Abstract
Case
We report a lethal case of a mamushi (Gloydius blomhoffii) bite. An 84‐year‐old woman was bitten by a mamushi snake on her right elbow. She was initially treated at another hospital, however, because the swelling expanded and her renal function worsened, she was transferred to our hospital. Compartment syndrome, thrombocytopenia, respiratory arrest, and elevated creatinine kinase and lactose dehydrogenase levels were seen; renal failure progressed, and ileus and peritonitis due to colon perforation occurred.
Outcome
The patient died 35 days after the bite. An autopsy revealed widespread necrosis of the ileum, colon, and liver.
Conclusion
This report supplies useful clinical information on the treatment of severe mamushi bite cases, and severe abdominal symptoms, such as ileus and melena, may be a lethal sign.
Keywords: Creatinine kinase, Gloydius blomhoffii, lethal case, mamushi bite, white blood cell count
Introduction
It is estimated that 10 out of 3,000 patients bitten by mamushi (Gloydius blomhoffii) in Japan die annually.1 However, the lethal cases of such mamushi bites are rarely reported, therefore, the processes to death are not well known to many physicians. Autopsy findings are also rarely reported.2 We experienced a lethal case of mamushi bite and herein report the clinical course and autopsy findings. We also analyzed the lethal signs of mamushi bite by undertaking a review of published reports.
Case
An 84‐year‐old woman was bitten on her right elbow by a mamushi snake while working on a farm at approximately 4.00 pm in mid‐August (day 1). She was initially admitted to another hospital, however, the swelling expanded and her renal function worsened on day 2. Therefore, the patient was transferred to our hospital at 9.00 am on the same day. Diffuse swelling and a wide s.c. hemorrhage was noted in the right upper limb and trunk (Fig. 1A–C). The initial blood analysis data are shown in Figure 1D. Acute renal failure, hepatic failure, rhabdomyolysis, and disseminated intravascular coagulation were noted. After admission, i.v. anti‐mamushi venom serum was given, and fluid expansion was started. However, the circulatory state did not improve and respiratory arrest occurred 3 h later. An examination by computed tomography revealed no cerebral lesion. Respiratory support and hemodialysis/continuous hemodiafiltration was started. Additional antiserum was injected later, however, compartment syndrome in the right forearm became evident, therefore, a decompression incision was made. From the time of admission, oliguria <100 mL/day had continued and generalized edema was marked. The lactate dehydrogenase (LD) and creatine kinase (CK) levels peaked at day 3, presenting as 79,400 IU/L (LD1, 7%; LD2, 5%; LD3, 3%; LD4, 10%; LD5, 75%) and 14,661 IU/L (BB, 0%; MB, 8%; MM, 92%), respectively. After admission, ileus presenting with evident bowel expansion (Fig. 1E) and occasional melena continued, and finally, abdominal free air was evident on day 19 (Fig. 1F). Emergency surgery revealed sigmoid colon necrosis, and it was resected (Fig. 1G). Elevation of the C‐reactive protein value accelerated, starting on day 20, and turbid ascites was collected on day 29. Enterococcus faecalis and Enterobacter aerogenes were also detected, thus bacterial peritonitis was diagnosed. From day 32, acidosis progressed, the surgical suture in the abdomen was opened, and on day 33, free air was observed in the portal vein and intestine wall ranging from the stomach to the colon (Fig. 1H, I). The patient died on day 35. The trends in the laboratory findings are summarized in Figure 2.
Figure 1.
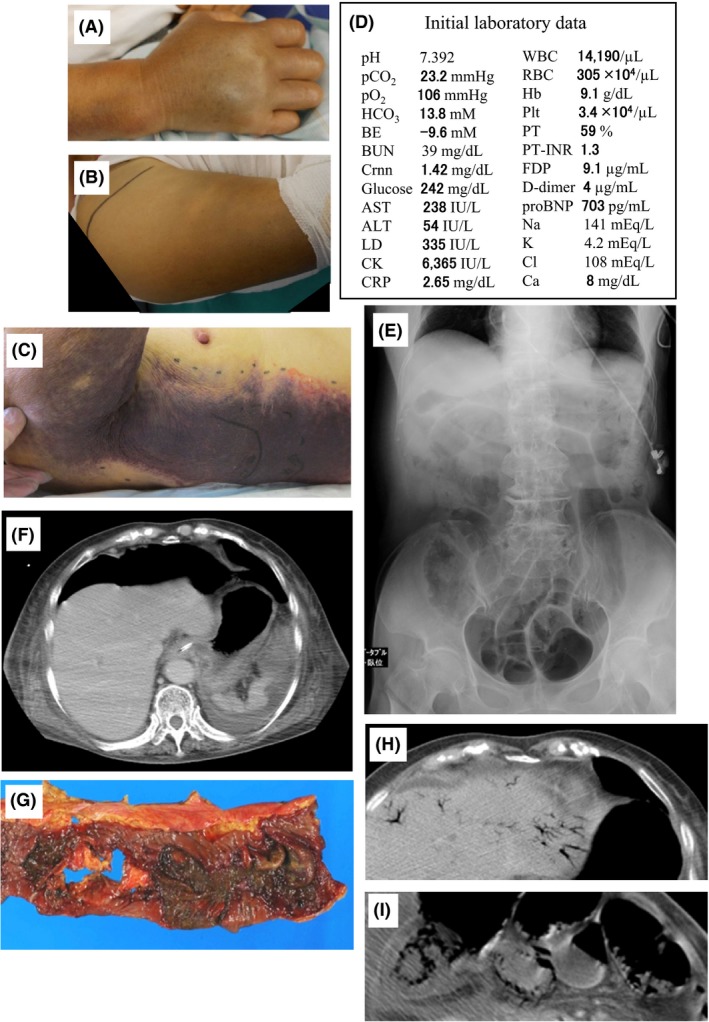
Clinical course of an 84‐year‐old woman following a mamushi (Gloydius blomhoffii) bite on the right elbow. Appearance of the hand (A), upper arm (B), and a vast s.c. hemorrhage on the trunk (C). The hand is severely swollen and the fingers show flexion contracture. Swelling extended distally from the elbow to the hand and proximally to the shoulder joint. D, Initial laboratory data on admission to our hospital. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BE, base excess; BUN, blood urea nitrogen; CK, creatine kinase; Crnn, creatinine; CRP, C‐reactive protein; FDP, fibrin degradation products; Hb, hemoglobin; LD, lactate dehydrogenase; pCO2, partial pressure of carbon dioxide; Plt, platelets; pO2, partial pressure of oxygen; pro BNP, amino‐terminal pro‐brain natriuretic peptide; PT, prothrombin time; PT‐INR, prothrombin time – international normalized ratio; RBC, red blood cells; WBC, white blood cells. E, Expanded bowels due to paralytic ileus on abdominal X‐ray. F, Free air in the abdomen. G, Excised necrotic sigmoid colon. Note multiple dark‐colored ulcers and a perforation. Air in the portal vein (H) and the walls of the intestine (I) on a computed tomography scan.
Figure 2.
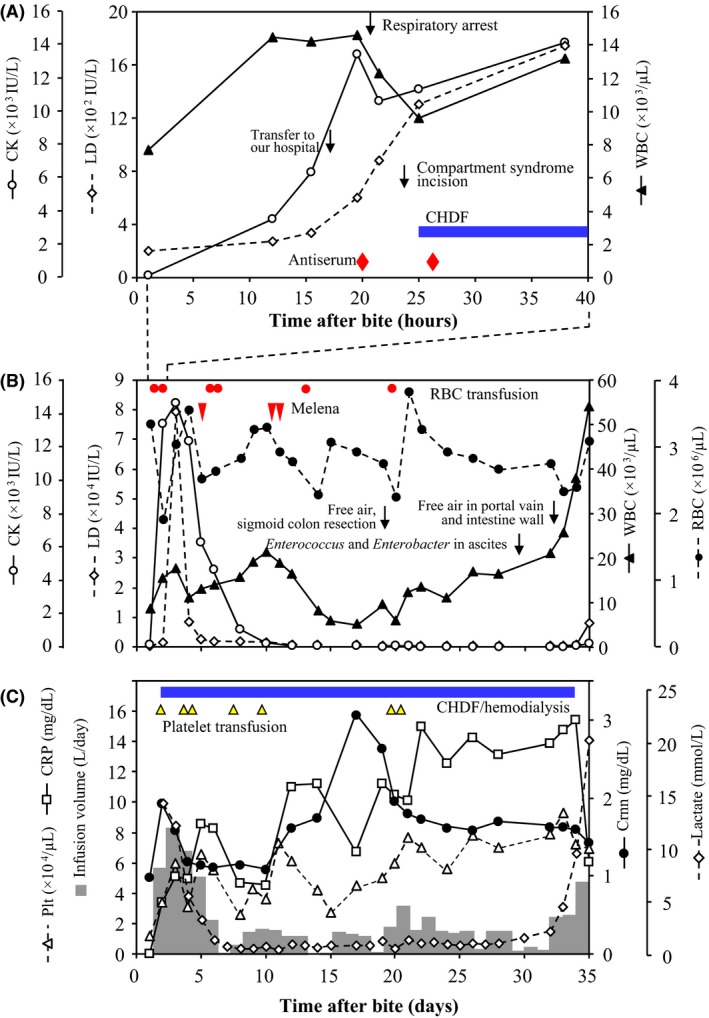
Trends in laboratory findings in an 84‐year‐old woman following a mamushi (Gloydius blomhoffii) bite on the right elbow. A, Trends in creatine kinase (CK) and lactate dehydrogenase (LD) levels and white blood cell (WBC) counts during 40 h after the mamushi bite. B, C, Trends in several parameters during the clinical course. Gray bars show infusion volume. Events are indicated by symbols. CHDF, continuous hemodiafiltration; Crnn, creatinine; CRP, C‐reactive protein; Plt, platelets; RBC, red blood cells.
An autopsy was carried out. The findings are summarized in Figure 3. The main macroscopic findings were as follows: small intestine, extensive patchy necrosis; ascending colon, multiple ulcers and bleeding; liver, acute necrosis in a wide area; and kidneys, cortical necrosis (Fig. 3A–D). Histologically, intestinal necrosis extended to the muscle layer, and the liver showed necrosis around the central vein (Fig. 3E, F). Glomerular necrosis was seen in a focal and wedge‐shaped pattern, thus indicating damage due to arteriolosclerotic change (data not shown). The brain was not examined. The cause of death was concluded to be peritonitis due to bowel necrosis and perforation.
Figure 3.
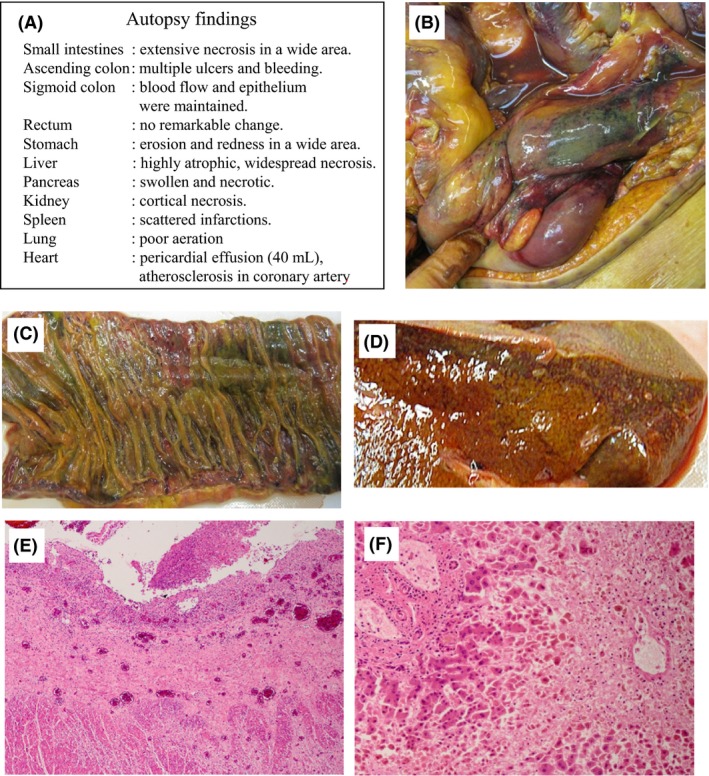
Autopsy findings in an 84‐year‐old woman who died following a mamushi (Gloydius blomhoffii) bite on the right elbow. A, Findings in each organ. B, Small intestine: some areas are necrotic and bleeding is prominent. C, In half of the area, submucosal bleeding and loss of epithelium are observed. D, Liver, diffuse central necrosis is shown. E, F, Microscopic findings. E, Small intestine, ulceration by the submucosa was seen and the necrotic area extended to the muscle layer. F, Liver, extensive necrosis around the central vein is prominent and many hepatocytes are lost. Structures in the portal area are maintained. This type of necrosis suggests intoxication.
Discussion
In addition to the present case, seven lethal cases have appeared in published reports since 1990.2, 3, 4, 5, 6, 7, 8 Two cases died within 4 days, and the causes of death were hyperkalemia and respiratory failure.6, 7 In one report, intestinal bleeding was described in relation to bleeding diathesis.6 Six cases survived during the acute phase and died after more than 1 week. In four out of the six cases, and in the present case, lower intestinal necrosis/bleeding appeared, which was suspected of being the cause of death (67%).2, 3, 4 In three cases, ileus was described or suspected (as in the present case).2, 3 However, of 25 severe but non‐lethal mamushi bite cases published since 1990, four reports (16%) described lower intestinal bleeding, but ileus was not mentioned.9, 10, 11, 12 Thus, severe lower intestinal troubles after an acute phase indicate a risk of death. Although not often described, ileus may be a lethal sign. Renal failure was invariably observed in severe cases and could not be recognized as a specific lethal symptom.
There were two life‐threatening crises during the course of the present patient. The first was marked thrombocytopenia, disseminated intravascular coagulation, and acute renal failure during the first several days. The clinical severity was reflected by the volume of transfusion (Fig. 2C). Thrombocytopenia can be caused by the direct injection of l‐amino acid oxidase in mamushi venom into the circulation.13 It is estimated that a considerable amount of venom had been injected into the circulation in this patient. The second became apparent after the appearance of free air due to bowel perforation, and this event resulted in death. The crisis was mainly reflected by elevation of the C‐reactive protein level and white blood cells (Fig. 2B, C). Injection of hemorrhagic factor‐1 into mice causes hemorrhagic bowel necrosis,14 therefore, hemorrhagic factor‐1 is suspected of being a causative agent, but the mechanism is not known. Potential lethal courses of mamushi bite are: (i) a proportion of patients die during the first crisis, likely by acute renal failure, (ii) those who survive the first crisis die during the long second crisis due to bowel necrosis.
A characteristic finding was an extreme elevation of the LD value (up to 79,000 IU/L). The LD5 isozyme was dominant, indicating potent liver damage by the venom, which was confirmed by an autopsy. Such an extreme LD value elevation in mamushi bite is rare. In our patient, the main origin of CK was the muscles (CK isozyme MM predominant), as in many other cases. The final elevation of the white blood cell count likely indicated the occurrence of a leukemoid reaction.
Approximately 10 deaths a year are attributed to mamushi bite in Japan, although only several cases have been published in the past 26 years. Therefore, the details of the progression from bite to death are unknown to most physicians. In this report, we suggested that severe bowel symptoms are signs of lethality, and by accumulating further reports, management of severe mamushi bite patients will be improved.
Conflict of interest
None.
[The copyright line for this article was changed on 28 October 2016 after original online publication]
References
- 1. Sakai A. Mamushi, habu, yamakagashi . Rinsho‐i. Med. Clin. Japan 2001; 27 (suppl): 1911–5. [Google Scholar]
- 2. Koutoku T, Kaguchi A, Kiyoi T, Nakamura T, Shin Y, Kimura T. A lethal case of mamushi bite. Nishi‐Nihon J. Dermatol. 2002; 64: 318–21. [Google Scholar]
- 3. Oishi M, Okamoto O, Fujiwara S et al A lethal case of mamushi bite. Jpn. J. Clin. Dermatol. 2008; 62: 407–10. [Google Scholar]
- 4. Abe M, Takai K, Tasaki M, Noda K, Shinohara I. A case of viper bite barely treated by the upper arm amputation. Jpn. J. Acute Med. 2001; 25: 368–70. [Google Scholar]
- 5. Kaneko N, Chida A, Okada Y. Initial management of viper bite: a report of two severe cases. J. Jpn. Soc. Emerg. Med. 2005; 8: 378–84. [Google Scholar]
- 6. Akishima S, Takahashi K, Hisakura K, Sakurai J. A case of critical injury by viperbite in a patient who underwent aortic valve replacement. Surgery 2005; 67: 329–32. [Google Scholar]
- 7. Uematsu M, Sawamura T, Hattori T, Yasufuku M. Review of 29 viper bites – advantage of antivenin therapy. J. Jpn. Soc. Clin. Surg. 1994; 55: 54–60. [Google Scholar]
- 8. Fujiwara I, Nomi S, Naitou K et al A study of 58 cases of viper bite. J. Jpn. Soc. Clin. Surg. 1992; 53: 1451–8. [Google Scholar]
- 9. Nakano M, Tanabe D, Kondo K, Noda K. A severe case of viper bite associated with stenotic ischemic enteritis. J. Clin. Surg. 2007; 62: 135–8. [Google Scholar]
- 10. Saito K, Sato K, Miyashou K, Kobayashi N, Kosaka N. A case of viper bite complicated with multiple organ failures. Jpn. J. Intensive Care Med. 1990; 14: 347–52. [Google Scholar]
- 11. Miura H, Hayano K, Inobe Y, Fukui H. Two severe cases of acute renal failure induced by mamushi viper venom. J. Jpn. Soc. Dial. Ther. 1991; 24: 651–5. [Google Scholar]
- 12. Kaihara R, Nagano Y, Kou T, Otsuka N, Yamaguchi K, Sakemi T. A case of severe mamushi viper venom‐induced disorder complicated with acute renal failure, rhabdomyolysis and ischemic colitis. J. Jpn. Soc. Dial. Ther. 1993; 26: 1337–40. [Google Scholar]
- 13. Takatsuka H, Sakurai Y, Yoshioka A et al Molecular characterization of l‐amino acid oxidase from Agkistrodon halys blomhoffii with special reference to platelet aggregation. Biochim. Biophys. Acta 2001; 1544: 267–77. [DOI] [PubMed] [Google Scholar]
- 14. Oshima G, Omori‐Sato T, Iwanaga S, Suzuki T. Studies on snake venom hemorrhagic factor I (HR‐I) in the venom of Agkistrodon halys blomhoffi. Its purification and biological properties. J. Biochem. 1972; 72: 1483–94. [DOI] [PubMed] [Google Scholar]


