Abstract
Aim
Early prediction of the neurological outcomes of patients with out‐of‐hospital cardiac arrest is important to select the optimal clinical management. We hypothesized that clinical data recorded at the site of cardiopulmonary resuscitation would be clinically useful.
Methods
This retrospective cohort study included patients with return of spontaneous circulation after cardiopulmonary resuscitation who were admitted to our university hospital between January 2000 and November 2013 or two affiliated hospitals between January 2006 and November 2013. Clinical parameters recorded on arrival included age (A), arterial blood pH (B), time from cardiopulmonary resuscitation to return of spontaneous circulation (C), pupil diameter (D), and initial rhythm (E). Glasgow Outcome Scale was recorded at 6 months and a favorable neurological outcome was defined as a score of 4–5 on the Glasgow Outcome Scale. Multiple logistic regression analysis was carried out to derive a formula to predict neurological outcomes based on basic clinical parameters.
Results
The regression equation was derived using a teaching dataset (total, n = 477; favourable outcome, n = 55): EP = 1/(1 + e− x), where EP is the estimated probability of having a favorable outcome, and x = (−0.023 × A) + (3.296 × B) − (0.070 × C) − (1.006 × D) + (2.426 × E) − 19.489. The sensitivity, specificity, and accuracy were 80%, 92%, and 90%, respectively, for the validation dataset (total, n = 201; favourable outcome, n = 25).
Conclusion
The 6‐month neurological outcomes can be predicted in patients resuscitated from out‐of‐hospital cardiac arrest using clinical parameters that can be easily recorded at the site of cardiopulmonary resuscitation.
Keywords: Cardiopulmonary arrest, logistic regression, neurological outcomes, prediction, return of spontaneous circulation
Introduction
Post‐cardiac arrest care, including percutaneous coronary intervention (PCI) and therapeutic hypothermia, after the return of spontaneous circulation (ROSC) can improve patient survival with good quality of life.1 Post‐cardiac arrest care may also reduce the morbidity and mortality associated with post‐cardiac arrest syndrome.1 However, intensive care physicians are increasingly being confronted with the question of whether to provide more intensive medical treatment to comatose patients who are resuscitated from out‐of‐hospital cardiac arrest (OHCA). This question arose because it is very difficult to predict the neurological outcomes of patients with OHCA at the early stage of ROSC. Therapeutic hypothermia was reported to improve the neurological outcomes in patients resuscitated from OHCA, and should be carried out immediately after successful ROSC.2, 3 The decision to use more invasive and intensive therapy, such as extracorporeal cardiopulmonary resuscitation (ECPR), must be made promptly in order to be effective.4 Therefore, it is important to predict the patient's neurological outcome as quickly as possible.
It was reported that biomarkers, such as interleukin (IL)‐6 and IL‐8 levels in cerebrospinal fluid and/or blood,5, 6 serum neuron‐specific enolase,6, 7 S‐100B protein,6, 8 glial fibrillary acidic protein,9 and creatine kinase‐BB isoenzyme10 were associated with neurological outcomes. However, it may take several hours to obtain the results of biochemical/laboratory tests, and such data have yet to enter practical use at the bedside. The timing of prediction also varies considerably among biomarkers. The predictors investigated in earlier reports included electrophysiological tests (e.g., electroencephalography and somatosensory evoked potentials) and neuroimaging (e.g., computed tomography and magnetic resonance imaging).11, 12, 13, 14, 15 However, it is impractical to perform electrophysiological tests in an emergency room immediately after ROSC. Neuroimaging examinations carried out immediately after ROSC are typically normal, and negative findings do not always predict good outcomes. Although clinical data, including the circumstances of OHCA and simple neurological tests, may show limited use as predictors, we can only obtain these clinical data immediately after ROSC.11
The purpose of this study was to establish a new model for predicting neurological outcomes using patient clinical data that are easy to obtain during the very early stage of ROSC in the emergency room.
Methods
Patients with OHCA
The study protocol was approved by the Institutional Review Board of the participating hospitals. The study was designed as a retrospective cohort study. Eligible patients received cardiopulmonary resuscitation (CPR), including chest compression and artificial respiration, from emergency medical services. Some patients received respiratory tract control with an appropriate device, and were treated with infusion solution and epinephrine.
Two datasets were established: derivation and validation. The derivation dataset consisted of patients with ROSC after OHCA who were admitted to our university hospital between January 2000 and November 2013. The validation dataset consisted of patients with ROSC after OHCA who were admitted to two affiliated hospitals between January 2006 and November 2013.
Data collection and assessment of patient outcomes
All patients were confirmed to have OHCA by medical staff (doctor, nurse, or emergency medical service) using an electrocardiographic monitor. We retrieved the following clinical parameters from medical records: age, sex, cause of cardiac arrest, presence of a witness, bystander CPR, initial rhythm, time from starting CPR to arrival at hospital, time from starting CPR to ROSC, pupil diameter on arrival at the hospital before the administration of epinephrine, initial arterial blood pH, use of therapeutic hypothermia, ECPR, PCI, and Glasgow Outcome Scale score at 6 months after ROSC.16 If epinephrine was given before the patient arrived at the hospital, the pupil diameter was recorded immediately before the administration of epinephrine during pre‐hospital CPR. If pupil diameter differed between the left and right eyes, the smaller diameter was recorded. Blood samples were obtained before or after ROSC within 10 min after arrival at the hospital. For patients who received ECPR, we regarded the time of starting ECPR as ROSC. Initial rhythm was classified as either ventricular fibrillation (Vf), which was defined as Vf and pulseless ventricular tachycardia (VT), or as non‐Vf, which was defined as pulseless electrical activity (PEA) and asystole.
Neurological outcomes were assessed in terms of Glasgow Outcome Scale score at 6 months after ROSC, which provides five categories. Favorable neurological outcomes included patients with good recovery and moderate disability, whereas unfavorable neurological outcomes included patients with severe disability, persistent vegetative state, or death.
Statistical analysis
To confirm whether the derivation and validation datasets were similar, we statistically compared their demographic and clinical parameters using the Mann–Whitney U‐test for continuous variables, or the χ2‐test and Fisher's exact probability test for categorical variables. P‐values were adjusted for multiple testing using Sidak's method.17 Taking into account the large sample sizes in both groups, we set the level of significance at P < 0.01.
Using the derivation dataset, multiple logistic regression analysis was carried out to derive an equation to predict favorable neurological outcome using the commonly recorded clinical parameters. Dummy variables were introduced for categorical variables, as follows: gender (male = 0; female = 1), witness (present = 1; absent = 0), bystander CPR (done = 1; not done = 0), Vf or VT in initial rhythm (yes = 1; no = 0), need for ECPR (yes = 1; no = 0), PCI (done = 1; not done = 0), and therapeutic hypothermia (done = 1; not done = 0). We used the stepwise selection method to develop the logistic regression equation. The estimated probability (EP) of having a favorable neurological outcome was computed from the clinical parameters for each patient. Diagnostic efficacy of EP in distinguishing unfavorable neurological outcomes was expressed as area under the curve (AUC) by receiver‐operating characteristic (ROC) analysis. The cut‐off value for EP was determined as a point of the optimal accuracy. In the next step, the equation was applied to the validation dataset and the diagnostic accuracy was evaluated by AUC and the rate of correct diagnosis.
All statistical analyses were carried out using StatFlex version 6 (Artech, Osaka, Japan).
Results
Characteristics of patients with OHCA
The derivation dataset initially consisted of 488 patients. However, clinical data or laboratory data could not be obtained in 11 patients, so these patients were excluded. Therefore, the final derivation dataset consisted of 477 patients (Fig. 1). The validation dataset initially consisted of 213 patients, but incomplete data were obtained from 12 patients. Therefore, the final validation dataset consisted of 201 patients (Fig. 1). The demographic and clinical characteristics of both datasets were comparable, except for age, sex, bystander CPR, and time from CPR to arrival at hospital, which were significantly different between the two datasets (Table 1).
Figure 1.
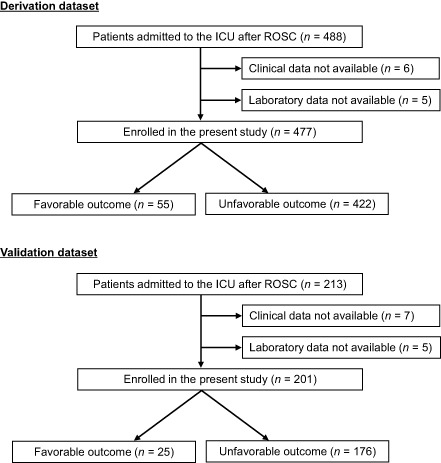
Patients with return of spontaneous circulation (ROSC) after cardiopulmonary resuscitation who were admitted to our university hospital between January 2000 and November 2013 (derivation dataset) or two affiliated hospitals between January 2006 and November 2013 (validation dataset). ICU, intensive care unit.
Table 1.
Characteristics of the derivation and validation datasets of patients with return of spontaneous circulation (ROSC) after cardiopulmonary resuscitation (CPR)
| Variables | Derivation dataset † (n = 477) | Validation dataset † (n = 201) | P‐value ‡ |
|---|---|---|---|
| Age, years | 64 ± 20 | 72 ± 16 | <0.01 |
| Sex | |||
| Male, n (%) | 318 (67) | 104 (52) | <0.01 |
| Cardiac etiology, n (%) | 219 (46) | 92 (46) | 0.97 |
| Witness, n (%) | 282 (59) | 133 (66) | 0.09 |
| Bystander CPR, n (%) | 129 (27) | 76 (38) | <0.01 |
| Initial rhythm | |||
| Vf and pulseless VT, n (%) | 87 (18) | 36 (18) | 0.92 |
| Time from CPR to arrival at hospital, min | 26 ± 15 | 21 ± 15 | <0.01 |
| Time from CPR to ROSC, min | 32 ± 19 | 30 ± 17 | 0.43 |
| Therapeutic hypothermia, n (%) | 99 (21) | 29 (14) | 0.06 |
| ECPR, n (%) | 38 (8) | 6 (3) | 0.02 |
| PCI, n (%) | 37 (8) | 13 (6) | 0.56 |
| Pupil size, mm | 4.7 ± 1.5 | 4.6 ± 1.4 | 0.39 |
| Arterial blood pH | 7.0 ± 0.2 | 7.0 ± 0.2 | 0.57 |
| Favorable neurological outcome, n (%) | 55 (12) | 25 (12) | 0.74 |
†Values are given as n (%) or as the mean ± SD. ‡P < 0.01 was considered statistically significant. ECPR, extracorporeal cardiopulmonary resuscitation; PCI, percutaneous coronary intervention; Vf, ventricular fibrillation; VT, ventricular tachycardia.
Multiple logistic regression model
Of 477 patients in the derivation dataset, 55 had favorable neurological outcomes (53 with good recovery and 2 with moderate disability) and 422 had unfavorable neurological outcomes (3 with severe disability, 79 with persistent vegetative state, and 340 died) (Fig. 1).
After stepwise variable selection, age (years, A), blood pH (B), time from starting CPR to ROSC (minutes, C), pupil diameter (mm, D), and Vf in the initial rhythm (yes = 1; no = 0, E) were significantly associated with favorable neurological outcomes.
The logistic regression equation derived was EP = 1/(1 + e− x), where EP represents the estimated probability of having a favorable neurological outcome, and x represents a linear combination of the relevant clinical parameters (i.e., variables A–E), for which the formula is shown below.
Based on the signs of the regression coefficients, A, C, and D were negatively associated with favorable neurological outcomes, while B and E were positively associated with favorable neurological outcomes. The odds ratio (OR) of each parameter for a specified size of change (Δ) in its value can be computed from the regression coefficient (b) as exp(b × Δ). Using the regression model coefficients, the OR for a change of −10 years of age was 1.26 (95% confidence interval [CI], 1.03–1.54), while the OR for a change in pH of 0.1 was 1.39 (95% CI, 1.11–1.74). The OR for a 2‐min decrease in the time from starting CPR to ROSC was 1.15 (95% CI, 1.07–1.23). The OR for a reduction in pupil diameter by 0.5 mm was 1.65 (95% CI, 1.37–1.99), and the OR for Vf rhythm was 11.31 (95% CI, 4.63–27.63). These findings are summarized in Table 2.
Table 2.
Significant predictors identified in the derivation dataset of patients with return of spontaneous circulation (ROSC) after cardiopulmonary resuscitation (CPR)
| Variables | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Age (per 10 years decrease) | 1.26 | 1.03–1.54 | 0.0281 |
| pH (per 0.1 increase) | 1.39 | 1.11–1.74 | 0.0037 |
| Time from starting CPR to ROSC (per 2 min decrease) | 1.15 | 1.07–1.23 | 0.0001 |
| Pupil diameter (per 0.5 mm reduction) | 1.65 | 1.37–1.99 | 0.0000 |
| Vf in initial rhythm | 11.31 | 4.63–27.63 | 0.0000 |
CI, confidence interval; Vf, ventricular fibrillation or pulseless ventricular tachycardia.
The ROC analysis was used to assess the efficacy of EP in predicting favorable neurological outcomes (Fig. 2). The AUC representing the accuracy of the prediction was as high as 0.95.
Figure 2.
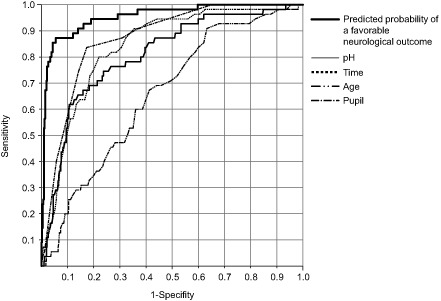
Receiver‐operating characteristic curve for the probability of favorable neurological outcomes based on the prediction model derived from the derivation dataset. The diagnostic efficacies of four individual parameters (pH, time, age, and pupil size), except for ventricular fibrillation or pulseless ventricular tachycardia (Vf) which was recorded as binary (yes = 1; no = 0), were compared with those of the estimated probability of favorable outcomes computed using the prediction model. Time denotes time from starting cardiopulmonary resuscitation to the return of spontaneous circulation.
The optimal cut‐off value for EP was 0.218 at a point of the optimal accuracy. For an EP exceeding 0.218, the neurological outcome was predicted to be favorable. The sensitivity, specificity, and accuracy of the prediction derived from the derivation dataset were 87%, 94%, and 93%, respectively.
Validation
The validation dataset (n = 201), which consisted of 25 patients with favorable outcomes and 176 patients with unfavorable outcomes, was used to assess the efficacy of the EP values computed from the prediction equation. The sensitivity, specificity, and accuracy of the model were 80%, 92%, and 90%, respectively. The ROC analysis of the EP values yielded an AUC of 0.94 (Fig. 3). The similar AUC for the validation and derivation datasets indicates good reproducibility of the equation in predicting the neurological outcome.
Figure 3.
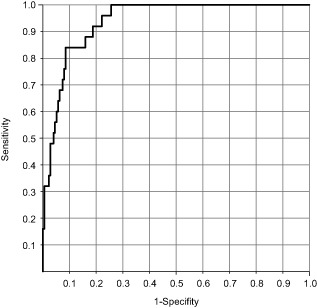
Receiver‐operating characteristic curve for the probability of favorable neurological outcomes in the validation dataset of patients with return of spontaneous circulation after cardiopulmonary resuscitation.
Discussion
In the present clinical study, the EP enabled us to predict the neurological outcome at 6 months after CPR, more quickly and accurately than the previously reported predictive models.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
We believe that the present EP should be helpful to assess whether aggressive therapeutic measures, such as PCI and/or therapeutic hypothermia, should be carried out as part of the patient's advanced life support. The EP will also help the family to understand the patient's likely neurological outcome at 6 months after CPR. Hayakawa et al.18 reported several prognostic indicators and an outcome prediction model for patients with ROSC after OHCA by discriminant logistic regression analysis. Criteria for inclusion in their study were a witnessed OHCA and presumed cardiac origin of the arrest. Because many patients lack a witness or cardiac cause in clinical settings, the inclusion criteria were not restricted in the present study. Therefore, we developed a model that can be applied to a broader range of patients. Of the 11 variables, five (age, arterial blood pH, time from starting CPR to ROSC, pupil diameter on arrival, initial Vf rhythm) were identified by the multiple logistic regression analysis to predict favorable neurological outcomes in the present study. Hayakawa et al.18 also reported that age and the time from collapse to ROSC were important predictors for outcomes at 1 month after OHCA, which were classified by the initial rhythm.
The sensitivity and specificity of predicting favorable neurological outcomes in the validation dataset were 80% and 92%, respectively. These values are sufficiently high to encourage the use of the new formula in routine clinical practice, particularly during the very early period after ROSC. Fischer et al. and others reported that pupil diameter, presence of a witness, bystander CPR, age, and initial rhythm were associated with patient outcomes.19, 20, 21, 22, 23, 24 Wijdicks et al.11 reported that the duration of CPR was associated with poor outcomes, but they could not clearly discriminate between patients with poor outcomes and those with favorable outcomes.
The number of patients with therapeutic hypothermia was 31 in favorable neurological outcome in derivation dataset. Of these 31 patients, 23 patients had a witness and Vf in initial rhythm. If therapeutic hypothermia reveals a 15% improvement in outcome for patients with a witness and Vf, as reported in the HACA study,3 approximately three patients may have benefited from therapeutic hypothermia in the present study. We estimate that the influence of therapeutic hypothermia would be limited in the prediction formula.
To improve the diagnostic accuracy by reducing the numbers of false‐negative and false‐positive cases, it may be useful to add other parameters, such as biomarkers (e.g., blood ammonia and lactate), electrophysiological tests, and neuroimaging data, which could be available immediately after ROSC.11, 12, 13, 14, 15, 25 However, in this study, because many of these parameters were not available or did not have satisfactory accuracy, we did not examine the effects of including them in the regression model. By contrast, IL‐6, IL‐8, S100B, neuron‐specific enolase, and glial fibrillary acidic protein levels could be used to assess EP, particularly in the later stages of resuscitation, because the appearance of these proteins in blood or cerebrospinal fluid occurs sometime after ROSC.5, 6, 7, 8, 9, 10
In the derivation dataset, seven patients with favorable outcomes were misclassified as having unfavorable outcomes. Two of these patients had acute coronary syndrome with a Vf rhythm that was unresponsive to defibrillation, and therefore required ECPR. The start of ECPR, which was defined as ROSC, was delayed in these patients compared with other patients who received ECPR. Nevertheless, the other parameters measured on arrival were generally good. Two patients also had acute coronary syndrome, but they were over 80 years of age and had a PEA rhythm. One patient was already hypothermic with a body temperature of 32°C on arrival. Therefore, the time to ROSC was relatively long, although the other parameters remained in appropriate ranges. The other two patients had hypercapnic respiratory failure with a PEA rhythm. From these observations, we should take care when using the EP for patients who had ECPR, hypothermia on arrival, or extreme hypercapnia with a non‐Vf rhythm, or who were elderly people with a non‐Vf rhythm.
This study has some limitations. First, it was not a multicenter clinical trial. Cardiopulmonary resuscitation and advanced therapeutic strategies, including therapeutic hypothermia or ECPR, were often carried out at our university and at the affiliated hospitals.1, 4 However, these advanced therapeutic strategies may differ among institutions. Therefore, the prediction formula reported here may not be applicable to all patients with ROSC in every institution. Second, only 80 patients (12%) of the total had favorable outcomes compared with 598 (88%) patients with unfavorable outcomes. Therefore, a prospective study with a larger number of patients is needed to confirm our results and establish a reproducible model.
Conclusions
Using multivariate logistic regression analysis, and including clinical parameters that are easily obtained at the site of CPR, we developed a model to predict neurologically favorable outcomes at 6 months after resuscitation from OHCA. The resulting model had a sensitivity and specificity of 80% and 92%, respectively.
Conflict of Interest
None.
Acknowledgements
The authors thank Mr. Keisuke Takada for his assistance on the statistical analysis, and Ms. Mika Abe for her editorial assistance.
References
- 1. Peberdy MA, Callaway CW, Neumar RW et al Part 9: post‐cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122: S768–786. [DOI] [PubMed] [Google Scholar]
- 2. Bernard SA, Gray TW, Buist MD et al Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002; 346: 557–563. [DOI] [PubMed] [Google Scholar]
- 3. Hypothermia after Cardiac Arrest Study Group . Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002; 346: 549–556. [DOI] [PubMed] [Google Scholar]
- 4. Cave DM, Gazmuri RJ, Otto CW et al Part 7: CPR techniques and devices: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122: S720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oda Y, Tsuruta R, Kasaoka S, Inoue T, Maekawa T. The cutoff values of intrathecal interleukin 8 and 6 for predicting the neurological outcome in cardiac arrest victims. Resuscitation 2009; 80: 189–193. [DOI] [PubMed] [Google Scholar]
- 6. Ekmektzoglou KA, Xanthos T, Papadimitriou L. Biochemical markers (NSE, S‐100, IL‐8) as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation. Resuscitation 2007; 75: 219–228. [DOI] [PubMed] [Google Scholar]
- 7. Almaraz AC, Bobrow BJ, Wingerchuk DM, Wellik KE, Demaerschalk BM. Serum neuron specific enolase to predict neurological outcome after cardiopulmonary resuscitation: A critically appraised topic. Neurologist 2009; 15: 44–48. [DOI] [PubMed] [Google Scholar]
- 8. Oda Y, Tsuruta R, Fujita M et al Prediction of the neurological outcome with intrathecal high mobility group box 1 and S100B in cardiac arrest victims. Resuscitation 2012; 83: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 9. Kaneko T, Kasaoka S, Miyauchi T et al Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation 2009; 80: 790–794. [DOI] [PubMed] [Google Scholar]
- 10. Tirschwell DL, Longstreth WT Jr, Rauch‐Matthews ME et al Cerebrospinal fluid creatine kinase BB isoenzyme activity and neurologic prognosis after cardiac arrest. Neurology 1997; 48: 352–357. [DOI] [PubMed] [Google Scholar]
- 11. Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S; Quality Standards Subcommittee of the American Academy of Neurology . Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 67: 203–210. [DOI] [PubMed] [Google Scholar]
- 12. Madl C, Grimm G, Kramer L et al Early prediction of individual outcome after cardiopulmonary resuscitation. Lancet 1993; 341: 855–858. [DOI] [PubMed] [Google Scholar]
- 13. Nakabayashi M, Kurokawa A, Yamamoto Y. Immediate prediction of recovery of consciousness after cardiac arrest. Intensive Care Med. 2001; 27: 1210–1214. [DOI] [PubMed] [Google Scholar]
- 14. Torbey MT, Selim M, Knorr J, Bigelow C, Recht L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke 2000; 31: 2163–2167. [DOI] [PubMed] [Google Scholar]
- 15. Choi SP, Park HK, Park KN et al The density ratio of grey to white matter on computed tomography as an early predictor of vegetative state or death after cardiac arrest. Emerg. Med. J. 2008; 25: 666–669. [DOI] [PubMed] [Google Scholar]
- 16. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975; 1: 480–484. [DOI] [PubMed] [Google Scholar]
- 17. Westfall PH, Young SS. Resampling‐Based Multiple Testing. New York: Wiley‐Interscience, 1993. [Google Scholar]
- 18. Hayakawa K, Tasaki O, Hamasaki T et al Prognostic indicators and outcome prediction model for patients with return of spontaneous circulation from cardiopulmonary arrest: The Utstein Osaka Project. Resuscitation 2011; 82: 874–880. [DOI] [PubMed] [Google Scholar]
- 19. Fischer M, Fischer NJ, Schüttler J. One‐year survival after out‐of‐hospital cardiac arrest in Bonn City: Outcome report according to the “Utstein Style”. Resuscitation 1997; 33: 233–243. [DOI] [PubMed] [Google Scholar]
- 20. Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004; 291: 870–879. [DOI] [PubMed] [Google Scholar]
- 21. Swor RA, Jackson RE, Cynar M et al Bystander CPR, ventricular fibrillation, and survival in witnessed, unmonitored out‐of‐hospital cardiac arrest. Ann. Emerg. Med. 1995; 25: 780–784. [DOI] [PubMed] [Google Scholar]
- 22. Schultz SC, Cullinane DC, Pasquale MD, Magnant C, Evans SR. Predicting in‐hospital mortality during cardiopulmonary resuscitation. Resuscitation 1996; 33: 13–17. [DOI] [PubMed] [Google Scholar]
- 23. Parish DC, Dane FC, Montgomery M, Wynn LJ, Durham MD. Resuscitation in the hospital: Differential relationships between age and survival across rhythms. Crit. Care Med. 1999; 27: 2137–2141. [DOI] [PubMed] [Google Scholar]
- 24. Bar‐Joseph G, Kette F, von Planta M, Wiklund L. Acid–base considerations and buffer therapy In: Paradis NA, Halperin HR, Kern KB, Wenzel V, Chamberlain DA. (eds). Cardiac Arrest: The Science and Practice of Resuscitation Medicine, 2nd edn Cambridge: Cambridge University Press, 2007; 674–697. [Google Scholar]
- 25. Shinozaki K, Oda S, Sadahiro T et al Blood ammonia and lactate levels on hospital arrival as a predictive biomarker in patients with out‐of‐hospital cardiac arrest. Resuscitation 2011; 82: 404–409. [DOI] [PubMed] [Google Scholar]


