Abstract
Aim
The present study aimed to elucidate the clinical characteristics of non‐convulsive status epilepticus (NCSE) in patients with altered mental status (AMS).
Methods
This single‐center retrospective study comprised 149 patients who were hospitalized between March 1, 2015 and September 30, 2015 at the emergency intensive care unit (ICU) of the Kagawa University Hospital (Kagawa, Japan). The primary outcome was NCSE incidence. The secondary outcome was the comparison of duration of ICU stay, hospital stay, and a favorable neurological outcome, as assessed using the modified Rankin Scale score, at discharge from our hospital between patients with and without NCSE. Favorable neurological outcome and poor neurological outcome were defined as modified Rankin Scale scores of 0–2 and 3–6, respectively.
Results
Simplified continuous electroencephalogram was used to monitor 36 patients (median age, 68 years; 69.4% males) with acute AMS; among them, NCSE was observed in 11 (30.1%) patients. Rates of favorable neurological outcome, duration of ICU stay, and hospital stay were not significantly different between the NCSE and non‐NCSE groups (P = 0.45, P = 0.30, and P = 0.26, respectively).
Conclusion
Approximately 30% of the patients with AMS admitted to emergency ICUs developed NCSE. The outcomes of AMS patients with and without NCSE did not differ significantly when appropriate medical attention and antiepileptic drugs were initiated. Simplified continuous electroencephalogram monitoring may be recommended in patients with AMS in emergency ICU to obtain early detection of NCSE followed by appropriate intervention.
Keywords: Altered mental status, electroencephalogram, intensive care unit, neurological outcome, non‐convulsive status epilepticus
Introduction
Non‐convulsive status epilepticus (NCSE) is defined as seizure activity on an electroencephalogram (EEG) without clinical findings associated with generalized convulsive status epilepticus.1 Non‐convulsive status epilepticus has been recognized as a relatively common condition among critically ill patients and those in an intensive care unit (ICU), with a prevalence of 7%2 to 10%3 among patients with continuous EEG monitoring. The condition must be diagnosed and rapidly treated to avoid significant morbidity and mortality.4, 5, 6 Conversely, our understanding of the clinical characteristics of NCSE presenting in ICUs in Japan is limited because of the insufficient recognition of NCSE by acute care physicians in ICUs. It is likely that a majority of NCSE cases remain undiagnosed if continuous EEG is not carefully monitored.7
Our institute provides a non‐invasive monitoring system combining two‐channel simplified continuous EEG (sEEG) and amplitude‐integrated EEG for the bedside monitoring of cerebral activities,8, 9 which facilitates the identification of NCSE in emergency ICU with two‐channel limitations. The present study aimed to elucidate the clinical characteristics of NCSE in patients with AMS.
Methods
Subjects
This study included all patients who underwent continuous EEG monitoring for at least 48 h between March 1, 2015 and September 30, 2015 at the emergency ICU in Kagawa University Hospital (Kagawa, Japan). Indications for continuous EEG included acutely ill patients with severely altered mental status (AMS), with or without subtle motor movements.1 Altered mental status was defined as any alteration in the level of responsiveness or alertness or arousability and could present as lethargy, delirium, confusion, agitation, coma, disinhibition, labile/blunted affects, or unexpected psychosis.10
Patients were excluded if they were in the first 24–48 h of withdrawal from aggressive treatment, if they could not continue an EEG examination of 48 h because of restlessness or patient demands, or if they were pregnant. Intubated patients were excluded if their consciousness was confirmed to be absolutely normal as judged by each acute care physician during sedation vacation and based on other findings.
Study design and setting
This single‐center study was a retrospective analysis of data collected by reviewing medical records. The study was approved by the institutional review board (IRB) of the Kagawa University Hospital and was carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The institutional review board waived the requirement for patient consent because of the retrospective nature of the study.
Diagnosis of NCSE by two‐channel sEEG
After admission to ICU, continuous EEG monitoring was undertaken as early as possible using a two‐channel EEG, which was displayed on the bedside monitor (BSM‐9101, AE‐918P; Nihon Kohden Corp., Tokyo, Japan). The EEG was recorded on a montage (Fp1‐C3, Fp2‐C4); the actual EEG waves were stored in the hard disk for 24 h and the electrodes were the non‐invasive disk type.
Patient diagnosis of epileptiform discharge was classified into three patterns, as described in the American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology, version 2012.11 These patterns were periodic discharges (PDs), rhythmic delta activity (RDA), and spike wave (SW; including both sharp wave and polyspike wave), with each pattern qualified based on their localization pattern such as “generalized” or “lateralized.” We regarded the wave pattern, displayed as an “evolution” such as changes in frequency, wave form, or localization, as important to diagnose NCSE. In particular, PDs were never diagnosed as NCSE without evolution.
We adopted the definition of status epilepticus from the generalized convulsive status epilepticus (GCSE) definition, which was provided by the Neuro Critical Care Society. We specified that if an epileptic discharge continued for >5 min or was repeated without an improvement in the mental status, it should be recorded as NCSE.1 In such cases, we routinely trialed antiepileptic drugs (AED) using either benzodiazepine (5 mg) or fosphenytoin (22.5 mg/kg) for NCSE diagnosis and reconfirmed when the epileptic discharges had disappeared and AMS was improved after these trials.7 We defined the patient who had epileptic discharges, which was diagnosed by our protocol, as NCSE even after GCSE.12
The international 10–20 systems EEG (BSM‐9101; Nihon Kohden Corp.) was used to complement the NCSE diagnosis using the two‐channel sEEG as needed to avoid incorrect diagnosis. The diagnosis of EEG findings and NCSE were determined by a neurointensivist and a neurosurgeon who were familiar with EEG.
Treatment of NCSE with AEDs
At our institution, NCSE is treated with AEDs and continuous administration of sedative drugs. The protocol is based on the guidelines of epilepsy published by the Neurocritical Care Society1.We defined first‐line treatment as an i.v. injection of diazepam and fosphenytoin as well as the enteral use of AEDs (levetiracetam, valproic acid, carbamazepine, phenytoin, and diazepam). Second‐line treatment comprised continuous i.v. injection of midazolam and propofol. For tertiary treatment, thiopental or thiamylal sodium was added, following the first‐ and second‐line treatment.
First‐line treatment is the key to resolving NCSE. Our protocol for first‐line treatment, besides conducting a AED trial, is as follows: we administered an initial dose of fosphenytoin at 22.5 mg/kg followed by 7.5 mg/kg/day for 3 days and added levetiracetam (1000–2000 mg/day) by enteral tube.
Data sampling
The following data were collected: age, gender, admission diagnosis, Glasgow Coma Scale score, Acute Physiology and Chronic Health Evaluation (APACHE) II score at admission, clinical symptoms, presence or absence of NCSE, modified Rankin Scale (mRS) at hospital discharge, duration of ICU stay, and duration of hospital stay.
Outcome measures
The primary outcome was the incidence of NCSE. Secondary outcomes were comparison of the duration of ICU stay, duration of hospital stay, and the incidence of a favorable neurological outcome (FO; mRS score of 0–2 at hospital discharge) between with NCSE group and without NCSE group. A poor neurological outcome (PO) was defined as an mRS score of 3–6 at hospital discharge.
Statistical analysis
Patient demographic factors and baseline characteristics were summarized using descriptive statistics. The distribution of each variable was compared between NCSE and non‐NCSE groups using the Mann–Whitney U‐test or Fisher's exact test, depending on the type of variable. Univariate analyses between the FO and PO groups were carried out to explore potential prognostic factors for FO. All statistical analyses were undertaken using JMP version 11 (SAS, Cary, NC, USA). A two‐sided probability value of <0.05 was considered to be statistically significant.
Results
Demographic factors and clinical characteristics
A total of 149 patients were hospitalized in the emergency ICU between March 1, 2015 and September 30, 2015 (Fig. 1). Simplified continuous EEG monitoring was used for 36 patients (median age, 68 years; 69.4% males) with AMS. Non‐convulsive status epilepticus was observed in 11 (30.1%) patients; 10 (27.8%) patients were discharged from the hospital with FO (Table 1).
Figure 1.
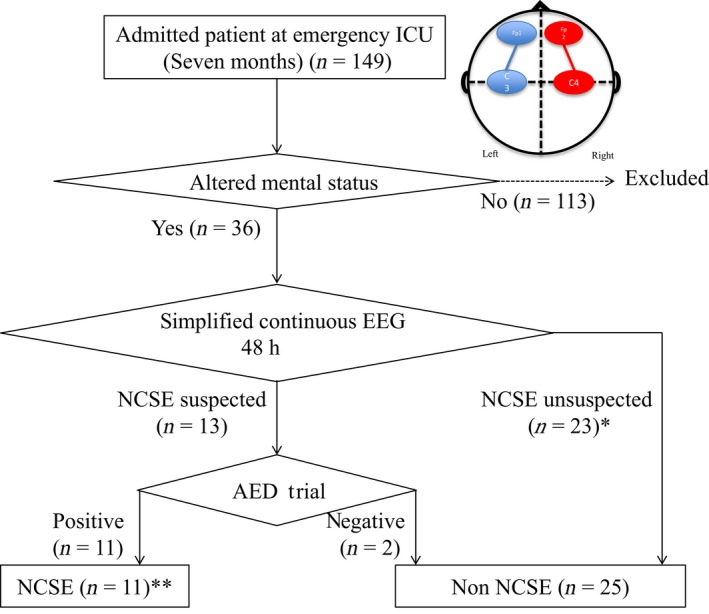
Flowchart of our study of non‐convulsive status epilepticus (NCSE) in patients with altered mental status admitted to a Japanese intensive care unit (ICU). The circle with quadrants shows the configuration of the bedside two‐channel, amplitude‐integrated electroencephalography (EEG) device: FP1–C3 and FP2–C2. *One case with lateralized periodic discharges was included; the lateralized periodic discharges disappeared when circulatory status was improved. **Six cases were included who underwent international 10–20 system EEG on the day same as that of the antiepileptic drug (AED) trial.
Table 1.
Baseline characteristics of 32 patients with altered mental status admitted to a Japanese intensive care unit (ICU)
| Characteristic | Total (n = 36) |
|---|---|
| Age, years | 68 (48–80) |
| Male | 25 (69.4) |
| Admission diagnosis | |
| Cerebral hemorrhage | 8 (22.2) |
| Infarction | 3 (8.3) |
| SAH | 2 (5.6) |
| Trauma | 4 (11.1) |
| PCAS | 5 (13.9) |
| GCSE | 4 (11.1) |
| Other | 10 (27.8) |
| GCS score | 7 (6–10) |
| APACHE II score | 25 (21–34) |
| NCSE | 11 (30.1) |
| Outcome | |
| ICU stay | 13 (6.3–22) |
| Hospital stay (in days) | 23.5 (12–36.5) |
| Favorable neurological outcome | 10 (27.8) |
Data are presented as median (interquartile range) for continuous variables and n (%) for categorical variables.
APACHE II, Acute Physiology and Chronic Health Evaluation; GCS, Glasgow Coma Scale; GCSE, generalized convulsive status epilepticus; NCSE, non‐convulsive status epilepticus; PCAS, post‐cardiac arrest syndrome; SAH, aneurysmal subarachnoid hemorrhage.
Details of patients with NCSE
The continuous EEG (cEEG) findings showed seven patients with spike and waves, and RDA in five patients (Table 2). Lateralized PDs (LPDs) were revealed in one patient. Non‐convulsive status epilepticus was resolved in all patients with AEDs, and their mental status recovered with disappearance of epileptic discharges. One patient (case 2) required thiopental. We followed the international 10–20 systems, and six cases were recorded and confirmed as NCSE on the same day as that of the AED trial.
Table 2.
Details of 11 patients admitted to a Japanese intensive care unit with non‐convulsive status epilepticus
| Case | Age, years | Gender | APACHE II score | Admission diagnosis | Clinical features | cEEG findings | AEDs | mRS at discharge |
|---|---|---|---|---|---|---|---|---|
| 1 | 81 | Male | 37 | PCAS | Altered mental status with myoclonus of the facial muscle | GPEDs, spike and wave | fPHT, LEV, propofol, diazepam | 6 |
| 2 | 38 | Male | 22 | GCSE | Altered mental status following generalized convulsive seizures and myoclonus of the facial muscle | Rhythmic delta activity | fPHT, LEV, propofol, midazolam, diazepam, thiopental | 1 |
| 3 | 76 | Female | 18 | Cerebral infarction | Altered mental status with the episodes of normal mentation | Spike and wave | fPHT, LEV, diazepam | 4 |
| 4 | 66 | Male | 24 | Post‐operative meningioma | Altered mental status following ;generalized convulsive seizures along with nystagmoid eye movement | LPDs | fPHT, LEV, diazepam | 2 |
| 5 | 71 | Male | 18 | Aspiration pneumonia | Altered mental status | Spike and wave | fPHT, PHT, LEV | 4 |
| 6 | 1 | Male | 43 | PCAS | Altered mental status | Rhythmic delta activity | VPA, midazolam | 4 |
| 7 | 79 | Male | 16 | Epilepsy | Altered mental status, aphasia | Spike and wave | LEV, CBZ, diazepam | 1 |
| 8 | 87 | Female | 34 | Pneumonia | Altered mental status, aphasia | Spike and wave, rhythmic delta activity | LEV, diazepam | 4 |
| 9 | 65 | Female | 19 | Epilepsy | Altered mental status following generalized convulsive seizures | Spike and wave, rhythmic delta activity | LEV, diazepam, propofol | 1 |
| 10 | 47 | Female | 22 | Refeeding syndrome | Altered mental status with myoclonus of the facial muscle along with nystagmoid eye movement | Spike and wave | LEV, diazepam | 4 |
| 11 | 75 | Male | 32 | Cerebral hemorrhage | Altered mental status following generalized convulsive seizures along with nystagmoid eye movement | Rhythmic delta activity | fPHT, LEV, propofol | 5 |
AED, antiepileptic drug; APACHE II, Acute Physiology and Chronic Health Evaluation; cEEG, continuous electroencephalogram; CBZ, carbamazepin; fPHT, fosphenytoin; GCSE, generalized convulsive status epilepticus; GPED, generalized periodic epileptiform discharge; LEV, levetiracetam; LPD, lateralized periodic discharge; mRS, modified Rankin Scale; PCAS, post‐cardiac arrest syndrome; VPA, valproic acid.
Comparison of baseline characteristics and outcomes between NCSE and non‐NCSE groups
No significant differences were observed in the baseline characteristics between these two groups or in the rate of achieving FO (P = 0.45), duration of ICU stay (P = 0.30), or duration of hospital stay (P = 0.26) (Table 3).
Table 3.
Comparison of baseline characteristics and outcomes between patients with altered metal status admitted to a Japanese intensive care unit (ICU) with non‐convulsive status epilepticus (NCSE) or not (Non‐NSCE)
| Characteristic | NCSE (n = 11) | Non‐NCSE (n = 25) | P‐value |
|---|---|---|---|
| Age, years | 71 (47–79) | 67 (48–82) | 0.85 |
| Male | 4 (36.4) | 7 (28.0) | 0.70 |
| Admission diagnosis | |||
| Cerebral hemorrhage | 1 (9.1) | 7 (28.0) | 0.22 |
| Infarction | 1 (9.1) | 2 (8.0) | |
| SAH | 0 (0) | 2 (8.0) | |
| Trauma | 0 (0) | 4 (16.0) | |
| PCAS | 2 (18.2) | 3 (12.0) | |
| GCSE | 1 (9.1) | 3 (12.0) | |
| Other | 6 (54.6) | 4 (16.0) | |
| GCS score | 7 (6–9) | 7 (6–10) | 0.79 |
| APACHE II score | 22 (18–34) | 26 (23–34) | 0.40 |
| ICU stay | 11 (5–15) | 13 (7–23) | 0.30 |
| Hospital stay (in days) | 18 (12–23) | 28 (10–39) | 0.26 |
| Favorable neurological outcome | 4 (36.4) | 6 (24.0) | 0.45 |
Data are presented as median (interquartile range) for continuous variables and n (%) for categorical variables.
APACHE II, Acute Physiology and Chronic Health Evaluation; GCS, Glasgow Coma Scale; GCSE, generalized convulsive status epilepticus; PCAS, post‐cardiac arrest syndrome; SAH, aneurysmal subarachnoid hemorrhage.
Comparison of baseline characteristics between FO and PO groups
When the FO and PO groups were compared (Table 4), the median APACHE II score in the FO group was significantly lower than that in the PO group (median [interquartile range]): 22 [18–24] vs. 28 [23–34], P < 0.01).
Table 4.
Comparison between favorable neurological outcome and poor neurological outcome in patients with altered metal status admitted to a Japanese intensive care unit (ICU)
| Characteristic | Favorable neurological outcome: mRS, 0–2 (n = 10) | Poor neurological outcome: mRS, 3–5 (n = 26) | P‐value |
|---|---|---|---|
| Age, years | 66 (48–79) | 70 (47–82) | 0.93 |
| Male | 1 (10.0) | 10 (38.5) | 0.13 |
| Admission diagnosis | |||
| Cerebral hemorrhage | 1 (10.0) | 7 (26.9) | 0.07 |
| Infarction | 0 (0) | 3 (11.5) | |
| SAH | 0 (0) | 2 (7.7) | |
| Trauma | 1 (10.0) | 3 (11.5) | |
| PCAS | 0 (0) | 5 (19.2) | |
| GCSE | 3 (30.0) | 1 (3.9) | |
| Other | 5 (50.0) | 5 (19.2) | |
| GCS score | 9 (7‐10) | 7 (4–10) | 0.16 |
| APACHE II score | 22 (18–24) | 28 (23–34) | <0.01 |
| NCSE | 4 (40.0) | 7 (26.9) | 0.45 |
| Outcome | |||
| ICU stay | 7 (4–14) | 14 (7–23) | 0.06 |
| Hospital (in days)stay | 12 (6–22) | 30 (15–40) | 0.02 |
Data are presented as medians (interquartile range) for continuous variables and n (%) for categorical variables.
APACHE II, Acute Physiology and Chronic Health Evaluation; GCS, Glasgow Coma Scale; GCSE, generalized convulsive status epilepticus; mRS, modified Rankin Scale; NCSE, non‐convulsive status epilepticus; PCAS, post‐cardiac arrest syndrome; SAH, aneurysmal subarachnoid hemorrhage.
Discussion
In the present study, approximately 30% of the patients with AMS in an emergency ICU in Japan developed NCSE. The outcomes of patients with AMS with and without NCSE did not differ significantly when appropriate medical attention and AED were initiated.
Kubota et al. reported the frequency of NCSE as 33% among patients who underwent cEEG with AMS in a stroke care unit in Japan.13 This result was similar to the frequency of NCSE of 30% reported in the present study. This may have been because of selection bias at our institute because approximately 69% of patients required neurointensive care. Because Kobata et al. reported that their advanced tertiary care center also provided neurocritical care to more than 50% of the patients,14 the rate of NCSE in the present study suggests the appropriate value in an emergency ICU in Japan.
In our report, the outcomes for patients with and without NCSE were not different, whereas previous studies reported poorer neurological outcomes for NCSE patients than for non‐NCSE patients.2, 5, 6 There are several reasons why our NCSE group achieved good outcomes. First, all patients with NCSE achieved resolution with AED treatment, and no patients with persistent status epilepticus were observed in the present study. Second, ICU at the Kagawa University Hospital is not only an ICU training facility for board‐certified intensivists, approved by the Japanese Society of Intensive Care Medicine, but also a neurointensivist‐managed ICU.15 Neurointensivists can provide neurological attention followed by appropriate treatment. Therefore, we believe that the prevention and early detection of the subsequent critical complications followed by appropriate intervention contributed to a better neurological outcome in patients with NCSE.
Third, the percentage of GCSE in the FO group was relatively higher than that in the PO group. Reports of less mortality from NCSE following GCSE than following other causes7 may have affected the outcomes of the present study.
Monitoring cerebral activities with cEEG is the principal screening method for NCSE.
In the Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care, EEG is strongly recommended in all patients with acute brain injury and in those with unexplained and persistent altered consciousness. Furthermore, cEEG monitoring in comatose ICU patients is suggested with weak recommendation.16 In the current study, we provided fundamental data of NCSE with sEEG (i.e., two‐channel cEEG), which will require further detailed examination in combination with the 10–20 system cEEG. Apparently, the best choice of examining NCSE for patients in intensive care is the universal application of 10–20 system cEEG. However, it is difficult because of limited facilities in Japanese ICUs. Therefore, sEEG monitoring may be recommended in patients with AMS in ICU.
Study limitations
The present study had several limitations. First, it was carried out retrospectively at a single treatment center, which introduced potential selection bias. Uncontrolled confounding factors may have also existed. Second, at our emergency ICU, we use a two‐channel EEG as a cEEG; however, the sensitivity of the two‐channel EEG is not high,17, 18 and we recognize that the gold standard of cEEG is an international 10–20 system. The true incidence of NCSE may have been underestimated. In the present study, out of 11 NCSE patients, 7 showed SWs. As SW is easier to diagnose than RDA and polymorphic delta activity, it might influence the sensitivity of two‐channel EEG. A similar multicenter study is needed for broader data collection. Third, a relatively small number of patients were included in this study, and the results require confirmation in a larger cohort. Beta‐error may have also existed. Fourth, this study did not precisely examine the duration of NCSE. Fifth, we diagnosed periodic discharge as epileptic, which could be expressed through NCSE. There is a possibility that these periodic patterns indicate only epiphenomenon of acute brain injury. But we made efforts to adapt periodic discharges with changing frequency and did not diagnose continuous RDA as epileptic discharge. Finally, it is possible that artifacts from EEG were not completely excluded because our system did not use video‐EEG monitoring. We attempted to exclude any artifact by collecting the nurse's statements.
Conclusions
Approximately 30% of patients with AMS admitted to emergency ICUs developed NCSE. Two‐channel EEG for 48 h would be a useful tool for detecting NCSE in the emergency setting. The outcomes of AMS patients with and without NCSE did not differ significantly when appropriate medical attention and antiepileptic drugs were initiated. Simplified continuous EEG monitoring may be recommended in patients with AMS in ICUs to obtain early detection of NCSE followed by appropriate intervention.
Conflict of interest
None.
Acknowledgments
We are grateful to all physicians and nurses at the study site for their important contribution to the successful completion of this study.
[The copyright line for this article was changed on 28 October 2016 after original online publication]
Reference
- 1. Brophy GM, Bell R, Claassen J et al Guidelines for the evaluation and management of status epilepticus. Neurocrit. Care 2012; 17: 3–23. [DOI] [PubMed] [Google Scholar]
- 2. Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit. Care Med. 2009; 37: 2051–6. [DOI] [PubMed] [Google Scholar]
- 3. Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004; 62: 1743–8. [DOI] [PubMed] [Google Scholar]
- 4. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003; 61: 1066–73. [DOI] [PubMed] [Google Scholar]
- 5. Krumholz A, Sung GY, Fisher RS, Barry E, Bergey GK, Grattan LM. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology 1995; 45: 1499–504. [DOI] [PubMed] [Google Scholar]
- 6. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 1996; 47: 83–9. [DOI] [PubMed] [Google Scholar]
- 7. Nagayama M. Nonconvulsive status epilepticus: clinical practice and pathophysiology. Brain Nerve 2013; 65: 561–72. [PubMed] [Google Scholar]
- 8. Friberg H, Westhall E, Rosen I, Rundgren M, Nielsen N, Cronberg T. Clinical review: continuous and simplified electroencephalography to monitor brain recovery after cardiac arrest. Crit. Care 2013; 17: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Egawa S, Hifumi T, Kawakita K et al Successful treatment of non‐convulsive status epilepticus diagnosed using bedside monitoring by a combination of amplitude‐integrated and two‐channel simplified electroencephalography. Acute Medicine & Surgery 2015; 3: 167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zehtabchi S, Abdel Baki SG, Omurtag A et al Prevalence of non‐convulsive seizure and other electroencephalographic abnormalities in ED patients with altered mental status. Am. J. Emerg. Med. 2013; 31: 1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirsch LJ, LaRoche SM, Gaspard N et al American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J. Clin. Neurophysiol. 2013; 30: 1–27. [DOI] [PubMed] [Google Scholar]
- 12. DeLorenzo RJ, Waterhouse EJ, Towne AR et al Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39: 833–40. [DOI] [PubMed] [Google Scholar]
- 13. Kubota Y, Nakamoto H, Kawamata T. Electroencephalographic features of nonconvulsive status epilepticus. Brain Nerve 2015; 67: 575–83. [DOI] [PubMed] [Google Scholar]
- 14. Kobata H. Significance of neurocritical care in the management of stroke. J Jpn Soc Intensive Care Med. 2012; 19: 325–30. [Google Scholar]
- 15. Egawa S, Hifumi T, Kawakita K et al Impact of neurointensivist‐managed intensive care unit implementation on patient outcomes after aneurysmal subarachnoid hemorrhage. J. Crit. Care 2016; 32: 52–5. [DOI] [PubMed] [Google Scholar]
- 16. Le Roux P, Menon DK, Citerio G. et al The international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the neurocritical care society and the european society of intensive care medicine. Neurocrit. Care 2014;21(Suppl 2):S282–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young GB, Sharpe MD, Savard M, Al Thenayan E, Norton L, Davies‐Schinkel C. Seizure detection with a commercially available bedside EEG monitor and the subhairline montage. Neurocrit. Care 2009; 11: 411–6. [DOI] [PubMed] [Google Scholar]
- 18. Nitzschke R, Muller J, Engelhardt R, Schmidt GN. Single‐channel amplitude integrated EEG recording for the identification of epileptic seizures by nonexpert physicians in the adult acute care setting. J. Clin. Monit. Comput. 2011; 25: 329–37. [DOI] [PubMed] [Google Scholar]


