotizing mediastinitis implies infection originating from the neck, most commonly an oropharyngeal or odontogenic focus, that spreads in the cervical fascial spaces and descends into the mediastinum. Early diagnosis is essential because descending necrotizing mediastinitis can rapidly progress to septic shock and organ failure. A comprehensive review of the current data of descending necrotizing mediastinitis in Japan was carried out using PubMed and ICHUSHI from the last 5 years. The symptoms, origins, comorbid conditions, treatment modalities, complications, and survival rates were analyzed. Tonsillar and pharyngeal origin was more identified compared to odontogenic origin. More than one‐third of patients were diabetic and 28% of them were not identified as having any comorbidity. Streptococcus sp. and anaerobes were most isolated, reflecting the microflora of the oral cavity. Of the broad antibiotics, carbapenem was the most used as treatment, and clindamycin was the most co‐given. Mediastinal drainage approach varied widely and the optimal approach is controversial. Twenty‐one patients were treated with video‐assisted thoracic surgical drainage and 15 cases by percutaneous catheter drainage, whereas transcervical approach was applied in 25 patients and thoracotomy was carried out in 21 patients. The overall mortality was 5.6%. Many authors advocated that the most effective management tool is a high degree of clinical suspicion followed by prompt and adequate drainage with intensive care including hemodynamic and nutritional support and repeat computer tomographic monitoring.
Keywords: Airway emergency, cervical necrotizing fasciitis, descending necrotizing mediastinitis, percutaneous catheter drainage, video‐assisted thoracoscopic drainage
Introduction
Cervical necrotizing fasciitis (CNF) can spread from oropharyngeal or odontogenic origin to the deep fascial planes of the neck. This polymicrobial infection is uncommon but rapidly progressive, destructive, and often fatal. Prompt diagnosis and treatment including secure airway, antibiotics, drainage, and intensive sepsis care contribute to improved survival. Nevertheless, the progression to mediastinum (descending necrotizing fasciitis, DNM) leads to a poor prognosis. Cervical necrotizing fasciitis with DNM was first described in 1938 by Pearse,1 who reported a 49% of mortality. The spread of the infection from the neck is attributed to negative intrathoracic pressure. Diagnostic criteria for DNM were proposed by Estrera et al.:2 (i) clinical evidence of severe oropharyngeal infection; (ii) characteristic radiographic features of mediastinitis; (iii) documentation of necrotizing mediastinal infection during operation or autopsy; and (iv) establishment of the relationship between DNM and the oropharyngeal process. Despite the technologic advances in diagnosis and treatment, DNM with sepsis has been reported to have high mortality.3 Recently, less invasive drainage methods for DNM, that is, video‐assisted thoracic surgical drainage (VATS),4 mediastinoscopy,5 and percutaneous catheter drainage,6, 7 have been reported. Although it is certain that early infection source control and drainage is crucial for DNM treatment, there are no guidelines in regard to drainage methods. In this review, the clinical features and treatment of DNM in Japan were overviewed.
Review of Published Data
A comprehensive review of the current data regarding DNM in Japan was carried out using PubMed (www.ncbi.nlm.nih.gov/pubmed) and ICHUSHI (Japan Medical Abstracts Society; http://search.jamas.or.jp/) over 5 years (2008–2013). The search terms were “descending necrotizing mediastinitis” and “cervical necrotizing fasciitis” with Japanese patients' reports. “Deep neck infection” or “neck abscess” were carefully evaluated and the DNM cases were identified and tabulated, as these terms have been broaden the inclusion criteria. Most cases consisted of a single case report, as the condition is not common. The symptom, origin, comorbid conditions, treatment modalities, complications, and survival rates were analyzed.
Eighty‐nine DNM cases (age, 63 ± 14 years) comprising 60 men and 29 women were identified in published reports. The data in Table 1 include days to admission and intervention from the first symptom, morbidity, and source of infection. In Table 2, bacteria, antibiotics, and drainage methods are clarified. Some case series did not provide sufficient information to separate individual cases; however, the intent was to investigate the clinical features and current treatment of DNM in Japan. The data or information of each case that was not provided in the published work was expressed as “NC, not clarified” in the tables.
Table 1.
Published cases of descending necrotizing mediastinitis in Japan 2008–2013: patients' characteristics
| Principal investigator | Year | Cases, n | Time to admission, days | Time to intervention, days | Symptom | Morbidity | Source of infection |
|---|---|---|---|---|---|---|---|
| Kageyama M8 | 2013 | 1 | 3 | 11 | ST, Dph | Others | PT |
| Takeno S9 | 2012 | 1 | 3 | 3 | F, TP | None | OD |
| 1 | 10 | 10 | NS | DM | OD | ||
| Kajiura K10 | 2012 | 1 | 5 | 5 | F, NP | Others | PT |
| Takamune Y11 | 2012 | 1 | 4 | 9 | NS, TS, Dph | DM | OD |
| Okamoto T12 | 2012 | 1 | 8 | 8 | ST, Dph | Others | PT |
| Ishinaga H13 | 2012 | 9 | NC | 5.0 | NC | DM 3 Others 3 | PT 8 Fistula 1 |
| Uemura T14 | 2011 | 1 | 7 | 7 | F, ST | DM | PT |
| 1 | NC | NC | NC | None | NC | ||
| 1 | NC | NC | NC | RD | NC | ||
| 1 | NC | NC | NC | DM | NC | ||
| Matsuhashi N15 | 2011 | 1 | NC | NC | ST, TS | None | OD |
| Ueda D16 | 2011 | 1 | 4 | 10 | ST, abdominal pain | None | OD |
| Oshima M17 | 2011 | 1 | 5 | 7 | NS, tachycardia | Others | OD |
| Nakata Y18 | 2011 | 6 | 5.5 ± 4.3 | 9.0 ± 4.5 | NC | None 5 Others 1 | PT 5 Unknown 1 |
| Nakamura K19 | 2011 | 1 | 7 | 7 | Dpn, back pain | None | PT |
| Nakanishi T20 | 2011 | 1 | 4 | 4 | Chest pain | Others | OD |
| Kodama M21 | 2011 | 1 | 5 | 5 | ST | None | PT |
| Kawakami M22 | 2010 | 1 | NC | 9 | NC | DM | NC |
| 1 | 8 | Others | |||||
| 1 | 3 | DM | |||||
| 1 | 3 | DM | |||||
| 1 | 1 | DM | |||||
| Oka S23 | 2010 | 1 | 2 | NC | NC | None | PT |
| 1 | 2 | 2 | None | PT | |||
| 1 | 8 | 8 | None | PT | |||
| 1 | 14 | 14 | Others | PT | |||
| 1 | 1 | 1 | None | PT | |||
| Fujiwara T24 | 2010 | 1 | 7 | 8 | Dsp, NS | DM | PT |
| Yamamoto M25 | 2010 | 1 | 8 | 8 | F, NP, TS | Others | OD |
| Fujimaki M26 | 2010 | 1 | 3 | 3 | F, ST, NS | None | Unknown |
| 1 | 3 | 4 | ST, NS, Dpn | None | PT | ||
| Yamaguchi Y27 | 2010 | 1 | 2 | 6 | F, NP, NS | Others | PT |
| Uno K28 | 2010 | 1 | 2 | 7 | F, ST, NP | None | PT |
| Sakagami T29 | 2010 | 1 | 4 | 4 | ST, Dpn | None | PT |
| Yamada K30 | 2010 | 1 | NC | 5 | F | St, Others | Unknown |
| Momota K31 | 2009 | 1 | 5 | 6 | NP, TS, Dph | DM,RD | OD |
| Sato K32 | 2009 | 1 | 3 | 5 | NP, NS, Dph | DM | Unknown |
| 1 | 5 | 12 | TP, NS | Others | OD | ||
| 1 | 4 | 7 | TP, NS | None | OD | ||
| 1 | 1 | 8 | F, NS | DM | PT | ||
| 1 | 5 | 8 | TP, NS | None | OD | ||
| Nario K33 | 2009 | 1 | 1 | 6 | F, ST, NS | RD | PT |
| 1 | 4 | 8 | F, Dph | None | Unknown | ||
| 1 | 4 | 4 | F, NS | Others | PT | ||
| Tanaka M34 | 2009 | 1 | 4 | 6 | F, Dph, Dpn | None | PT |
| 1 | 3 | 6 | ST, Dph | Others | PT | ||
| 1 | 2 | 2 | ST, Dph, Dpn | None | PT | ||
| Kawai Y35 | 2009 | 1 | 4 | 8 | NS, Dph, Dpn | Others | OD |
| Araki W36 | 2009 | 1 | 1 | 7 | F, NP | DM | PT |
| Hatano A37 | 2009 | 1 | 5 | 10 | ST, NP, NS | DM | PT |
| Murakami I38 | 2009 | 1 | 3 | 7 | ST, Dph, Dpn | DM | PT |
| Hagino H39 | 2009 | 1 | 4 | 9 | TP, TS, Dpn, NS | St | OD |
| Kodama Y40 | 2008 | 1 | 7 | 16 | NP, NS | Others | OD |
| Yoshifuku K41 | 2008 | 1 | 4 | 4 | ST, NP, NS, Dph | DM | PT |
| Ito S42 | 2008 | 1 | 3 | 3 | F, NS, Dpn | DM | PT |
| 1 | 5 | 5 | F, NS | Others | PT | ||
| 1 | 5 | 5 | F, NS | DM, Others | PT | ||
| 1 | 7 | 7 | ST, NS, Dph | DM | PT | ||
| 1 | 6 | 6 | F, ST, Dph | None | PT | ||
| Usubuchi H43 | 2008 | 1 | 7 | 7 | NS, Dpn, Dph | DM | PT |
| Suzuki A44 | 2008 | 1 | 2 | 8 | ST, Dph | Others | PT |
| Sumi Y7 | 2008 | 14 | 5.2 ± 2.5 | 5.2 ± 2.5 | NC | DM 7 St 1 | PT 10 OD 2 Salivary 2 |
| Sum/mean ± SD | 89 | 4.5 ± 2.5 | 6.5 ± 2.9 | DM 30 | PT55 OD18 |
DM, diabetes mellitus; Dph, dysphagia; Dpn, dyspnea; F, fever; NP, neck pain; NS, neck swelling; NC, not clarified; OD, odontogenic infection; PT, pharynx and tonsil; RD, renal dysfunction; St, steroids; ST, sore throat; TP, tooth pain; TS, trismus.
Table 2.
Published cases of descending necrotizing fasciitis in Japan, 2008–2013: microbiology, treatment, outcome
| Investigator | Bacteria | Antibiotics | Drainage time | Mediastinal drainage methods | Outcome | ||
|---|---|---|---|---|---|---|---|
| Streptococcus spp. | Anaerobe | Others/ND | |||||
| Kageyama M8 | ND | PM | 1 | VATS | Survived | ||
| Takeno S9 | 1 | PM, CLDM, LZD | 2 | VATS | Survived | ||
| 1 | PM, CLDM, LZD | 2 | VATS | Survived | |||
| Kajiura K10 | 1 | CP | 3 | THC | Survived | ||
| Takamune Y11 | 1 | PM, CLDM | 3 | VATS | Survived | ||
| Okamoto T12 | ND | PM | 1 | THC, VATS | Survived | ||
| Ishinaga H13 | 2 | 1 | Coryne 1, Others 3 | CP, CLDM | 2.0 ± 0.7 | THC 6 MS 2 TC 1 | Survived |
| Uemura T14 | Kleb, Enterococcus | PM | 2 | VATS | Survived | ||
| 1 | NC | NC | NC | Survived | |||
| 1 | 1 | NC | NC | VATS | Survived | ||
| 1 | NC | NC | VATS | Survived | |||
| Matsuhashi N15 | Coryne | PM, CLDM | 3 | TC | Survived | ||
| Ueda D16 | 1 | 1 | CP, CLDM | 2 | THC | Survived | |
| Oshima M17 | 1 | NC | 2 | TC, THC | Survived | ||
| Nakata Y18 | NC | NC | NC | NC | Survived | ||
| Nakamura K19 | 1 | PM, CLDM, FLCZ | 2 | None | Dead | ||
| Nakanishi T20 | 1 | 2 | PM, PC | NC | CD | Dead | |
| Kodama M21 | ND | NC | 2 | VATS | Survived | ||
| Kawakami M22 | 1 | NC | NC | TC | Survived | ||
| 1 | 2 | MRSA | TC | Survived | |||
| 1 | 1 | TC | Survived | ||||
| 1 | Kleb | TC | Survived | ||||
| 1 | 2 | TC | Survived | ||||
| Oka S23 | MSSA | NC | 3 | VATS | Survived | ||
| 1 | MSSA | 2 | VATS | Survived | |||
| 1 | 2 | VATS | Survived | ||||
| ND | 1 | TC | Survived | ||||
| 1 | 1 | TC | Survived | ||||
| Fujiwara T24 | 1 | CP, CLDM | 2 | VATS | Survived | ||
| Yamamoto M25 | 1 | 2 | CP, CLDM | 2 | TC | Survived | |
| Fujimaki M26 | ND | PM, CLDM | 2 | THC | Survived | ||
| 1 | PM, CLDM | NC | TC | Dead | |||
| Yamaguchi Y27 | 1 | PM, CLDM | 3 | TC, VATS | Survived | ||
| Uno K28 | ND | PC, CLDM | 2 | TC, VATS | Survived | ||
| Sakagami T29 | 1 | 1 | PM, CLDM, FLCZ | 3 | TC, VATS | Survived | |
| Yamada K30 | 1 | Kleb | PM | 2 | TC | Survived | |
| Momota K31 | 1 | 1 | CP, CLDM | 2 | TC, THC | Dead | |
| Sato K32 | 1 | PM, CLDM | 1 | THC | Survived | ||
| 1 | 1 | PM | 2 | TC | Survived | ||
| 1 | 1 | PM | 2 | THC | Survived | ||
| 1 | PM, CLDM | 1 | SB, THC | Survived | |||
| 1 | 1 | PM, CLDM | 2 | TC, SB | Survived | ||
| Nario K33 | ND | PM, CLDM | 1 | VATS | Survived | ||
| ND | PM | 1 | THC | Survived | |||
| 1 | 2 | PM | 2 | TC, MS | Survived | ||
| Tanaka M34 | NC | NC | 3 | THC | Survived | ||
| 2 | VATS | Survived | |||||
| 3 | VATS | Survived | |||||
| Kawai Y35 | 1 | 2 | PC, CLDM | 2 | TC | Survived | |
| Araki W36 | 1 | PM, CLDM | 2 | NC | Survived | ||
| Hatano A37 | MSSA | CP, CLDM | 2 | TC, SB | Survived | ||
| Murakami I38 | 1 | PM, CLDM | 3 | TC, VATS | Survived | ||
| Hagino H39 | ND | PM, CLDM | 5 | TC, SB | Survived | ||
| Kodama Y40 | 1 | CP | 3 | TC | Survived | ||
| Yoshifuku K41 | 1 | PM, CLDM | 3 | THC | Survived | ||
| Ito S42 | 1 | PC, CLDM, FLCZ | 3 | THC | Survived | ||
| MSSA, Enterococcus | PC, CP, CLDM | 2 | THC | Survived | |||
| 1 | 1 | PM | 1 | NC | Survived | ||
| Kleb | PC, ISP | 1 | NC | Survived | |||
| ND | PM, CLDM | NC | NC | Survived | |||
| Usubuchi H43 | 1 | PM, CLDM | 3 | THC | Survived | ||
| Suzuki A44 | ND | CP, CLDM | 2 | THC, MS | Survived | ||
| Sumi Y7 | 10 | 8 | Kleb, MRSA | PM, PC | NC | CD 14 | 1 Dead,14 Survived |
| Summary | 51 | 33 | ND 11 | PM 30, CLDM 29 CP 9, PC 7 | 2.12 ± 0.8 | TC25, THC21, VATS21, CD15 | 5 Dead, 84 Survived |
Streptococcus spp.: S. constellatus, S. intermedius, S. agalactiae, S. mitis, the other Streptococcus sp. Anaerobic bacteria: Peptostreptococcus sp, Prevottela sp, Fusobacterium sp, Bacteroides sp. CD, catheter drainage; CLDM, clindamycin; Coryne, Corynebacterium; CP, cephem; FLCZ, fluconazole; ISP, isepamicin; Kleb, Klebsiella sp.; LZD, linezolid; MRSA, methicillin‐resistant Staphylococcus aureus; MS, mediastinoscopic drainage; MSSA, methicillin‐sensitive Staphylococcus aureus; NC, not clarified; ND, not detected; PC, penicillin; PM, carbapenem; SB, subxiphoidal drainage; TC, transcervical approach; THC, thoracotomy; VATS, video‐assisted thoracic surgical drainage.
Incidence
Necrotizing fasciitis is most common in extremities, perineum, and trunk. Less than 5% of cases involve the cervicofacial region.45, 46 Mediastinal extension (DNM) was reported to occur in 40–45% of CNF patients.3 Many investigators blame the rarity of the condition for its late recognition and less aggressive initial treatment.
Origin
Odontogenic sources, tonsillar and pharyngeal abscesses, sialadenitis, injury by a foreign body, or catheterization are common origins of DNM. The primary origin of DNM is unknown in some cases. In our review cases, tonsillar and pharyngeal origin (n = 55) was identified more often than odontogenic origin (n = 18) (Table 1).
Clinical Presentation
Initial signs and symptoms are universally mild and non‐specific. The clinical signs related to cervical localization are pain, dysphagia, anorexia, dyspnea, tachypnea, fever, odynophagia, hoarseness, erythema, anterior neck edema, and crepitus. The diagnosis of DNM can be difficult, owing to the vagueness of the symptoms.47 Symptoms of mediastinal infection include chest discomfort, respiratory insufficiency, and any septic signs.48
Microbiology
The microbiologic findings are complex and polymicrobial with aerobes and anaerobes, reflecting the microflora of the oral cavity. The most common aerobic bacteria are Streptococcus spp. (S. constellatus, S. intermedius, S. agalactiae, S. mitis). The common anaerobic bacteria include Peptostreptococcus, Bacteroides fragilis, Prevotella, and Fusobacterium.49 In our review, Streptococcus spp. were most often isolated (Table 2). There were no bacteria detected in 11 patients, probably because antibiotics were prescribed before their admission.
Risk Factors
Many DNM patients are immunocompromised, with comorbidities such as diabetes mellitus, malnutrition, advanced age, renal failure, liver cirrhosis, and underlying malignancy. However, there are a certain number of DNM patients without any identified comorbidities. In our review, 30 patients (33.7%) were diabetic and 25 patients (28.1%) without any comorbidities identified. Descending necrotizing fasciitis predominantly affects males.50 In our study, 67% of DNM patients were male.
The factors associated with mediastinal progression (DNM) in CNF patients have been studied. Age, comorbidity (diabetes), pharyngeal origin, oral intake of glucocorticoids before hospital admission, production of gas by infecting organism, and the number of spaces involved, especially the retropharyngeal space, are predicting factors for mediastinal spreading.7, 51, 52
Pathways of Spread of Infections from Neck to Mediastinum
With the absence of barriers in the fascial planes, the mediastinal expansion may be due to mechanical or chemical properties or both of the gas produced by the bacteria, or may directly indicate virulence of the causative bacteria.51 Understanding of the pathway begins from the knowledge of the anatomy of fascial planes and cervical spaces (Fig. 1). There are three potential pathways to the mediastinum:54 (i) the pretracheal route to the anterior mediastinum; (ii) the lateral pharyngeal route to the middle mediastinum; and (iii) the retropharyngeal route to the posterior mediastinum.
Figure 1.
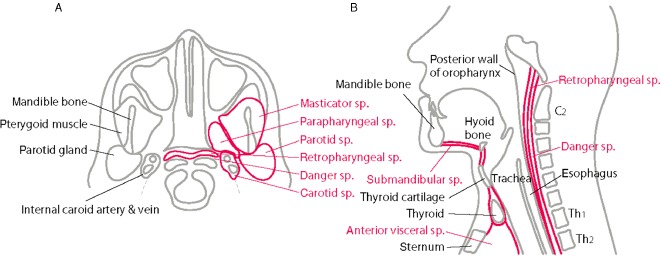
Cervical spaces important for mediastinal progression (reproduced from Sumi (2013),53 “Current treatment for burn injury”). A, Axial schematic view: parapharyngeal space (sp.) is the hub for deep space infections, which communicates with the major spaces: submandibular space, retropharyngeal space, and carotid space. B, Sagittal schematic view: submandibular space to anterior mediastinum (anterior visceral space), retropharyngeal space to the posterior mediastinum via danger space.
Submandibular space
This space extends from the floor of the mouth to fascial attachments at the hyoid bone. The infection in this space is typically odontogenic and called Ludwig's angina, which refers to its historically lethal nature.55
Anterior visceral space
This space contains the thyroid, trachea, and esophagus extending from thyroid cartilage into superior mediastinum. Infection down to this route can lead to pericarditis and empynema.
Parapharyngeal space
This space serves as an anatomic hub for deep space infections, which communicates with spaces that are important route to mediastinum: submandibular space, retropharyngeal space, carotid space.52
Carotid space
The carotid space is located in the posterior of the parapharyngeal space and includes the internal carotid artery, jugular vein, and cranial nerves, extending from the skull base to the aortic arch. The route to the anterior and middle mediastinum through this space is called Lincoln's pathway.56
Retropharyngeal space
This space extends from the skull base to the level of the Th2 vertebral body. Posterior to this space is the danger space, which goes to the level of the diaphragm, providing a pathway into the posterior mediastinum. Abscesses in the retropharyngeal space often rupture in the danger space and result in posterior mediastinitis.
Diagnosis
Diagnosis of DNM requires a high index of suspicion, which can be very difficult clinically. Studies have shown that only 15–34% of patients with CNF/DNM have an accurate diagnosis on admission.57 In our review cases, the time until admission from first symptoms was 4.5 ± 2.5 days and the time until intervention was 6.5 ± 2.9 days. This discrepancy is due to a lack of recognition of DNM on admission and/or progression to DNM from CNF without adequate treatment. Careful examination, as below, and a high level of suspicion for DNM are key components to a prompt diagnosis.
Laboratory data
Not only data to indicate inflammatory response (white blood cell counts, C‐reactive protein, procalcitonin), but to assess the general condition, including renal and respiratory function, unknown risk factors (HbA1c), complicated disseminated intravascular coagulation, and nutrition status, must be delivered. It is crucial to diagnose sepsis and organ failure and recommended to evaluate the lactate level and oxygenation by arterial blood gas analysis.58
Imaging
Imaging is an essential tool to diagnose the location and extent of infection and to define the management for CNF and DNM.
-
Chest X‐ray:
Chest X‐ray can indicate not only airway malposition, that is, tracheal shift due to cervical edema, but widened mediastinum, and pleural effusion. It may be useful in the demonstration of s.c. emphysema and gas extending from cervical spaces to mediastinum.
-
Contrast‐enhanced multidetector row computed tomography:
Contrast‐enhanced computed tomography (CT) of the neck and chest is a golden standard to evaluate the spread of infection. The features of CNF/DNM include diffuse thickening of the cutis and subcutis, reticular enhancement of the s.c. fat, thickening and enhancement of cervical fasciae, platysma, muscles, and mediastinal fat, s.c. gas, fluid collections, pericardial effusion, and pleural effusion (Figs 2 and 3). Identification of continuity of an infectious process from the neck into the thorax establishes the diagnosis of DNM. Understanding the pathway of mediastinal extension is pivotal to establish the effective drainage. Multiplanar reconstruction imaging with multidetector CT is useful.54, 59 Endo et al. proposed a classification of the extent of DNM based on CT findings and facilitate management.60 Type 1 is a localized infection above the carina, can be managed with transcervical drainage, Type 2A is characterized by diffuse anterior mediastinal involvement, requires transcervicotomy and subxiphoid approach and Type 2B involves both the anterior and posterior mediastinum, requires thoracotomy. Repeat CT allows for visualization of the extent of the infection and identification of new or untreated space involvement.
Figure 2.
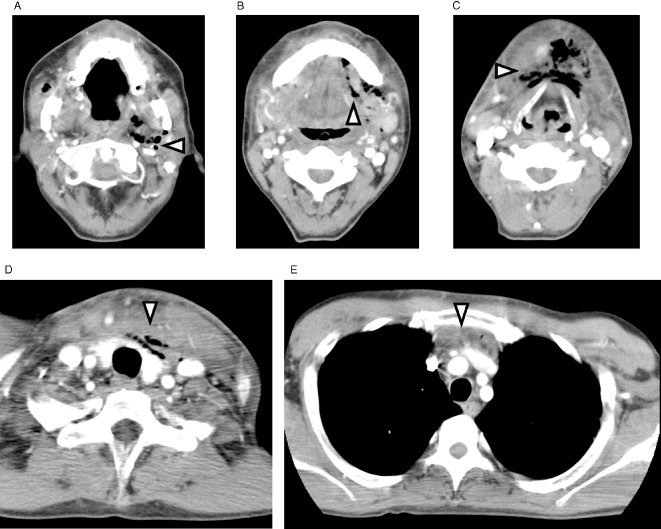
Contrasted computed tomography of neck and chest: anterior mediastinitis due to odontogenic source (reproduced from Sumi (2013),53 “Current treatment for burn injury”). A, Gas collections in left masticator, parapharyngeal, and carotid spaces. B, C, Diffuse thickening of the subcutis, reticular enhancement of s.c. fat, and gas collections in submandibular space. D, Reticular enhancement of s.c. fat, and gas collections in pretracheal and anterior visceral space. E, Thickening and enhancement of anterior mediastinal fat and fluid collections.
Figure 3.
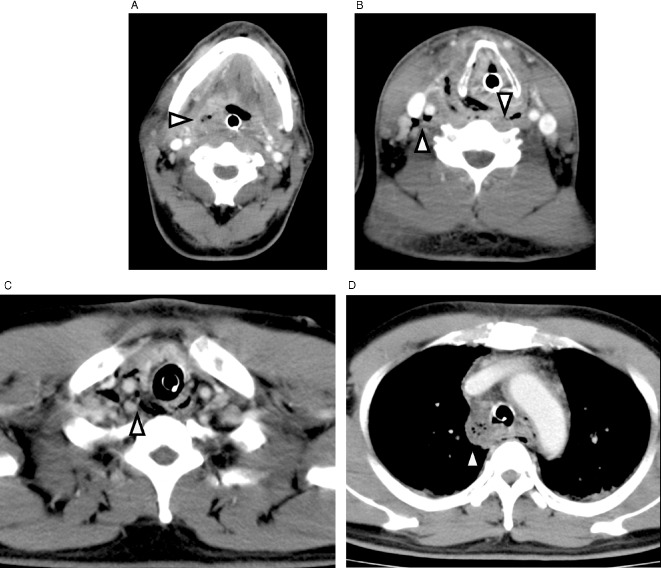
Contrasted computed tomography of neck and chest showing posterior mediastinitis due to tonsillar abscess (reproduced from Sumi (2013),53 “Current treatment for burn injury”). A, Diffuse thickening and gas collection in retropharyngeal space. B, C, Diffuse thickening and gas collection in carotid and danger space. D, Diffuse thickening of mediastinal fat and gas collection in posterior mediastinum.
Gram stain and culture
It is essential to identify the bacteria that causes CNF and DNM. As soon as possible, Gram stain of infectious area and blood should be carried out. Cultures for aerobes and anaerobes are required. Without open wounds, aspiration or debridement of infectious areas is needed to obtain a specimen.
Histology
The histological findings are confirmatory. It shows superficial fascial necrosis and dense infiltration of neutrophils in deeper parts of s.c. tissue and fascia. Subcutaneous fat edema and necrotic vasculitis may be present.
Prognosis
The mortality rates are exceedingly high in the case of CNF with DNM and septic shock. Sarna et al.3 reported that, in 100 cases of CNF, the mortality rate for CNF with DNM was 41% compared with 20% for CNF alone. Descending necrotizing mediastinitis approximately triples the risk of developing septic shock (23% versus 7%). Delay in diagnosis increases mortality,61 and those who survive need more extensive surgery and reconstruction. With early diagnosis, outcome is much improved and significant long‐term disability is reduced or prevented.62 In our review series, the mortality rate (5.6%) was much lower than in previous reports, perhaps because most of the cases were single case reports. Four patients died from multiple organ failure and one died from cervical hemorrhage (Table 2).
Treatment
Once DNM is diagnosed, the patient should be admitted to the intensive care unit for frequent monitoring and sepsis management, or referred to the right facilities capable of critical care.
Airway management
Airway management is critical because the CNF process may produce neck edema, which may result in the difficulty of intubation. The awake intubation techniques that use direct visualization with bronchoscopy are the most prudent approach. Sometimes tracheotomy is unavoidable to secure the airway. There is a notion that the tracheotomy should be carried out very carefully to avoid extending the infection into the tracheotomy.62 In our review cases, seven patients (7.9%) presented with airway emergency and 36 patients (40%) were treated with either urgent or planned tracheotomy (data not shown).
Antibiotics
Antibiotic therapy should be started immediately.58 Because polymicrobial CNF/DNM is more commonly seen, antibiotics need to be broad enough to cover Gram‐positive cocci,Gram‐negative rods, and anaerobic bacteria. Several empirical regimens include piperacillin–tazobactam and vancomycin and clindamycin with ceftriaxone or carbapenems have been described in the published reports.63, 64 It is suggested that, in cases of DNM complicated by streptococcal toxic shock syndrome, the use of protein synthesis inhibitors such as clindamycin proves more effective than penicillin.65 In our review, carbapenem was given most often, and clindamycin was co‐given most often (Table 2). Antibiotic therapy should be tailored as cultures and sensitivities become available. The duration of i.v. antibiotic therapy would be at least 7–10 days.65 Not only before the treatment, but during the clinical course, the culture must be taken, as there may be antibiotic‐resistant bacteria growth with open wounds.
Source control
Once the origin is identified, the management of original infection needs to be done. Extracting the tooth, removal of the foreign body, and drainage of retropharyngeal abscess by intraoral incision may be considered.
Drainage and debridement
Early aggressive intervention and medical optimization can halt the progression of DNM and to septic shock, drastically improving survival. Cervical drainage in the involved area is indisputable. However, the optimal form of mediastinal drainage remains controversial. Mediastinal drainage approach varies widely, namely, thoracotomy, median stenotomy, clamshell incision, a subxiphoid approach, a transcervical approach, VATS, mediastinoscopy, and percutaneous catheter drainage. Many authors have recommended that for advanced DNM (i.e., Endo type II),60 the optimal treatment should include radical surgical debridement of affected tissue through an open thoracic approach.66, 67, 68, 69, 70 However, these invasive methods are high‐risk approaches for critically ill patients with overwhelming sepsis and may lead to unfavorable outcomes with complications.
In 2004, Isowa et al. first reported the successful management of DNM patients with VATS.71 At around that time, more and more authors advocated VATS as one of the treatments for DNM and emphasized the excellent visualization of the entire thoracic cavity, the lower degree of invasiveness, and favorable outcome.4, 72, 73 The VATS technique has the universal advantages of minimally invasive surgery, such as little pain, better cosmetics, and faster recovery.
Nakamori et al. first reported the effectiveness of percutaneous catheter drainage as a novel treatment for DNM a decade ago.6 This technique is superior in terms of pain control, prevention of protein leakage from the wound site, and less secondary infection by antibiotic‐resistant bacteria. Although some questioned the reliability diagnosing the CNF/DNM without surgical exploration and how the necrotic tissue is debrided with this less invasive technique,62 the mortality with their method is promising (0%) and 3.1% in our follow‐up report.7
Although the diagnostic criteria by Estrera et al.2 is widely accepted, documentation of necrotizing mediastinal infection during operation or autopsy is not always applied with less invasive drainage methods.
In this review, many cases with less invasive drainage approaches were uncovered. Twenty‐one patients were treated with VATS and 15 cases by catheter drainage, the transcervical approach was taken in 25 patients, and thoracotomy was carried out in 21 patients (Table 2).
Of five non‐survivors, thoracotomy was carried out in two patients, and the other two patients were treated by catheter drainage. There was no significant difference in mortality among mediastinal drainage methods. Repeat CT scanning allows us to monitor new or untreated space involvement and encourage frequent drainage. In our review, 2.12 times of drainage was required, and some cases required repeated drainage more than three times (Table 2).
Sepsis management
Aggressive hemodynamic and nutritional support and intensive care according to the surviving sepsis campaign58 are the cornerstones for successful management of DNM.
Wound management and reconstruction
After the open debridement, meticulous daily wound care is essential. A daily regimen of wound irrigation and dressing change is recommended. Topical negative pressure therapy can reduce edema and stimulate the formation of granulation tissue. Once the infection has resolved and healthy granulation tissue is present, the wound may be closed with skin flaps or split‐thickness grafts.
Hyperbaric oxygen
The use of hyperbaric oxygen (HBO) therapy has been advocated in published reports.74, 75 No patient was treated with HBO therapy in this review series, mostly because of less accessibility of HBO devices. The HBO is directly bactericidal to the anaerobic bacteria and should increase polymorphonuclear cell function. There still remains the need for a randomized controlled trial of the use of HBO to substantiate its adjunctive role in the treatment of CNF/DNM.74, 75
Complications
Complications include compromised airway, jugular vein thrombosis, suppurative jugular thrombophlebitis (Lemierre's syndrome),76 carotid artery erosion and rupture, septic shock, empyema, and bronchocavitary fistula.
Conclusion
Descending necrotizing fasciitis is a lethal condition if we do not diagnose promptly and start a multidisciplinary approach as soon as possible. Early diagnosis, drainage management, and aggressive monitoring and resuscitation in the intensive care unit as well as use of CT to guide repeated intervention are centerpieces to achieve a favorable outcome. Although the optimal approach to the mediastinum is not standardized, more recently, less invasive approaches successfully using thoracoscopic or mediastinoscopic or percutaneous catheter drainage have been reported.
Conflict of Interest
None.
References
- 1. Pearse HE. Mediastinitis following cervical suppuration. Ann. Surg. 1938; 108: 588–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Estrera AS, Landay MJ, Grisham JM, Sinn DP, Platt MR. Descending necrotizing mediastinitis. Surg. Gynecol. Obstet. 1983; 157: 545–552. [PubMed] [Google Scholar]
- 3. Sarna T, Sengupta T, Miloro M, Kolokythas A. Cervical necrotizing fasciitis with descending mediastinitis: literature review and case report. J. Oral Maxillofac. Surg. 2012; 70: 1342–1350. [DOI] [PubMed] [Google Scholar]
- 4. Son HS, Cho JH, Park SM, Sun K, Kim KT, Lee SH. Management of descending necrotizing mediastinitis using minimally invasive video‐assisted thoracoscopic surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2006; 16: 379–382. [DOI] [PubMed] [Google Scholar]
- 5. Ohno K, Yamasaki Y, Hatanaka N, Yamamoto S, Naitoh H, Kuwata K. [Mediastinoscopic drainage for descending necrotizing mediastinitis]. Jpn J. Thorac. Cardiovasc. Surg. 1998; 46: 175–178. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 6. Nakamori Y, Fujimi S, Ogura H et al Conventional open surgery versus percutaneous catheter drainage in the treatment of cervical necrotizing fasciitis and descending necrotizing mediastinitis. AJR Am. J. Roentgenol. 2004; 182: 1443–1449. [DOI] [PubMed] [Google Scholar]
- 7. Sumi Y, Ogura H, Nakamori Y et al Nonoperative catheter management for cervical necrotizing fasciitis with and without descending necrotizing mediastinitis. Arch. Otolaryngol. Head Neck Surg. 2008; 134: 750–756. [DOI] [PubMed] [Google Scholar]
- 8. Kageyama M, Takizawa Y, Sugiyama K et al A case of descending necrotizing mediastinitis. Pract. Otorhinolaryngol. 2013; 106: 167–171. (In Japanese.) [Google Scholar]
- 9. Takeno S, Yamashita S, Yamamoto S, Tokuishi K, Moroga T, Kawahara K. Feasibility of minimum invasive thoracoscopic mediastinal drainage for the patients with septic shock due to descending necrotizing mediastinitis. J. Jpn. Soc. Surg. Infect. 2012; 9: 75–81. (In Japanese.) [Google Scholar]
- 10. Kajiura K, Sakiyama S, Toba H, Kawakami Y, Kenzaki K, Kondo K. A patient with descending necrotizing mediastinitis saved by drainage performed three times. J. Jpn. Chest Surg. 2012; 26: 433–438. (In Japanese.) [Google Scholar]
- 11. Takamune Y, Hiraki A, Akasaki K, Yoshitake Y, Nakayama H, Shinohara M. A case of descending necrotizing mediastinitis caused by post‐extraction infection. Jpn. J. Oral. Maxillofac. Surg. 2012; 58: 332–336. (In Japanese.) [Google Scholar]
- 12. Okamoto T, Funai K, Sekihara K, Shimizu K, Shiiya N. A case of descending necrotizing mediastinitis secondary to a neck abscess. J. Jpn. Chest Surg 2012; 26: 510–514. (In Japanese.) [Google Scholar]
- 13. Ishinaga H, Otsu K, Sakaida H et al Descending necrotizing mediastinitis from deep neck infection. Eur. Arch. Otorhinolaryngol. 2013; 270: 1463–1466. [DOI] [PubMed] [Google Scholar]
- 14. Uemura T, Nakano T, Hoshida T, Satomi S. A case of descending necrotizing mediastinitis treated by thoracoscopic mediastinal drainage. J. Jpn. Surg. Assoc. 2011; 72: 619–623. (In Japanese.) [Google Scholar]
- 15. Matsuhashi N, Tachi M, Sakuratani T et al A case of descending necrotizing mediastinitis assumed associated with tooth extraction‐induced deep cervical infection. J. Jpn. Coll. Surg. 2011; 36: 920–924. (In Japanese.) [Google Scholar]
- 16. Ueda D, Tamura J, Baba N. A case of descending necrotizing mediastinitis differentiated from Boerhaave's disease. J. Jpn. Surg. Assoc. 2011; 72: 2232–2236. (In Japanese.) [Google Scholar]
- 17. Oshima M, Saito H, Kiuchi R et al Descending necrotizing mediastinitis extended to empyema. Kyobu Geka 2011; 64: 142–145. (In Japanese.) [PubMed] [Google Scholar]
- 18. Nakata Y, Ogawa T, Owaki S, Shimizu T. Patients with a deep neck abscess—a clinical study of the risk factor complicated with mediastinum abscess. JJS Infect. Dis. Otolaryngol. 2011; 30: 111–114. (In Japanese.) [Google Scholar]
- 19. Nakamura K, Yamamoto N, Gon G, Oyama M, Shimada M, Oka H. A case of descending necrotizing mediastinitis due to α streptococcus (Group A). JAEM 2011; 31: 931–936. (In Japanese.) [Google Scholar]
- 20. Nakanishi T, Wada T, Kiguchi T et al An autopsy case of cervical necrotizing fasciitis and descending necrotizing mediastinitis treated by percutaneous catheter drainage. Med. J. Osaka Gen. Med. Cen. 2011; 34: 35–40. (In Japanese.) [Google Scholar]
- 21. Kodama M, Nonaka M, Usuda R. A case of successfully treated descending necrotizing mediastinitis. Geka. 2011; 73: 1103–1107. (In Japanese.) [Google Scholar]
- 22. Kawakami M, Motoyoshi K, Ukumori T, Takahashi H, Wakisaka H. A clinical analysis of deep neck abscess. Otolaryngol. Head Neck Surg. 2010; 82: 613–617. (In Japanese.) [Google Scholar]
- 23. Oka S, Hanagiri T, Takenaka M et al Surgical treatment for patients with descending necrotizing mediastinitis. Kyobu Geka 2010; 63: 1022–1025. (In Japanese.) [PubMed] [Google Scholar]
- 24. Fujiwara T, Kataoka K, Matsuura M. Two patients with descending necrotizing mediastinitis treated by video‐assisted thoracoscopic mediastinal drainage. JSES 2010; 15: 79–84. (In Japanese.) [Google Scholar]
- 25. Yamamoto M, Hayashi M, Fujino K. Deep‐neck infection with necrotizing internal jugular vein treated with vacuum‐assisted closure. Pract. Otorhinolaryngol. 2010; 103: 1141–1145. (In Japanese.) [Google Scholar]
- 26. Fujimaki M, Ito S, Oba S, Iizuka T, Kusunoki T, Ikeda K. Two cases of descending necrotizing mediastinitis secondary to a deep neck abscess. Pract. Otol. 2010; S126: 73–79. (In Japanese.) [Google Scholar]
- 27. Yamaguchi Y, Nishida K, Kobayashi M, Takeuchi Y. A case report of cervical necrotizing fasciitis extending to the mediastinum. Otolaryngol. Head Neck Surg. 2010; 82: 413–417. (In Japanese.) [Google Scholar]
- 28. Uno K, Saitou K, Okubo K et al A case of deep cervical infection with mediastinitis and axillary abscess. Otolaryngol. Head Neck Surg. 2010; 82: 303–308. (In Japanese.) [Google Scholar]
- 29. Sakagami T, Takemura H, Motoki N, Tomoda K. A case of deep neck abscess extending to the mediastinum. JJS Infect. Dis. Otolaryngol. 2010; 28: 137–140. (In Japanese.) [Google Scholar]
- 30. Yamada K, Kitao K, Mizoguchi T et al A case of deep neck abscess with disseminated intravascular coagulation (DIC). J. Sapporo City Gen. Hosp. 2010; 70: 61–66. (In Japanese.) [Google Scholar]
- 31. Momota Y, Satomura K, Tokuyama R, Yuasa T, Kudo K, Takano H. A case of pericoronitis of lower wisdom tooth leading to descending necrotizing mediastinitis. Shikoku Dent. Res. 2009; 21: 413–419. (In Japanese.) [Google Scholar]
- 32. Sato K, Izumi S, Sato K, Takahashi S. Five cases with a mediastinal abscess occurring from a deep neck abscess. Pract. Otorhinolaryngol. 2009; 102: 975–981. (In Japanese.) [Google Scholar]
- 33. Nario K, Hosoi H, Sasai H, Kamakura A, Kurokawa M, Miyahara H. Three cases of advanced deep neck infection extending to the mediastinum. Pract. Otorhinolaryngol. 2009; 102: 145–152. (In Japanese.) [Google Scholar]
- 34. Tanaka M, Ueno M, Machida Y et al Descending necrotizing mediastinitis. Kyobu Geka 2009; 62: 1073–1077. (In Japanese.) [PubMed] [Google Scholar]
- 35. Kawai Y, Nakamura T, Asai H, Konobe T, Okuchi K. A case of descending necrotizing fasciitis treated by transcervical drainage. Shujutsu 2009; 63: 527–530. (In Japanese.) [Google Scholar]
- 36. Araki W, Ikeda K, Shimizu T et al A case report of type 2 diabetes mellitus with descending necrotizing mediastinitis caused by deep cervical abscess. J. Jpn. Diabetes Soc. 2009; 52: 569–573. (In Japanese.) [Google Scholar]
- 37. Hatano A, Ui N, Shigeta Y, Iimmura J, Rikitake M. Clinical analysis of deep neck space infections. O.R.L. Tokyo 2009; 52: 23–33. (In Japanese.) [Google Scholar]
- 38. Murakami I, Fukusaki T, Sugamura M, Yamano T, Sueta N, Nakagawa T. A case of peritonsillar abscess complicated with mediastinitis. JJS Infect. Dis. Otolaryngol. 2009; 27: 177–180. (In Japanese.) [Google Scholar]
- 39. Hagino H, Asai H, Miyamoto H, Watanabe K, Usami T, Ueda M. A case of descending necrotizing mediastinitis associated with odontogenic infection who suffered cardiac arrest. Hosp. Dent. 2009; 21: 147–151. (In Japanese.) [Google Scholar]
- 40. Kodama Y, Ono K, Arashiyama T, Oseki K, Tsuchida M, Takagi R. A case of descending necrotizing mediastinitis arising from odontogenic infection with NSAID‐induced gastric ulcer in a very elderly patient. Jpn. J. Oral Maxillofacial. Surg. 2008; 54: 541–545. (In Japanese.) [Google Scholar]
- 41. Yoshifuku K, Nagano H, Kurono Y. A case of deep cervical and mediastinal abscess successfully treated by repeated surgical treatment. Pract. Otorhinolaryngol. 2008; 101: 367–373. (In Japanese.) [Google Scholar]
- 42. Ito S, Inoue M, Saito M, Yamayoshi T, Kidogawa H. Management of descending necrotizing mediastinitis. J. Jpn. Soc. Emer. Med. 2008; 11: 486–493. (Japanese). [Google Scholar]
- 43. Usubuchi H, Ohta Y, Kanazawa T, Tsubaki K, Iino Y, Yamada S. A case of toxic shock‐like syndrome due to Group A streptococcal infection of a neck lesion. Pract. Otorhinolaryngol. 2008; 101: 555–559. (In Japanese.) [Google Scholar]
- 44. Suzuki A, Suzuki T, Yoshino T, Shibata Y, Saito Y, Kawabata A. Case of descending necrotizing mediastinitis secondary to cervical abscess. Toyota J. Med. 2008; 18: 123–126. (In Japanese.) [Google Scholar]
- 45. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J. Bone Joint Surg. Am. 2003; 85‐A: 1454–1460. [PubMed] [Google Scholar]
- 46. Ord R, Coletti D. Cervico‐facial necrotizing fasciitis. Oral Dis. 2009; 15: 133–141. [DOI] [PubMed] [Google Scholar]
- 47. Sandner A, Borgermann J, Kosling S, Silber RE, Bloching MB. Descending necrotizing mediastinitis: early detection and radical surgery are crucial. J. Oral Maxillofac. Surg. 2007; 65: 794–800. [DOI] [PubMed] [Google Scholar]
- 48. Moreira J. Severe sepsis and septic shock. N. Engl. J. Med. 2013; 369: 2063. [DOI] [PubMed] [Google Scholar]
- 49. Brook I, Frazier EH. Microbiology of mediastinitis. Arch. Intern. Med. 1996; 156: 333–336. [PubMed] [Google Scholar]
- 50. Garatea‐Crelgo J, Gay‐Escoda C. Mediastinitis from odontogenic infection. Report of three cases and review of the literature. Int. J. Oral Maxillofac. Surg. 1991; 20: 65–68. [DOI] [PubMed] [Google Scholar]
- 51. Petitpas F, Blancal JP, Mateo J et al Factors associated with the mediastinal spread of cervical necrotizing fasciitis. Ann. Thorac. Surg. 2012; 93: 234–238. [DOI] [PubMed] [Google Scholar]
- 52. Kang SK, Lee S, Oh HK et al Clinical features of deep neck infections and predisposing factors for mediastinal extension. Korean J. Thorac. Cardiovasc. Surg. 2012; 45: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sumi Y. Cervical Necrotizing Fasciitis and Descending Necrotizing Mediastinitis. Current Treatment for Burn Injury, 2nd edn Tokyo: Chugaiigakusha, 2013. [Google Scholar]
- 54. Pinto A, Scaglione M, Scuderi MG, Tortora G, Daniele S, Romano L. Infections of the neck leading to descending necrotizing mediastinitis: role of multi‐detector row computed tomography. Eur. J. Radiol. 2008; 65: 389–394. [DOI] [PubMed] [Google Scholar]
- 55. Patterson HC, Kelly JH, Strome M. Ludwig's angina: an update. Laryngoscope 1982; 92: 370–378. [DOI] [PubMed] [Google Scholar]
- 56. al‐Ebrahim KE. Descending necrotising mediastinitis: a case report and review of the literature. Eur. J. Cardiothorac Surg. 1995; 9: 161–162. [DOI] [PubMed] [Google Scholar]
- 57. Lin C, Yeh FL, Lin JT et al Necrotizing fasciitis of the head and neck: an analysis of 47 cases. Plast. Reconstr. Surg. 2001; 107: 1684–1693. [DOI] [PubMed] [Google Scholar]
- 58. Dellinger RP, Levy MM, Rhodes A et al Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013; 41: 580–637. [DOI] [PubMed] [Google Scholar]
- 59. Scaglione M, Pinto A, Giovine S, Di Nuzzo L, Giuliano V, Romano L. CT features of descending necrotizing mediastinitis—a pictorial essay. Emerg. Radiol. 2007; 14: 77–81. [DOI] [PubMed] [Google Scholar]
- 60. Endo S, Murayama F, Hasegawa T et al Guideline of surgical management based on diffusion of descending necrotizing mediastinitis. Jpn J. Thorac. Cardiovasc. Surg. 1999; 47: 14–19. [DOI] [PubMed] [Google Scholar]
- 61. Panda NK, Simhadri S, Sridhara SR. Cervicofacial necrotizing fasciitis: can we expect a favourable outcome? J. Laryngol. Otol. 2004; 118: 771–777. [DOI] [PubMed] [Google Scholar]
- 62. Weiss A, Nelson P, Movahed R, Clarkson E, Dym H. Necrotizing fasciitis: review of the literature and case report. J. Oral Maxillofac. Surg. 2011; 69: 2786–2794. [DOI] [PubMed] [Google Scholar]
- 63. Cirino LM, Elias FM, Almeida JL. Descending mediastinitis: a review. Sao Paulo Med. J. 2006; 124: 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lanisnik B, Cizmarevic B. Necrotizing fasciitis of the head and neck: 34 cases of a single institution experience. Eur. Arch. Otorhinolaryngol 2010; 267: 415–421. [DOI] [PubMed] [Google Scholar]
- 65. Lancerotto L, Tocco I, Salmaso R, Vindigni V, Bassetto F. Necrotizing fasciitis: classification, diagnosis, and management. J. Trauma Acute Care Surg. 2012; 72: 560–566. [DOI] [PubMed] [Google Scholar]
- 66. Wheatley MJ, Stirling MC, Kirsh MM, Gago O, Orringer MB. Descending necrotizing mediastinitis: transcervical drainage is not enough. Ann. Thorac. Surg. 1990; 49: 780–784. [DOI] [PubMed] [Google Scholar]
- 67. Freeman RK, Vallieres E, Verrier ED, Karmy‐Jones R, Wood DE. Descending necrotizing mediastinitis: an analysis of the effects of serial surgical debridement on patient mortality. J. Thorac. Cardiovasc. Surg. 2000; 119: 260–267. [DOI] [PubMed] [Google Scholar]
- 68. Kiernan PD, Hernandez A, Byrne WD et al Descending cervical mediastinitis. Ann. Thorac. Surg. 1998; 65: 1483–1488. [DOI] [PubMed] [Google Scholar]
- 69. Marty‐Ane CH, Berthet JP, Alric P, Pegis JD, Rouviere P, Mary H. Management of descending necrotizing mediastinitis: an aggressive treatment for an aggressive disease. Ann. Thorac. Surg. 1999; 68: 212–217. [DOI] [PubMed] [Google Scholar]
- 70. Kocher GJ, Hoksch B, Caversaccio M, Wiegand J, Schmid RA. Diffuse descending necrotizing mediastinitis: surgical therapy and outcome in a single‐centre series. Eur. J. Cardiothorac Surg. 2012; 42: e66–72. [DOI] [PubMed] [Google Scholar]
- 71. Isowa N, Yamada T, Kijima T, Hasegawa K, Chihara K. Successful thoracoscopic debridement of descending necrotizing mediastinitis. Ann. Thorac. Surg. 2004; 77: 1834–1837. [DOI] [PubMed] [Google Scholar]
- 72. Min HK, Choi YS, Shim YM, Sohn YI, Kim J. Descending necrotizing mediastinitis: a minimally invasive approach using video‐assisted thoracoscopic surgery. Ann. Thorac. Surg. 2004; 77: 306–310. [DOI] [PubMed] [Google Scholar]
- 73. Chen KC, Chen JS, Kuo SW et al Descending necrotizing mediastinitis: a 10‐year surgical experience in a single institution. J. Thorac. Cardiovasc. Surg. 2008; 136: 191–198. [DOI] [PubMed] [Google Scholar]
- 74. Langford FP, Moon RE, Stolp BW, Scher RL. Treatment of cervical necrotizing fasciitis with hyperbaric oxygen therapy. Otolaryngol. Head Neck Surg. 1995; 112: 274–278. [DOI] [PubMed] [Google Scholar]
- 75. Krenk L, Nielsen HU, Christensen ME. Necrotizing fasciitis in the head and neck region: an analysis of standard treatment effectiveness. Eur. Arch. Otorhinolaryngol. 2007; 264: 917–922. [DOI] [PubMed] [Google Scholar]
- 76. Dool H, Soetekouw R, van Zanten M, Grooters E. Lemierre's syndrome: three cases and a review. Eur. Arch. Otorhinolaryngol. 2005; 262: 651–654. [DOI] [PubMed] [Google Scholar]


