Abstract
Case
A 47‐year‐old man ingested 60 mL of a topical solution (3,000 mg minoxidil) and presented with prolonged hypotension. Treatment with dopamine hydrochloride and noradrenaline provided blood pressure control. Serum unchanged minoxidil concentrations at 4 and 16 h after ingestion were 4,994 and 33.9 ng/mL, respectively. Urine concentrations of unchanged minoxidil, minoxidil‐O‐glucuronide, and minoxidil‐N‐O‐sulfate at 16 h after ingestion were 360.4, 1,953, and 104.5 ng/mL, respectively.
Outcome
The serum unchanged minoxidil concentration rapidly decreased over a short interval. However, the patient needed to receive vasopressor support for the first 4 days after being admitted to the hospital. The urine minoxidil‐O‐glucuronide concentration was higher than the concentrations of unchanged minoxidil and minoxidil‐N‐O‐sulfate.
Conclusion
Although the serum concentration of unchanged minoxidil rapidly decreased, ingesting large amounts of a topical minoxidil solution can have serious and prolonged cardiovascular effects. Analyzing the minoxidil‐O‐glucuronide concentration in urine is useful for diagnosing minoxidil poisoning.
Keywords: Antihypertensive agents, blood pressure, minoxidil glucuronide, minoxidil sulfate
Introduction
The amine oxide compound 6‐(1‐piperidinyl)‐2,4‐pyrimidinediamine‐3‐oxide (minoxidil) (Fig. 1) has been used clinically as an antihypertensive and antialopecia agent. A topical solution with 2% or 5% minoxidil is used to treat androgenic alopecia. Few reports on minoxidil intoxication, including minoxidil and its metabolite concentrations in biological samples, exist.1, 2 Identifying the toxic causative agent is necessary to definitively diagnose poisoning in clinical settings; additionally, reporting and interpreting the analytical findings of toxic substances is needed to carry out a clinical assessment. Herein, we present serum and urine analyses of minoxidil and its metabolites in a patient who ingested a topical minoxidil solution.
Figure 1.
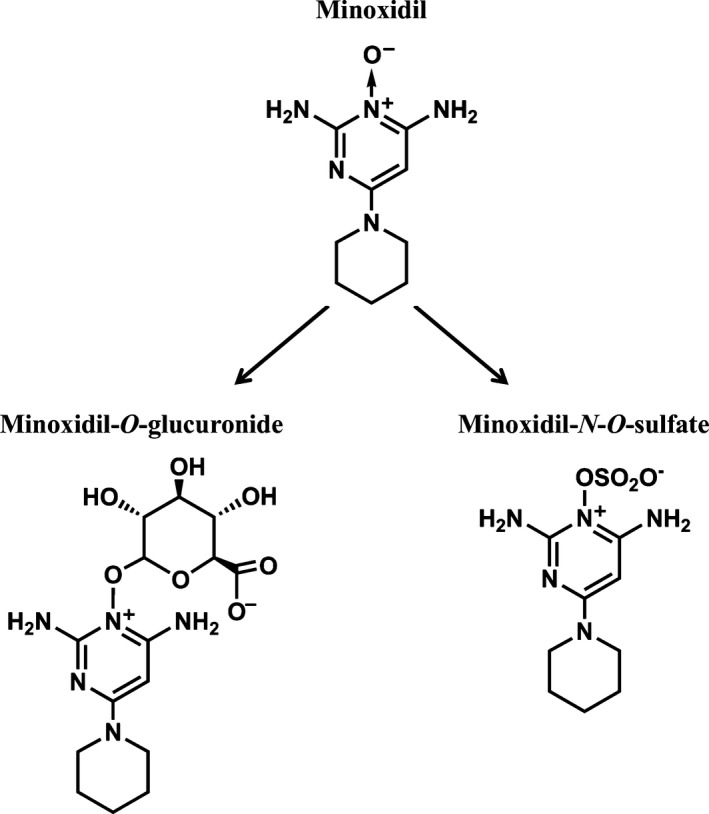
Structures of minoxidil and its metabolites. Minoxidil is metabolized by the liver to minoxidil‐O‐glucuronide and minoxidil‐N‐O‐sulfate. Minoxidil and these metabolites are excreted by the kidney. Minoxidil‐N‐O‐sulfate is a pharmacologically active metabolite.
Case
A 47‐year‐old man took 10 tablets of over‐the‐counter headache medicine containing acetaminophen and ibuprofen for his long‐duration headache. Afterward, at around 12:00 (noon), he ingested approximately 60 mL RiUP X5® in an attempt to commit suicide. He then drove a car and caused a traffic accident. When the police officer undertook a cognitive interview, the patient presented with epigastric discomfort and was transferred to our hospital at 4 h after ingestion. He vomited large amounts of black vomitus in the ambulance. Upon arriving at the hospital, he developed nausea and dizziness. Physical examinations revealed the following: Glasgow Coma Scale score, 14 (E3V5M6); heart rate, 92 b.p.m.; blood pressure, 50/19 mmHg; body temperature, 34.9°C; and SpO2, 95%. No abnormalities were observed in the chest radiographs, head computed tomography images, chest computed tomography images, or electrocardiograms. Figure 2 shows the patient's clinical data. The patient, who had no prior history of renal failure, presented with acute renal failure due to dehydration, and rapid fluid therapy was administered. The patient was given an infusion of 4,120 mL fluid replacement volume from arrival to 7 h after arrival, and his obtained urine volume was approximately 20 mL during the 7‐h period after arrival. To treat the hypotension, a 10‐mg i.v. dose of etilefrine hydrochloride and continuous infusion of dopamine hydrochloride were administered, but his blood pressure was resistant to the therapy. Continuous infusion of noradrenaline and a low dose of dopamine hydrochloride were given to control the patient's blood pressure and increase his urine output, respectively; afterwards, his blood pressure stabilized and a sufficient urine volume was obtained. The patient was discharged 6 days after hospitalization.
Figure 2.
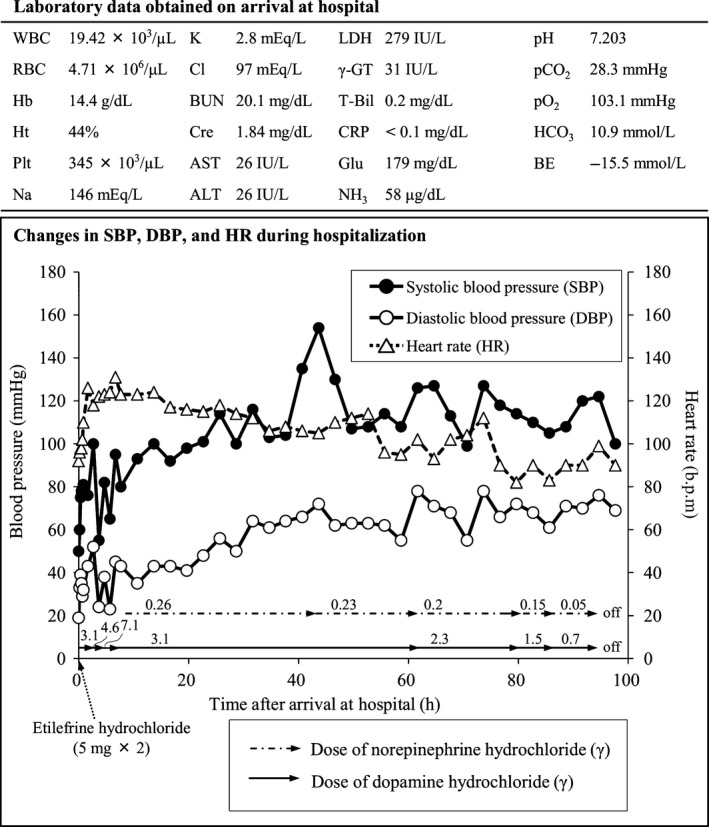
Clinical data of a 47‐year‐old man who ingested 60 mL of a topical solution (3,000 mg minoxidil) and presented with prolonged hypotension, including laboratory data obtained on arrival at the hospital and changes in his blood pressure and heart rate during hospitalization. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BE, base excess; BUN, blood urea nitrogen; Cl, chloride; Cre, creatinine; CRP, C‐reactive protein; Glu, glucose; γ‐GT, γ‐glutamyl transpeptidase; Hb, hemoglobin; HCO3, bicarbonate ion; Ht, hematocrit; K, potassium; LDH, lactate dehydrogenase; Na, sodium; NH3, ammonia; pCO2, carbon dioxide partial pressure; pO2, oxygen partial pressure; Pt, blood platelet; RBC, red blood cell; T‐Bil, total bilirubin; WBC, white blood cell.
We analyzed the unchanged minoxidil, minoxidil‐O‐glucuronide, and minoxidil‐N‐O‐sulfate levels in the serum and urine samples using a liquid chromatograph (UFLC; Shimadzu, Kyoto, Japan)–tandem mass spectrometer (3200QTRAP; AB Sciex, Framingham, MA, USA). Quantification analyses of these compounds were carried out on multiple reaction monitoring (MRM) with the MRM transition of m/z 210 > m/z 164 in positive ion mode. Minoxidil levels in the serum and urine were extracted according to the QuEChERS method.3 Unchanged minoxidil was analyzed in the serum and urine samples; minoxidil‐O‐glucuronide and minoxidil‐N‐O‐sulfate were only analyzed in the urine samples. The serum concentrations of unchanged minoxidil at 4 and 16 h after ingestion were 4,994 and 33.90 ng/mL, respectively. The urine concentrations of unchanged minoxidil, minoxidil‐O‐glucuronide, and minoxidil‐N‐O‐sulfate at 16 h after ingestion were 360.4 ng/mL, 1,953 ng/mL, and 104.5 ng/m, respectively. The urine concentrations of minoxidil‐O‐glucuronide and minoxidil‐N‐O‐sulfate were calculated by subtracting the urine concentration of unchanged minoxidil without glucuronidase or sulfatase enzyme treatment from the concentration of unchanged minoxidil produced by treatment with glucuronidase or sulfatase enzymes, respectively. Figure 3 shows the MRM chromatograms of unchanged minoxidil in the serum at 4 and 16 h after ingestion.
Figure 3.
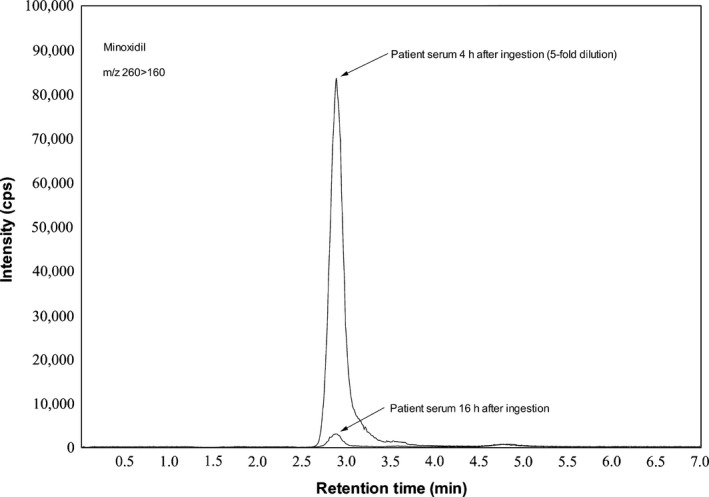
Multiple reaction monitoring chromatograms of unchanged minoxidil obtained after extracting the serum from the intoxication case using liquid chromatography–tandem mass spectrometry. Column, SeQuant® ZIC®‐pHILIC (150 × 2.1 mm internal diameter, 3.5 μm particle size; Merck, Darmstadt, Germany). Mobile phase, a mixture of 30 mmol/L ammonium formate (pH 4.0) and acetonitrile (40:60, v/v). Flow rate, 0.2 mL/min. Column oven, 40°C. Injection volume, 2 μL.
Discussion
Here, we report the levels of unchanged minoxidil and minoxidil metabolites and the effect of the metabolites on prolonged hypotension in minoxidil poisoning. At least 95% of orally administered minoxidil is absorbed from the gastrointestinal tract and is metabolized to the direct‐acting vasodilator minoxidil‐N‐O‐sulfate4 and the less pharmacologically active minoxidil‐O‐glucuronide (Fig. 1). Minoxidil has a large volume of distribution (mean of 197 L in healthy subjects).5 RiUP X5® contains 5 g minoxidil per 100 mL of solution. In our case, the patient ingested 60 mL of the solution, equaling 3,000 mg minoxidil, which is approximately 80–300 times greater than the recommended therapeutic oral dose for controlling hypertension (10–40 mg daily dose for adults). The serum concentrations of unchanged minoxidil in intoxication cases have been reported as follows: 150 ng/mL at 3 h after ingesting 100 mg minoxidil (tablet) in a 2‐year‐old child,1 and 1,668 ng/mL at 3 h after ingesting 650 mg minoxidil (topical solution) in an adult.2 Here, the serum concentration of unchanged minoxidil at 4 h after ingestion was 4,994 ng/mL. Compared to the concentrations reported at similar time intervals, the concentration we observed is the highest serum concentration reported to date. The serum concentration of unchanged minoxidil in our patient at 16 h after ingestion rapidly decreased to approximately 1/150 of that recorded at 4 h after ingestion. Generally, the half‐life of minoxidil in the serum of healthy volunteers is approximately 1 h.5, 6 In our case, the half‐life of minoxidil in the serum appeared to be similar to the reported half‐life.
For the serum minoxidil‐O‐glucuronide, the average half‐life for healthy volunteers is 1.7 h,5 thus the serum half‐life of minoxidil‐O‐glucuronide is similar to that of minoxidil. In terms of urinary excretion, approximately 20% and 40% of an oral dose are excreted in the urine as unchanged minoxidil and minoxidil‐O‐glucuronide, respectively.5, 6 Here, the urine concentrations of unchanged minoxidil, minoxidil‐O‐glucuronide, and minoxidil‐N‐O‐sulfate at 16 h after ingestion were 360.4, 1,953, and 104.5 ng/mL, respectively. The urine concentration of minoxidil‐O‐glucuronide was higher than the concentrations of unchanged minoxidil and minoxidil‐N‐O‐sulfate. Minoxidil‐N‐O‐sulfate is an unstable compound; moreover, minoxidil sulfate is easily hydrolyzed to the parent drug in an aqueous solution.7 These findings suggest that analyzing unchanged minoxidil in the serum and urine in addition to analyzing minoxidil‐O‐glucuronide in the urine is useful for making a certain diagnosis of minoxidil poisoning.
The patient was required to be on vasopressor support for the first 4 days of hospitalization; continuous infusion of noradrenaline appears to be useful for controlling the blood pressure. The prolonged antihypertensive effect has been reported in other minoxidil intoxication cases, with the hypotensive effect lasting up to 3 days.2 However, the short half‐lives of minoxidil and minoxidil‐O‐glucuronide do not explain the prolonged hypotensive effect. Considering that minoxidil has a large volume of distribution, persistent effects at the site of action, such as in the smooth muscles, may lead to the effect. Although no pharmacokinetic studies have been carried out on minoxidil‐N‐O‐sulfate, it is more potent and has a faster onset of activity with regard to the hypotensive effect than minoxidil4 and may also be sequestrated in smooth muscles, perhaps leading to the effect. It appears likely that the prolonged hypotensive effect in this patient was related to a delay in elimination of the active metabolite due to acute renal failure.
Conclusion
Although the serum concentration of unchanged minoxidil rapidly decreased, ingesting large amounts of a topical minoxidil solution can lead to serious and prolonged cardiovascular symptoms. Analyzing minoxidil‐O‐glucuronide in the urine is useful for diagnosing minoxidil poisoning.
Conflict of Interest
None.
References
- 1. Isles C, Mackay A, Barton PJM, Mitchell I. Accidental over dose of minoxidil in a child. Lancet 1981; 1: 97. [DOI] [PubMed] [Google Scholar]
- 2. Poff SW, Rose SR. Minoxidil overdose with ECG changes: case report and review. J. Emerg. Med. 1992; 10: 53–7. [DOI] [PubMed] [Google Scholar]
- 3. Usui K, Hayashizaki Y, Hashiyada M, Funayama M. Rapid drug extraction from human whole blood using a modified QuEChERS extraction method. Leg. Med. (Tokyo) 2012; 14: 286–96. [DOI] [PubMed] [Google Scholar]
- 4. McCall JM, Aiken JW, Chidester CG, DuCharme DW, Wendling MG. Pyrimidine and triazine 3‐oxide sulfate: a new family of vasodilators. J. Med. Chem. 1983; 26: 1791–3. [DOI] [PubMed] [Google Scholar]
- 5. Adams MH, Poynor WJ, Garnett WR et al Pharmacokinetics of minoxidil in patients with cirrhosis and healthy volunteers. Biopharm. Drug Dispos. 1998; 19: 501–15. [DOI] [PubMed] [Google Scholar]
- 6. Fleishaker JC, Andreadis NA, Welshman IR, Wright CE 3rd. The pharmacokinetics of 2.5‐ to 10‐mg oral dose of minoxidil in healthy volunteers. J. Clin. Pharmacol. 1989;29:162–7. [DOI] [PubMed] [Google Scholar]
- 7. Carrum G, Abernethy DR, Sadhukhan M, Wright CE 3rd. Minoxidil analysis in human plasma using high‐performance liquid chromatography with electrochemical detection. J. Chromatogr. 1986; 381: 127–35. [DOI] [PubMed] [Google Scholar]


