Abstract
Case
A 61‐year‐old man with an unremarkable medical history was admitted with fever 7 days after being bitten by his dog. On day 3, he showed altered mental status, and laboratory data showed progressive hemolytic anemia, thrombocytopenia, hyperbilirubinemia, renal dysfunction, coagulopathy, and schistocytosis. Severe sepsis complicated with thrombotic microangiopathy caused by Capnocytophaga canimorsus was suspected.
Outcome
Plasma exchange was applied to treat the thrombotic microangiopathy and resulted in platelet count increase and improved renal function, hyperbilirubinemia, and schistocytosis. Blood culture results confirmed the presence of C. canimorsus. The patient was discharged in good condition.
Conclusion
Capnocytophaga canimorsus is rare cause of severe sepsis, and should be suspected even in immunocompetent patients with dog‐bite history. Capnocytophaga canimorsus infection may be complicated by thrombotic microangiopathy, for which plasma exchange should be considered prior to definitive diagnosis of thrombotic microangiopathy.
Keywords: Acute kidney injury, Capnocytophaga canimorsus, dog bite, sepsis, thrombotic microangiopathy
Background
Capnocytophaga canimorsus (C. canimorsus), a facultative anaerobic, gram‐negative bacteria, is found in the normal oral flora of dogs. This bacterium, commonly transmitted by dog bites, can cause severe sepsis, especially in immunocompromised patients, and the overall mortality rate is reportedly approximately 30%.1, 2 Dogs are one of the world's most popular pets. Thus, health care providers should recognize this pathogen and disease.
Severe C. canimorsus sepsis can be complicated by thrombotic microangiopathy (TMA), which requires specific treatment such as plasma exchange (PE), plasma transfusion, and molecular‐targeted drugs.1, 3, 4 However, C. canimorsus is difficult to isolate and identify.2 Therefore, the diagnosis of C. canimorsus infection and the associated TMA in patients with severe sepsis may be missed and appropriate treatment may be delayed. Here we present a case of severe sepsis caused by C. canimorsus complicated by TMA in an immunocompetent man who was bitten by a dog, and underwent PE based on presumptive diagnosis with effective improvement of his pathological status.
Case
A 61‐year‐old man with an unremarkable medical history was admitted to a nearby local hospital for a high fever that developed 7 days after being bitten by his dog. Capnocytophaga canimorsus sepsis was the clinically assumed diagnosis, despite no evidence of an immunocompromised history, and the patient received antibiotic therapy. For diagnostic confirmation, a blood sample was sent to the National Institute of Infectious Diseases for polymerase chain reaction (PCR) testing. The day after admission, he developed renal dysfunction (serum creatinine, 4.96 mg/dL and anuria) and thrombocytopenia (14,000/μL). Therefore, he was transferred to our institution for intensive care. On admission, he had no consciousness disturbance, and normal vital signs (respiratory rate, 12/min; heart rate, 74 b.p.m.; blood pressure, 126/80 mmHg; and body temperature, 36.7°C). His left little finger had a black wound from the dog bite, without obvious local sign of infection. Laboratory data revealed leukocytosis (11,700/μL), thrombocytopenia (3,000/μL), a high level of lactate dehydrogenase (5673 U/L), acute kidney injury (serum creatinine level, 6.54 mg/dL, anuria), and coagulopathy (fibrin/fibrinogen degradation products, 56.6 μg/dL). The fibrinogen level was not decreased (296 mg/dL) (Table 1). Schistocytes were also detected in the peripheral blood sample. The patient was diagnosed with severe sepsis and antibiotic therapy was started with meropenem and clindamycin. Continuous renal replacement therapy was applied for acute kidney injury with anuria, uncontrollable acidosis, and electrolyte abnormality. On day 3, he presented with altered mental status and hypoxic respiratory failure requiring mechanical ventilatory support. Further laboratory data showed progressive anemia, hyperbilirubinemia, a sustained high level of lactate dehydrogenase, and schistocytosis (Table 1). A clinical diagnosis of severe sepsis complicated with TMA was made based on these findings.
Table 1.
Changes in laboratory data in a 61‐year‐old man with severe sepsis complicated by thrombotic microangiopathy caused by Capnocytophaga canimorsus
| At admission to previous hospital | At admission to our hospital | Day 3 | Day 7 | Reference ranges | |
|---|---|---|---|---|---|
| WBC, /μL | 14,600 | 11,700 | 12,700 | 16,700 | 4000−9000 |
| Hb, g/dL | 10.8 | 10.4 | 7.5 | 8.1 | 14.0−18.0 |
| PLT, /μL | 14,000 | 3000 | 14,000 | 57,000 | 150,000–350,000 |
| T‐Bil, mg/dL | 3.98 | 5.6 | 15.3 | 5.2 | 0.2−1.0 |
| AST, U/L | 1997 | 957 | 346 | 37 | 8−38 |
| LDH, U/L | 4081 | 5673 | 5334 | 355 | 106−220 |
| BUN, mg/dL | 75.0 | 100 | 71 | 35 | 8−20 |
| Cr, mg/dL | 4.96 | 6.54 | 4.62 | 3.15 | 0.44−1.15 |
| CRP, mg/dL | 39.1 | 40.3 | 23.2 | 6.2 | 0.0−0.3 |
| PCT, ng/mL | – | 315.8 | 85.1 | 12.0 | 0.00−0.40 |
| FDP, μg/mL | 199.5 | 98.2 | 88.2 | 32.3 | 0.0−4.9 |
| PT‐INR | 1.66 | 1.15 | 1.08 | 0.95 | <1.15 |
| APTT, s | 49.3 | 39.3 | 49.6 | 33.7 | 29.6−40.8 |
| FBG, mg/dL | 269 | 296 | 257 | 275 | 200−400 |
| AT, % | 101 | 77 | 65 | 63 | 80–120 |
Platelet transfusion was applied from day 1 to day 3 and platelet counts (PLT) were increased on day 3 compared with those on admission. APTT, activated partial thromboplastin time; AST, aspartate transaminase; AT, anti‐thrombin activity; BUN, blood urea nitrogen; Cr, creatinine; CRP, C‐reactive protein; FBG, fibrinogen; FDP, fibrin/fibrinogen degradation products; Hb, hemoglobin; LDH, lactate dehydrogenase; PCT, procalcitonin; PT‐INR, prothrombin time – international normalized ratio; T‐Bil, total bilirubin; WBC, white blood cells.
As the patient's condition deteriorated rapidly, we decided to initiate PE, despite the lack of a definite diagnosis from among several TMA disorders, including thrombotic thrombocytopenic purpura, hemolytic–uremic syndrome, atypical hemolytic–uremic syndrome, or other secondary TMA. We continued PE from days 3 to 6 based on the clinical improvement, which resulted in significant platelet count increase and improved renal function, hyperbilirubinemia, and schistocytosis (Fig. 1, Table 1). The blood culture on admission at the previous hospital showed Capnocytophaga species, and the PCR results also detected C. canimorsus. The antibiotic therapy was changed to ampicillin–sulbactam on day 10 and continued to day 14. A disintegrin‐like and metalloprotease with thrombospondin type 1 motifs 13 (ADAMTS13) activity on day 3 (without preceding plasma transfusion) was reported as 39% on day 12. Renal replacement therapy was discontinued on day 21. He was discharged in good condition on day 44. The patient was finally diagnosed with secondary TMA due to C. canimorsus infection.
Figure 1.
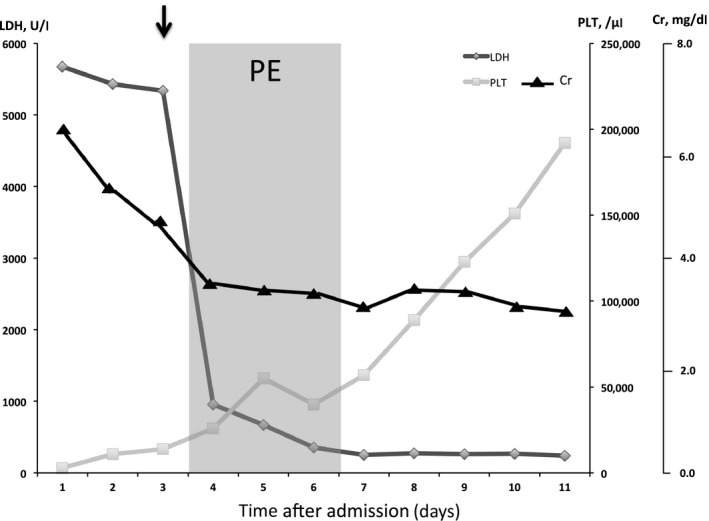
Clinical course and changes in laboratory data. The 61‐year‐old male patient was diagnosed with severe sepsis complicated by thrombotic microangiopathy on day 3 (black arrow). Plasma exchange was applied on days 3–6 to treat the thrombotic microangiopathy and resulted in improved platelet count (PLT; square), serum creatinine level (Cr; triangle), and lactate dehydrogenase (LDH; rhombus) level.
Discussion
We present a case of dog bite‐induced C. canimorsus severe sepsis with TMA in an immunocompetent man. Plasma exchange was applied based on presumptive clinical diagnosis of TMA and dramatically improved the patient's condition.
Polymerase chain reaction testing has been reported to be the most sensitive method for the definitive diagnosis of C. canimorsus infection,2 which is only undertaken in highly suspicious cases, and bacteriological detection requires at least 2–7 days of culture.5 Although asplenic status, alcoholism, and immunosuppression have been suggested as potential risk factors for C. canimorsus infection,1 it can also occur in immunocompetent patients without pre‐existing conditions.6 Although C. canimorsus does not produce beta‐lactamase and antibiotic therapy with penicillin, ampicillin, or cephalosporins has been suggested to be effective,7 the mortality of its infection was reported up to 30%.2 Insufficient recognition among health care providers, uncommon presentation, and difficulty in definitive diagnosis may cause diagnostic delay. It may then lead to subsequent organ failure, disseminated intravascular coagulation (DIC), and TMA.
Thrombotic microangiopathy syndrome's clinical features include microangiopathic hemolytic anemia, thrombocytopenia, and acute organ injury.8 The pathological features are vascular damage that is manifested by arteriolar and capillary thrombosis with abnormalities in the endothelium and vessel wall. Thrombotic microangiopathy syndrome includes ADAMTS13 deficiency‐, Shiga toxin‐, and complement‐mediated TMA. ADAMTS13 activity is severely decreased in patients with ADAMTS13 deficiency‐mediated TMA (<5% in most patients), and Shiga toxin‐mediated TMA is mainly caused by enterohemorrhagic Escherichia coli infection. Complement‐mediated TMA is a heterogeneous disorder characterized by resulting functional deficiency in complement factor H and the absence of enterohemorrhagic E. coli infection and low ADAMTS13 activity. Thrombotic microangiopathy related to other conditions, infection, collagen disease, and so on, is considered as secondary TMA. Plasma exchange within 24 h of diagnostic confirmation has been recommended as the initial treatment for TMA syndrome.9 The role of PE is to give ADAMTS13 and von Willebrand factor, to remove ADAMTS13 inhibitor and uncleaved ultralarge von Willebrand factor in ADAMTS13 deficiency‐mediated TMA. In complement‐mediated TMA, the purpose of PE is to give normal complement regulators and to remove abnormal complement factor H and anticomplement factor H antibody. In addition, efficacy of PE was reported in adult Shiga toxin‐mediated TMA with central nervous system symptoms.10 Thus, in adult TMA cases with central nervous system symptoms, empiric PE might be recommended.
Our patient was diagnosed with TMA, and ADAMTS13 deficiency‐ and Shiga toxin‐mediated TMA were ruled out. Disseminated intravascular coagulation often complicates severe sepsis, resulting in thrombocytopenia and coagulopathy; DIC may have contributed to the patient's pathophysiological condition, including thrombocytopenia and schistocytes. Significant hemolytic anemia is rarely observed in patients with DIC and fibrinogen levels are often decreased. In this case, the fibrinogen level remained within the normal range at all times (Table 1). Therefore, this patient would be diagnosed with possible secondary TMA due to C. canimorsus infection.
Conclusion
Capnocytophaga canimorsus is a rare cause of severe sepsis that can occur even in healthy people. Key to suspicion is dog‐bite history. Capnocytophaga canimorsus infection may also be complicated by TMA, which is difficult to diagnose. Health care providers should consider applying PE when TMA is suspected in patients with severe symptoms, prior to definitive diagnosis.
Conflict of Interest
None.
[The copyright line for this article was changed on 28 October 2016 after original online publication]
References
- 1. Ma A, Goetz MB. Capnocytophaga canimorsus sepsis with associated thrombotic thrombocytopenic purpura. Am. J. Med. Sci. 2013; 345: 78–80. [DOI] [PubMed] [Google Scholar]
- 2. Janda JM, Graves MH, Lindquist D, Probert WS. Diagnosing Capnocytophaga canimorsus infections. Emerg. Infect. Dis. 2006; 12: 340–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noris M, Mescia F, Remuzzi G. STEC‐HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 2012; 8: 622–33. [DOI] [PubMed] [Google Scholar]
- 4. Noris M, Remuzzi G. Atypical hemolytic‐uremic syndrome. N. Engl. J. Med. 2009; 361: 1676–87. [DOI] [PubMed] [Google Scholar]
- 5. Ciantar M, Spratt DA, Newman HN, Wilson M. Assessment of five culture media for the growth and isolation of Capnocytophaga spp. Clin. Microbiol. Infect. 2001; 7: 158–60. [DOI] [PubMed] [Google Scholar]
- 6. Hantson P, Gautier PE, Vekemans MC et al Fatal Capnocytophaga canimorsus septicemia in a previously healthy woman. Ann. Emerg. Med. 1991; 20: 93–4. [DOI] [PubMed] [Google Scholar]
- 7. Jolivet‐Gougeon A, Sixou JL, Tamanai‐Shacoori Z, Bonnaure‐Mallet M. Antimicrobial treatment of Capnocytophaga infections. Int. J. Antimicrob. Agents 2007; 29: 367–73. [DOI] [PubMed] [Google Scholar]
- 8. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N. Engl. J. Med. 2014; 371: 654–66. [DOI] [PubMed] [Google Scholar]
- 9. Scully M, Goodship T. How I treat thrombotic thrombocytopenic purpura and atypical hemolytic uraemic syndrome. Br. J. Haematol. 2014; 164: 759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colic E, Dieperink H, Titlestad K, Tepel M. Management of an acute outbreak of diarrhea‐associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: an observational study. Lancet 2011; 378: 1089–93. [DOI] [PubMed] [Google Scholar]


