Dear Editor,
A 58‐year‐old Asian woman hung herself. When emergency medical service arrived, she was under cardiopulmonary arrest with asystolic rhythm. Sixty minutes later, her circulation was restored and she was then transferred from a rural hospital to our hospital. When the patient was brought to our hospital, she was unconscious, and her pupils were dilated and had no light reflex. Computed tomography images showed diffuse brain swelling and unclear corticomedullary junctions, and electroencephalography revealed no brain activity.
After admission to the intensive care unit, noradrenaline and vasopressin were started, and were continued for 2 months due to persistent vasodilatory shock. On day 7, we started glutamine, fiber, and oligosaccharide (GFO; Otsuka Pharmaceutical Factory, Inc., Naruto City, Japan) through a nasogastric tube, and on day 10, watery diarrhea ensued after receiving low residual liquid diet. On day 12, we changed the enteral nutrient to HINE E‐GEL (Otsuka Pharmaceutical Factory, Inc.), a low residual liquid diet changing from a liquid to a jelly in a low pH environment. The next day, diarrhea stopped. After HINE E‐GEL was given consistently, soft stool was excreted regularly, without vomiting. Because serum phosphorus increased, we started other low residual liquid nutrients. As the patient developed watery diarrhea thereafter, we continued giving HINE E‐GEL with lanthanum carbonate. On day 28, a plain abdominal x‐ray revealed that her stomach was filled with calcified substances (Fig. 1). A few days later, she started vomiting. To dissolve the bezoar, 500 mL commercially available Coca‐Cola (Coca‐Cola East Japan Co, Ltd., Tokyo, Japan) was administered through a nasogastric tube once a day according to several previous reports.1 The bezoar was reduced gradually thereafter.
Figure 1.
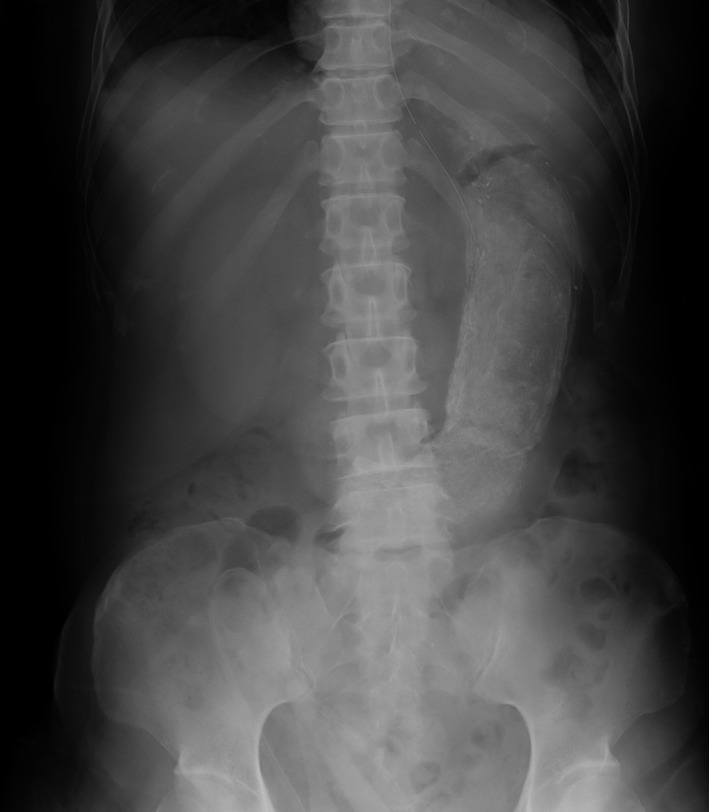
Abdominal x‐ray of a 68‐year‐old critically ill woman who developed gastric bezoar following feeding with low residual liquid diet in jelly form. On hospital day 28, the abdominal x‐ray showed her stomach was dilated and filled with calcified substances.
Gastric bezoars in critically ill patients have been rarely reported. Some case reports showed intestinal bezoars were caused by reduced gastrointestinal motility, which most frequently occurred after gastrointestinal surgery.2, 3 Although our patient had not undergone surgery, her intestinal motility might have been impaired because of her critical condition. Another risk factor for bezoar is feeding substances. Aluminum hydroxide, sucralfate, cholestyramine, and nifedipine are supposed to be the causes of pharmacobezoar.4 Our patient enterally received lanthanum carbonate, esomeprazole, hydrocortisone, and bifidobacterium. Among them, only lanthanum carbonate was reported as a cause of a pharmacobezoar in a patient who swallowed chewable tablets without chewing repeatedly, resulting in formation of a rectal bezoar;5 our patient was administered lanthanum carbonate dissolved in water through a nasogastric tube. To our knowledge, liquid diet in jelly form has not been reported as a cause of bezoar. Clinicians should be aware that low residual liquid diet changing from a liquid to a jelly in a low pH environment, which is widely used to prevent gastro‐esophageal regurgitation and diarrhea, can cause gastric bezoar in critically ill patients with reduced intestinal motility.
Conflict of Interest
None.
Acknowledgments
Written informed consent was obtained from the patient's family for publication of this case report.
[The copyright line for this article was changed on 28 October 2016 after original online publication]
References
- 1. Chung YW, Han DS, Park YK et al Huge gastric diospyrobezoars successfully treated by oral intake and endoscopic injection of Coca‐Cola. Dig. Liver Dis. 2006; 38: 515–7. [DOI] [PubMed] [Google Scholar]
- 2. Dedes KJ, Schiesser M, Schafer M et al Postoperative bezoar ileus after early enteral feeding. J Gastrointest Surg. 2006; 10: 123–7. [DOI] [PubMed] [Google Scholar]
- 3. Iwamuro M, Okada H, Matsueda K et al Review of the diagnosis and management of gastrointestinal bezoars. World J Gastrointest Endosc. 2015; 7: 336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor JR, Streetman DS, Castle SS. Medication bezoars: a literature review and report of a case. Ann. Pharmacother. 1998; 32: 940–6. [DOI] [PubMed] [Google Scholar]
- 5. Black T, Philips G, Burbridge R. Pharmacobezoar in a patient on an oral phosphate binder. Gastrointest. Endosc. 2013; 77: 511–2. [DOI] [PubMed] [Google Scholar]


