Abstract
Case
A 61‐year‐old woman was diagnosed with deep cervical abscess and enlarged mediastinal abscess. These required a protracted period of mechanical ventilation and neck and thoracic drainage surgery with daily wound lavage, necessitating the administration of large amounts of fentanyl and dexmedetomidine. After extubation, fentanyl was discontinued but dexmedetomidine was continued, and she developed hypertension, tachycardia, tachypnea, and hyperthermia within several hours; therefore, she was diagnosed with opioid withdrawal syndrome. Her symptoms failed to improve with either an increased dexmedetomidine dose or a diltiazem infusion for symptomatic management. Ultimately, 20 mg nifedipine was given through a nasogastric tube, which led to a resolution of withdrawal symptoms.
Outcome
This is the first case of calcium channel blockers attenuating opioid withdrawal syndrome symptoms in a human.
Conclusion
Calcium channel blockers might be alternative therapy to refractory opioid withdrawal syndrome. Case accumulation in the future is expected.
Keywords: Calcium channel blocker, fentanyl, nifedipine, opioid withdrawal syndrome
Introduction
A significant problem associated with prolonged opioid use in critically ill patients is the development of opioid withdrawal syndrome (OWS). Although the optimal treatment for OWS is not well defined in critically ill patients, reports suggest that α‐2 agonists have some efficacy. Here, we report a case in which a calcium channel blocker (CCB) that was refractory to α‐2 agonist therapy was effective in treating OWS.
Case Report
A 61‐year‐old euthyroid woman with a history of Graves' disease following isotope treatment presented with fever and dyspnea. Laryngoscopy revealed marked laryngeal edema, and tracheal intubation was carried out for airway protection. Contrast‐enhanced computed tomography on day 1 showed an abscess from the mediastinum to the oropharynx, and the patient was diagnosed with deep cervical abscess. The causative microorganism was identified as α‐Streptococcus spp. from dental caries. The patient was treated with antibiotics and mechanical ventilation in the intensive care unit. Fentanyl and dexmedetomidine (DEX), analgesic and sedative, respectively, were continually administered to the patient targeting the Richmond Agitation–Sedation Scale from 0 to −2 and, if necessary, supplemental propofol was administered.
Unfortunately, conservative therapy failed to improve descending necrotizing mediastinitis, and she underwent neck drainage surgery with two Penrose tubes and thoracic drainage with a 20‐French gauge (Fr) thoracic tube on day 4, followed by daily wound lavage. However, contrast‐enhanced computed tomography on day 8 revealed an enlarged mediastinal abscess. On day 9, video‐assisted mediastinal drainage was carried out with a 15‐Fr nelaton catheter, with continued daily wound lavage via the nelaton catheters.
Subsequently, deep cervical abscess and her hemodynamic and respiratory status improved but marked laryngeal edema hindered extubation. Because of persistent laryngeal edema, analgesia and sedation continued for the management of mechanical ventilation to protect the airway. We achieved an ideal Richmond Agitation–Sedation Scale from 0 to −2 throughout this period. A large amount of fentanyl was used for the treatment of wound lavage and prevention of accidental removal of drainage catheters and the tracheal tube.
On day 30, laryngeal edema had reduced and extubation was feasible, and at that time, we administered DEX and fentanyl. First, fentanyl administration was terminated, and because of mild respiratory depression due to fentanyl, we administered 0.1 mg naloxone and carried out extubation following confirmation of improved respiratory status. The total fentanyl dose was 1.6 mg/kg for 30 days. Treatment with DEX (0.3 μg/kg/h) was continued after extubation. Gradual increase in blood pressure, heart rate, respiratory rate, and body temperature was observed several hours after extubation. Her arterial blood gas analysis showed a favorable status, (pH, 7.437; pCO2, 47.2 mmHg; pO2, 151.3 mmHg [O2 mask, 5 L/min], HCO3, 31.3 mmol/L) and airway obstruction, infection, or thyroid abnormality (thyroid stimulating hormone, 6.54 μU/mL; free‐thyroxine, 0.53 ng/dL) were not observed. The patient was diagnosed with OWS, and the DEX dose was increased to 0.8 μg/kg/h. However, her OWS symptoms did not improve; therefore, a diltiazem infusion was given for symptomatic management. Despite maximal administration of diltiazem (30 mg/h), her symptoms persisted; therefore, 20 mg nifedipine was additionally administered through a nasogastric tube on day 32. Although the patient suffered from transient hypotension, OWS symptoms, including hypertension, tachycardia, tachypnea, and hyperthermia, were resolved with no recurrence (Fig. 1). On day 38, she was discharged from the intensive care unit. At present, she is being followed‐up at our hospital for her wounds.
Figure 1.
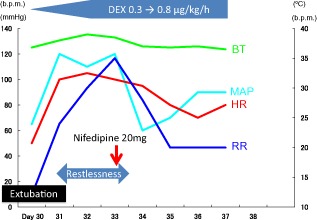
Clinical course of symptoms and treatment of opioid withdrawal syndrome. BT, body temperature; DEX, dexmedetomidine; HR, heart rate; MAP, mean arterial pressure; RR, respiratory rate.
Discussion
Critically ill patients who are mechanically ventilated often require prolonged opioid administration. In turn, extended opioid use increases the risks of opioid complications, including OWS.1 Iatrogenic OWS has an incidence of 35%–57%, with prior exposure to sedative and analgesic agents.2, 3 Moreover, OWS itself increases morbidity, hospital costs, and the duration of hospital stay.4
Opioid withdrawal syndrome symptoms are caused by hyperactivity of the central nervous system that has been suppressed by long‐term administration of opioids.4 Symptoms of OWS include central nervous system irritability, gastrointestinal distress, and autonomic dysfunction.5 Cammarano et al. proposed diagnostic criteria for OWS that requires more than two of the following symptoms and signs:2 restlessness, nausea, anxiety, myoclonus, delirium, tachycardia, hypertension, fever, seizures, and tachypnea.
The risk factors for iatrogenic OWS include extended exposure, elevated total cumulative doses, rapid tapering, and abrupt discontinuation.1, 2 With regard to fentanyl, the risk increases when the total cumulative dose exceeds 1.5 mg/kg. In this case, the dose was approximately 1.6 mg/kg.
Although the optimal treatment for OWS in critically ill patients is unknown, the α‐2 agonist has been widely used. Many OWS symptoms stem from noradrenergic hyperactivity in the central nervous system. Through a negative feedback process, α‐2 agonists that stimulate α‐2a receptors on presynaptic neurons in the locus coeruleus suppress catecholamine release from postganglionic adrenergic nerve terminals and relieve autonomic OWS symptoms.1
Other reports suggest that OWS can be effectively treated with methadone, buprenorphine, benzodiazepines, and supportive therapy.6 Moreover, DEX, an α‐2 agonist, has reported to be effective for OWS treatment, similar to clonidine.1, 7 In our case, although DEX was given prior to extubation and after development of OWS, it was unable to resolve OWS symptoms. It is possible that the patient developed resistance to DEX because of prolonged treatment (for 3 weeks). The α‐2 agonist, DEX, was ineffective for treating OWS; therefore, an alternative was necessary.
In patients with OWS, voltage‐dependent calcium channels play an essential role in nerve impulse transmission such as neurotransmitter release.8, 9 In experiments using animal models, verapamil, nifedipine, and nimodipine were effective for treating OWS.8, 10, 11 However, there have been no reports stating that CCB effectively treated OWS in humans. In our case, diltiazem was given as supportive therapy, which was ineffective. Diltiazem is considered most effective in preventing rather than treating OWS;12 therefore, if diltiazem was given previously, the patient may not have developed OWS.
However, subsequent treatment with nifedipine was effective in attenuating OWS. In rats, nifedipine abolished the signs of OWS through the following proposed mechanism. Nifedipine is a dihydropyridine CCB, and the density of cortical dihydropyridine binding sites is elevated in rats in a state of morphine withdrawal by naloxone administration after chronic morphine treatment.13
Furthermore, Michaluk et al.14 reported that the coadministration of different types of CCB can completely block OWS in morphine‐dependent rats. In this case, the coadministration of nifedipine and diltiazem may have acted synergistically to treat OWS. The precise mechanism is still unknown; however, CCB displayed a beneficial effect on OWS.
This case showed that CCBs can effectively attenuate OWS symptoms in humans. Calcium channel blockers might be alternative therapy to refractory OWS. Additional studies with large sample size are needed to explore the efficacy of CCB to treat OWS in humans.
Conflict of Interest
None.
References
- 1. Honey BL, Benefield RJ, Miller JL et al α2‐Receptor agonists for treatment and prevention of iatrogenic opioid abstinence syndrome in critically ill patients. Ann. Pharmacother. 2009; 43: 1506–1511. [DOI] [PubMed] [Google Scholar]
- 2. Cammarano WB, Pittet JF, Weitz S et al Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit. Care Med. 1998; 26: 676–684. [DOI] [PubMed] [Google Scholar]
- 3. Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit. Care Med. 1999; 27: 196–199. [DOI] [PubMed] [Google Scholar]
- 4. Wijdicks EF, Sharbrough FW. New‐onset seizures in critically‐ill patients. Neurology 1993; 43: 1042–1043. [DOI] [PubMed] [Google Scholar]
- 5. Ista E, van Dijk M, Gamel C et al Withdrawal symptoms in critically ill children after long‐term administration of sedatives and/or analgesics: a first evaluation. Crit. Care Med. 2008; 36: 2427–2432. [DOI] [PubMed] [Google Scholar]
- 6. Tetrault JM, O'Connor PG. Substance abuse and withdrawal in the critical care setting. Crit. Care Clin. 2008; 24: 767–788. [DOI] [PubMed] [Google Scholar]
- 7. Upadhyay SP, Mallick PN, Elmatite WM et al Dexmedetomidine infusion to facilitate opioid detoxification and withdrawal in a patient with chronic opioid abuse. Indian J. Palliat. Care 2011; 17: 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee SC, Yoburn BC. The effect of nimodipine on opioid antagonist‐induced upregulation and supersensitivity. Pharmacol. Biochem. Behav. 2000; 66: 347–351. [DOI] [PubMed] [Google Scholar]
- 9. Drdla R, Sandkühler J. Long‐term potentiation at C‐fibre synapses by low‐level presynaptic activity in vivo . Mol. Pain 2008; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blackburn‐Munro G, Brown CH, Neumann ID et al Verapamil prevents withdrawal excitation of oxytocin neurones in morphine‐dependent rats. Neuropharmacology 2000; 39: 1596–1607. [DOI] [PubMed] [Google Scholar]
- 11. Vitcheva V, Mitcheva M. Effects of nifedipine on behavioral and biochemical parameters in rats after multiple morphine administration. Methods Find. Exp. Clin. Pharmacol. 2004; 26: 631–634. [DOI] [PubMed] [Google Scholar]
- 12. Kishioka S, Inoue N, Nishida S et al Diltiazem inhibits naloxone‐precipitated and spontaneous morphine withdrawal in rats. Eur. J. Pharmacol. 1996; 316: 7–14. [DOI] [PubMed] [Google Scholar]
- 13. Antkiewicz‐Michaluk L, Michaluk J, Romanska I et al Cortical dihydropyridine binding sites and behavioural syndrome in morphine‐abstinent rats. Eur. J. Pharmacol. 1990; 180: 129–135. [DOI] [PubMed] [Google Scholar]
- 14. Michaluk J, Karolewicz B, Antkiewicz‐Michaluk L et al Effects of various Ca2+ channel antagonists on morphine analgesia, tolerance and dependence, and on blood pressure in the rat. Eur. J. Pharmacol. 1998; 352: 189–197. [DOI] [PubMed] [Google Scholar]


