Abstract
Aim
The aims of this study were to investigate outcomes of abdominal trauma in patients with hemorrhagic shock requiring emergency laparotomy and clarify the beneficial effects of intra‐aortic balloon occlusion (IABO) for intra‐abdominal hemorrhage in patients with critically uncontrollable hemorrhagic shock (CUHS).
Methods
We reviewed 44 hemorrhagic shock patients who underwent emergency laparotomy for intra‐abdominal hemorrhage over a 6‐year period. Of these patients, we examined data for 19 subjects who underwent IABO during initial resuscitation to control massive intra‐abdominal bleeding leading to CUHS.
Results
The average Injury Severity Score and probability of survival (Ps) of the 44 patients were 27.6 ± 15.4 and 0.735 ± 0.304, respectively, and the overall survival rate was 77.3%. The differences in the Glasgow Coma Scale, lactate level, prothrombin time – international normalized ratio, and Ps between the two groups (21 responders and 23 non‐responders) were statistically significant (P < 0.05). Intra‐aortic balloon occlusion was attempted in 19 of 23 patients (82.6%) with CUHS, and there were no statistically significant differences in presenting Glasgow Coma Scale, body temperature, lactate, prothrombin time – international normalized ratio, or Revised Trauma Score between the survivors (n = 12) and non‐survivors (n = 7). The only significant differences between these two groups were observed in Injury Severity Score (P = 0.047) and Ps (P = 0.007). In all patients, the balloons were successfully placed in 8.1 ± 3.3 min in the thoracic aorta, and a significant increase in systolic blood pressure was observed immediately after IABO.
Conclusion
The IABO procedure can be life‐saving in the management of patients with CUHS arising from intra‐abdominal hemorrhage, permitting transport to surgery.
Keywords: Critically uncontrollable hemorrhagic shock, intra‐abdominal hemorrhage, intra‐aortic balloon occlusion, trauma
Introduction
The control of hemorrhage is the primary therapeutic intervention in patients with abdominal trauma. Saving the life of a patient who has experienced a severe abdominal injury is extremely difficult. In order to circumvent a lethal, bloody, and vicious circle of acidosis, hypothermia, and coagulation failure, hemorrhage should be controlled as soon as possible.1 Some authors have reported the efficacy of resuscitative emergency thoracotomy with aortic cross‐clamping in patients with severe abdominal trauma.2, 3, 4 However, there are only a few reports of the use of intra‐aortic balloon occlusion (IABO) to treat abdominal trauma.5, 6 In addition, Brenner et al.7 reported that a reappraisal of the need for resuscitative endovascular balloon occlusion of the aorta is required. It is reportedly a feasible and effective means for patients in shock from blunt and penetrating mechanisms. We use the IABO procedure in patients with abdominal bleeding based on the individual circumstances, including at the accident scene and in the emergency room (ER) and operating room.
The aims of this study were to investigate the outcomes of abdominal trauma in patients with hemorrhagic shock requiring emergency laparotomy and clarify the beneficial effects of IABO for intra‐abdominal hemorrhage in patients with critically uncontrollable hemorrhagic shock (CUHS).
Methods
This study was carried out as a retrospective review of clinical records at the trauma center at Wakayama Medical University Hospital (Wakayama, Japan). The total number of patients with severe injury (Injury Severity Score [ISS] > 16) at our center during the study period was 1125; of these, the number with severe abdominal injury (Abbreviated Injury Scale > 4) was 53. A total of 44 patients with intra‐abdominal hemorrhagic shock were treated with emergency laparotomy between January 2007 and December 2012.
We divided these 44 patients into the responder group and the non/transient responder group according to the TRAUMA criteria.8 Patients with a systolic blood pressure (SBP) >90 mmHg and a heart rate <100 b.p.m. at the accident scene or in the ER who were stabilized after initial resuscitation were defined as responders. Patients with SBP <90 mmHg or heart rate >100 b.p.m. despite the use of initial resuscitation with 2 L i.v. crystalloids at the accident scene or in the ER were defined as non/transient responders, and we initiated the blood transfusion while simultaneously performing IABO using a percutaneous occlusion balloon catheter (Senko Medical Instrument Mfg. Co. Ltd., Tokyo, Japan). The IABO procedure was carried out in the ER, operating room, or at the accident scene, where a medical doctor and nurse were dispatched by helicopter. Our center has a system of doctor‐staffed helicopters. In this system, a helicopter that has been configured for emergency medical service care with an on‐board emergency physician and nurse is rushed to the accident scene.4 At the accident scene, the physician determines the need for IABO based on a physical examination and focused assessment with sonography for trauma (FAST). In this series, the IABO procedure was carried out in the emergency room (n = 15), operating room immediately before laparotomy (n = 2), and on site with a flight doctor (n = 2).
In all situations, the interventions were undertaken by emergency physicians. A 0.032‐inch wire was inserted through the sheath in the femoral artery and was advanced through the femoral artery to the thoracic aorta by observation. Similar to the process used to insert the wire, the balloon was placed over the wire up to 50–70 cm for placement in the thoracic aorta and then slowly inflated with saline solution until friction with the aortic wall was felt. Stannard et al.9 published a description of resuscitative endovascular balloon occlusion of the aorta in which the occlusion zone was defined as zone I, which extends from the origin of the left subclavian artery to the celiac artery. In the present analysis, absent femoral pulses were verified bilaterally in order to ensure the efficacy of aortic occlusion, and we subsequently measured the patient's brachial arterial pressure. The duration of inflation was permitted to last up to 20 min, after which the balloon was deflated for 5 min. We also used one‐third to two‐thirds inflation if the patient's blood pressure was stabilized. As such, intermittent balloon inflation and deflation is required until hemodynamic stability is restored.
The data of blood tests were collected from the initial data on arrival. The ISS, Revised Trauma Score (RTS), and probability of survival (Ps) were calculated with commonly used formulas. Continuous data are presented as mean ± standard deviation. We used χ2‐tests for categorical data and Wilcoxon rank–sum tests for continuous data. To compare improvement in the SBP of pre‐ and post‐IABO, we used Wilcoxon signed–rank tests. Statistical significance was defined as a P‐value of less than 0.05. All statistical analyses were carried out with the JMP 9 software package (SAS Institute Inc., Cary, NC, USA).
Results
The characteristics of the 44 patients with hemorrhagic shock are shown in Table 1. We divided the 44 patients into two groups (responders and non‐responders) based on their response to the initial resuscitation attempt and compared the background factors of these two groups. The differences in Glasgow Coma Scale, lactate, prothrombin time – international normalized ratio, and Ps were statistically significant (P < 0.05). There were no significant differences in hemoglobin, platelets, ISS, or RTS between the two groups (responders and non‐responders). However, there was a tendency for ISS and RTS in non‐responders (32 ± 17.2 and 6.44 ± 1.35, respectively) to be higher than in responders (23 ± 12.0 and 7.05 ± 1.17, respectively).
Table 1.
Characteristics of 44 patients with intra‐abdominal hemorrhagic shock treated with emergency laparotomy
| All patients (n = 44) | Responder (n = 21) | Non‐responder (n = 23) | P‐value | |
|---|---|---|---|---|
| Age, years | 54 ± 19 | 53 ± 18 | 56 ± 20 | 0.698 |
| GCS | 13 ± 3.5 | 13 ± 3.5 | 12 ± 3.4 | 0.027 |
| BT, °C | 36.1 ± 1.1 | 36.3 ± 0.73 | 35.8 ± 1.4 | 0.241 |
| Lactate, mmol/L | 5.1 ± 4.1 | 3.9 ± 3.8 | 6.1 ± 4.1 | 0.016 |
| Hb, g/dL | 10.3 ± 3.0 | 11.0 ± 2.9 | 9.8 ± 3.2 | 0.200 |
| PT‐INR | 1.3 ± 0.57 | 1.18 ± 0.32 | 1.43 ± 0.73 | 0.030 |
| Plate, 104/μL | 18.0 ± 8.2 | 20.5 ± 9.3 | 15.7 ± 6.4 | 0.169 |
| ISS | 27 ± 15 | 23 ± 12.0 | 32 ± 17.2 | 0.106 |
| RTS | 6.7 3 ± 1.29 | 7.05 ± 1.17 | 6.44 ± 1.35 | 0.119 |
| Ps (%) | 73.5 ± 30.4 | 81.4 ± 27.4 | 66.1 ± 31.8 | 0.039 |
BT, body temperature; GCS, Glasgow Coma Scale; Hb, hemoglobin; ISS, Injury Severity Score; Plate, platelet count; Ps, probability of survival; PT‐INR, prothrombin time – international normalized ratio; RTS, Revised Trauma Score.
The course of treatment and prognosis for the 44 patients with hemorrhagic shock requiring emergency laparotomy are shown in Figure 1. A total of 23 patients had CUHS, with a survival rate of 60.9%, which was significantly poorer than that (95.2%) observed in the 21 patients without CUHS (P = 0.0102). The IABO procedure was carried out in 19 of 23 patients (82.6%) with CUHS; all balloons were successfully placed in an adequate position without complications. Two patients experienced possibly preventable trauma‐related death, with a global mortality rate of 36.8% (7 of 19 patients; 5 of these 7 patients died due to progressive intra‐abdominal hemorrhagic shock after surgery and 2 patients died due to brain edema and cardiac injury). We were unable to insert the balloon in 4 patients, and thoracic aorta cross‐clamping instead of IABO was carried out in 1 of these patients. Two of these 4 patients showed sudden changes in their vital signs while being transferred to the operating room, while the final patient had absent pulses in the femoral arteries. We were able to rescue only 2 of the 4 patients who were not treated with IABO. We should have used thoracic aorta cross‐clamping in the 3 patients in whom the IABO device could not be inserted.
Figure 1.
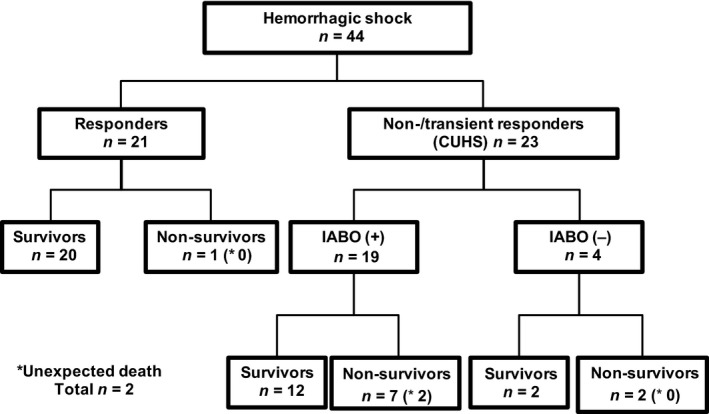
Management of 44 patients with hemorrhagic shock requiring emergency laparotomy.
The characteristics of the 19 patients treated with IABO are summarized in Table 2. There were no statistically significant differences in the presenting Glasgow Coma Scale, body temperature, lactate, prothrombin time – international normalized ratio, or RTS values between the survivors and non‐survivors. The only significant differences between the two groups were observed in the ISS (P = 0.047) and Ps (P = 0.007).
Table 2.
Characteristics of 19 patients with intra‐abdominal hemorrhagic shock treated with intra‐aortic balloon occlusion
| All patient (n = 19) | Survivors (n = 12) | Non‐survivors (n = 7) | P‐value | |
|---|---|---|---|---|
| GCS | 12 ± 3.0 | 13 ± 3.2 | 12 ± 2.6 | 0.119 |
| BT, °C | 35.7 ± 1.5 | 35.8 ± 1.6 | 35.3 ± 1.1 | 0.339 |
| Lactate, mmol/L | 5.9 ± 4.0 | 4.8 ± 3.8 | 7.6 ± 3.7 | 0.189 |
| PT‐INR | 1.46 ± 0.79 | 1.56 ± 0.98 | 1.28 ± 0.19 | 0.937 |
| ISS | 33 ± 18 | 26 ± 14 | 44 ± 19 | 0.047 |
| RTS | 6.6 ± 1.3 | 6.7 ± 1.4 | 6.4 ± 1.2 | 0.464 |
| Ps, % | 67 ± 30 | 80 ± 26 | 44 ± 21 | 0.007 |
BT, body temperature; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; Ps, probability of survival; PT‐INR, prothrombin time – international normalized ratio; RTS, Revised Trauma Score.
The treatment courses of the 19 patients are shown in Table 3. A total of 12 patients presented with shock upon arrival to the ER, including 7 survivors and 5 non‐survivors. In contrast, 7 patients presented with shock during treatment in the ER, 5 of whom survived and 2 did not. Although the time from the onset of shock to surgery was not significantly different between the survivors and non‐survivors, this parameter tended to be shorter (86 ± 51 min) in the survivors than in the non‐survivors (100 ± 30 min). The time from IABO to inflation was 8.1 ± 3.3 min, and the time from inflation of IABO to surgery was 20 ± 15 min. There were no statistically significant differences in these times between the survivors and non‐survivors. The volume of red blood cells within the 24 h from the accident was not significantly different between the survived and non‐survived groups (P = 0.126). Also fresh frozen plasma and platelet concentrate were not significantly different between the survived and non‐survived groups (P = 0.417, P = 0.761). We were able to repeat inflation/deflation of the balloon and/or use one‐third to two‐thirds inflation to shorten the total occlusion time, thus allowing us to carry out the operation while ensuring a stable patient condition. We undertook eight damage control surgery (DCS) procedures in 19 non‐responders and were able to rescue 4 of these patients.
Table 3.
Treatment course of 19 patients with intra‐abdominal hemorrhagic shock treated with intra‐aortic balloon occlusion (IABO)
| All patients (n = 19) | Survivors (n = 12) | Non‐survivors (n = 7) | P‐value | |
|---|---|---|---|---|
| Vital shock (SBP <90 mmHg or heart rate >100 b.p.m.) | ||||
| On arrival at emergency department | 12 | 7 | 5 | 0.568 |
| During treatment | 7 | 5 | 2 | |
| Time from shock to operation, min | 91 ± 44 | 86 ± 51 | 100 ± 30 | 0.374 |
| Time from insertion of IABO to inflation of balloon, min | 8.1 ± 3.3 | 7.7 ± 3.1 | 8.7 ± 3.7 | 0.574 |
| Time from inflation of IABO to operation, min | 20 ± 15 | 23 ± 17 | 16 ± 12 | 0.330 |
| Time from onset to initial resuscitation, min | 34 ± 22 | 35 ± 26 | 32 ± 10 | 0.611 |
| Length of operation time, min | 104 ± 46 | 110 ± 39 | 94 ± 59 | 0.219 |
| Damage control surgery | 8/19 | 4/12 | 4/7 | 0.311 |
SBP, systolic blood pressure.
The improvements in SBP in the 19 patients treated with IABO are shown in Table 4. The increase in blood pressure obtained after IABO was statistically significant compared with that observed before IABO in both the survivors and non‐survivors. Furthermore, the systolic blood pressure values of both the survivors and non‐survivors improved to equivalent levels, namely 122 ± 20 mmHg and 125 ± 25 mmHg, respectively, and IABO had the effect of transiently increasing blood pressure in both groups.
Table 4.
Improvements in systolic blood pressure in 19 patients with intra‐abdominal hemorrhagic shock treated with intra‐aortic balloon occlusion (IABO)
| Before IABO, mmHg | After IABO, mmHg | P‐value | |
|---|---|---|---|
| Survivors (n = 12) | 78 ± 10 | 122 ± 20 | <0.001 |
| Non‐survivors (n = 7) | 67 ± 12 | 125 ± 25 | 0.008 |
Discussion
In this study, the SBP values increased in all patients after IABO; the mean SBP increased from 75 ± 10 mmHg to 124 ± 22 mmHg. Some authors have already reported similarly significant mean increases in SBP after IABO, allowing for the control of hemorrhagic shock.10, 11, 12, 13 Irahara et al.6 used IABO in patients with traumatic hemorrhagic shock and reported that the SBP increased from 65.5 ± 4.7 mmHg to 123.1 ± 10.5 mmHg. Martinelli et al.10 used IABO in patients with hemorrhagic shock due to pelvic fractures and found that the SBP increased from 41 ± 26 mmHg to 111 ± 47 mmHg. Importantly, complications related to IABO insertion have been reported (spinal cord damage, abdominal viscera ischemia, and organ failure).14 Recently, Saito et al.15 reported that 3 of 24 patients who underwent IABO had complication, 1 with external iliac artery injury and 2 with lower limb ischemia. The three patients required amputation. Other investigators have noted that some patients treated with IABO developed complications of balloon rupture.10, 11 However, there were no complications related to the use of IABO in the present study. Therefore, because IABO leads to an increase in SBP, we assume that the IABO should be inserted quickly in patients with CUHS caused by intra‐abdominal hemorrhage. We could insert the IABO in 19 of 23 patients classified as non/transient responders. In this study, it took only approximately 8 min to insert and inflate the IABO, and the time from IABO inflation to surgery was approximately 20 min, which is adequately short. In contrast, the time from the onset of shock to surgery was approximately 91 min. During this time, we monitored the patient's response to fluid resuscitation and/or transfusion. Although the blood pressure values were stabilized transiently with initial resuscitation in most patients, the blood pressure again became unstable. Therefore, we think that a lot of time is required to decide whether to insert an IABO device. Recently, Norii et al.16 and Inoue et al.17 reported that IABO was associated with higher mortality compared with patients matched by propensity score who did not undergo IABO. Norii et al. described IABO as a “last ditch” effort to salvage dying trauma patients. In contrast, Inoue et al. described IABO not as a last‐ditch effort, but could be a potentially effective device when it was integrated into surgery or transcatheter embolization without delay. We also think that IABO is only a device that can control bleeding transiently, not stop bleeding, and should be considered as part of treatment strategy for abdominal trauma patients. Thus, we believe that the decision to carry out IABO and surgery should be made as soon as possible in patients with CUHS who are critically unstable, and the indications for IABO at the accident scene should be extended.
In general, an emergency thoracotomy (ET) for aortic cross‐clamping is regarded to be an effective management strategy of patients in extreme shock. It has been reported that the overall survival rate after ET in trauma patients is only 18–31%.3, 18 Recently, Moore et al.19 reported that the use of IABO in patients with non‐compressible hemorrhage from the abdomen and pelvis is feasible and can effectively control hemorrhage. In addition, they reported that patients undergoing IABO had fewer early deaths and improved overall survival compared with patients undergoing ET (37.5% versus 9.7%, P = 0.003). In addition, in our study, we were unable to insert an IABO device in four patients, two of whom (50%) could not be rescued. One of these two patients was treated with ET, whereas the other showed sudden changes in vital signs. Therefore, we believe that ET should be quickly performed in patients in whom it is not possible to safely insert an IABO device.
The advantage of IABO is the ability to restore and maintain hemodynamics in patients with hemorrhagic shock. Therefore, after IABO, the patient can be safely transferred to the operating room within the hospital or from the accident scene to the emergency medical care center by a doctor‐staffed helicopter. In this study, we were able to temporarily control the bleeding, carry out surgical treatment in a bloodless field, and identify the site of bleeding by inflating/deflating the balloon. Miura et al.20 also reported their successful experience with this device. In their study, IABO was attempted in patients with massive intra‐abdominal hemorrhage following hepatopancreatobiliary surgery. After IABO, the operative field became clearly visible, and the sites of bleeding were easily found and controlled. In addition, because decompression of the abdominal wall tamponade by laparotomy can cause sudden cardiovascular collapse,21, 22 we believe that IABO can be applied to prevent sudden cardiac arrest after laparotomy in patients with CUHS.
Conclusions
It is possible to quickly insert an IABO device, which allows the blood pressure to be significantly increased. In order to maintain the patient's blood pressure during the operation, inflation/deflation can be repeated or the balloon can be inflated to half‐volume. Our findings suggest that IABO can be used to stabilize the hemodynamics of the patient, provide a good view of the operative field, and detect the site of bleeding. Therefore, we believe that the survival rate can be increased by using IABO devices. However, the decision to undertake IABO and subsequent surgery must be made as quickly as possible after trauma in order to reduce the duration of occlusion.
Conflict of interest
None.
References
- 1. Moore FA, Mckinley BA, Moore EE. The next generation in shock resuscitation. Lancet 2004; 363: 1988–96. [DOI] [PubMed] [Google Scholar]
- 2. Seamon MJ, Pathak AS, Bradley KM et al Emergency department thoracotomy: still useful after abdominal exsanguination? J. Trauma 2008; 64: 1–7. [DOI] [PubMed] [Google Scholar]
- 3. Pahle AS, Pedersen BL, Skaga NO, Pillgram‐Larsen J. Emergency thoracotomy saves lives in a Scandinavian hospital setting. J. Trauma 2010; 68: 599–603. [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto H, Mashiko K, Hara Y et al Role of resuscitative emergency field thoracotomy in the Japanese helicopter emergency medical service system. Resuscitation 2009; 80: 1270–4. [DOI] [PubMed] [Google Scholar]
- 5. Gupta BK, Khaneja SC, Flores L, Eastlick L, Longmore W, Shaftan GW. The role of intra‐aortic balloon occlusion in penetrating abdominal trauma. J. Trauma 1989; 29: 861–5. [DOI] [PubMed] [Google Scholar]
- 6. Irahara T, Sato N, Moroe Y, Fukuda R, Iwai Y, Unemoto K. Retrospective study of the effectiveness of Intra‐Aortic Balloon Occlusion (IABO) for traumatic haemorrhagic shock. World J. Emerg. Surg. 2015; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner ML, Moore LJ, DuBoss JJ et al A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J. Trauma Acute Care Surg. 2013; 75: 506–11. [DOI] [PubMed] [Google Scholar]
- 8. Alarcon LH, Puyana JC, Peitzman AB. Management of shock In: Mattox KL, Moore EE, Feliciano DV. (eds). Trauma, 7th edn New York: McGraw‐Hill, 2013; 189–215. [Google Scholar]
- 9. Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J. Trauma 2011; 71: 1869–72. [DOI] [PubMed] [Google Scholar]
- 10. Martinelli T, Thony F, Decléty P et al Intra‐aortic balloon occlusion to salvage patients with life‐threatening hemorrhagic shocks from pelvic fractures. J. Trauma 2010; 68: 942–8. [DOI] [PubMed] [Google Scholar]
- 11. Matsuda M, Tanaka Y, Matsukawa R et al Transbrachial arterial insertion of aortic occlusion balloon catheter in patients with shock from ruptured abdominal aortic aneurysm. J. Vasc. Surg. 2003; 38: 1293–6. [DOI] [PubMed] [Google Scholar]
- 12. Karkos CD, Bruce IA, Lambert ME. Use of the intra‐aortic balloon pump to stop gastrointestinal bleeding. Ann. Emerg. Med. 2001; 38: 328–31. [DOI] [PubMed] [Google Scholar]
- 13. Harma M, Harma M, Kunt AS, Andac MH, Demir N. Balloon occlusion of the descending aorta in the treatment of severe post‐partum haemorrhage. Aust. N. Z. J. Obstet. Gynaecol. 2004; 44: 170–1. [DOI] [PubMed] [Google Scholar]
- 14. Avaro JP, Mardelle V, Roch A et al Forty‐minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J. Trauma 2011; 71: 720–6. [DOI] [PubMed] [Google Scholar]
- 15. Saito N, Matsumoto H, Yagi T et al Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J. Trauma Acute Care Surg. 2015; 78: 897–903. [DOI] [PubMed] [Google Scholar]
- 16. Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score‐adjusted untreated patients. J. Trauma Acute Care Surg. 2015; 78: 721–8. [DOI] [PubMed] [Google Scholar]
- 17. Inoue J, Shiraishi A, Yoshiyuki A, Haruta K, Matsui H, Otomo Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: a propensity score analysis. J. Trauma Acute Care Surg. 2016; 80: 559–67. [DOI] [PubMed] [Google Scholar]
- 18. Millikan JS, Moore EE. Outcome of resuscitative thoracotomy and descending aortic occlusion performed in the operating room. J. Trauma 1984; 24: 387–92. [DOI] [PubMed] [Google Scholar]
- 19. Moore LJ, Brenner M, Kozar RA et al Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J. Trauma Acute Care Surg. 2015; 79: 523–30. [DOI] [PubMed] [Google Scholar]
- 20. Miura F, Takada T, Ochiai T et al Aortic occlusion balloon catheter technique is useful for uncontrollable massive intraabdominal bleeding after hepato‐pancreato‐biliary surgery. J. Gastrointest. Surg. 2006; 10: 519–22. [DOI] [PubMed] [Google Scholar]
- 21. Sankaran S, Lucas C, Walt AJ. Thoracic aortic clamping for prophylaxis against sudden cardiac arrest during laparotomy for acute massive hemoperitoneum. J. Trauma 1975; 15: 290–6. [DOI] [PubMed] [Google Scholar]
- 22. Ledgerwood AM, Kazmers M, Lucas CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J. Trauma 1976; 16: 610–5. [DOI] [PubMed] [Google Scholar]


