Abstract
Ambulatory blood pressure monitoring (ABPM) can identify phenotypes that cannot be measured in the clinic. Determining race and sex disparities in ABPM measures among HIV+ individuals may improve strategies to diagnose and treat hypertension in this high risk population. We compared ABPM measures between 24 African American and 25 white HIV+ adults (36 men and 13 women). Awake systolic BP (SBP) and diastolic BP (DBP) were similar in African Americans and whites. After multivariable adjustment, sleep SBP and DBP were 9.7 (95%confidence interval [95%CI]: 4.7, 14.8) mmHg and 8.4 (95%CI: 4.3, 12.5) mmHg higher, respectively, among African Americans compared with whites. After multivariable adjustment, SBP and DBP dipping ratios were 5.2% (95%CI: 1.7%, 8.7%) and 6.1% (95%CI 2.0%, 10.3%) smaller among African Americans compared with whites. After multivariable adjustment, awake and sleep SBP and DBP were higher in men compared to women. There was no difference in SBP or DBP dipping ratios comparing men and women. The prevalence of awake masked hypertension was 42% in men versus 17% in women, and the prevalence of sleep masked hypertension was 57% among African Americans versus 18% among whites. These data suggest that ABPM measures differ by race and sex in HIV+ adults.
Keywords: Ambulatory Blood Pressure Monitoring, HIV, Health Status Disparities, African Americans
Introduction
Ambulatory blood pressure monitoring (ABPM) has been identified as the reference standard for the confirmation of hypertension by the United States Preventive Services Task Force.1 Individuals undergoing ABPM wear a BP cuff and monitor and BP is typically measured every 15 to 60 minutes for 24 hours.2, 3 Mean BP measured through ABPM has a stronger association with risk for cardiovascular disease (CVD) events, compared to measurements taken in the clinic setting.1, 2 HIV+ individuals have a 1.5 to 2 fold higher CVD risk compared with their HIV- counterparts.4, 5 The increased CVD risk in HIV+ individuals is not explained by traditional CVD risk factors, including clinic-measured BP.4 ABPM may be particularly useful for HIV+ adults, as this population has a high psychosocial burden, and a high prevalence of autonomic dysfunction and sleep disturbance.6 Each of these are risk factors for ABPM phenotypes associated with high CVD risk, including nocturnal hypertension, a non-dipping BP pattern, and masked hypertension.6 In a recent systematic review, only eight studies of ABPM in HIV+ adults were identified.7 While a high prevalence of nocturnal hypertension and non-dipping BP were present among HIV+ participants, none of these studies included African Americans.7
In the US, African Americans have a higher incidence and prevalence of HIV infection compared to other race and ethnic groups.8, 9 African Americans also have a higher prevalence of nocturnal hypertension and non-dipping BP compared with whites.10 Men have several risk factors for abnormal ABPM phenotypes, but there are few data comparing ABPM phenotypes between men and women.11 Identifying race and sex disparities in ABPM phenotypes among HIV+ individuals may improve strategies to diagnose and treat hypertension in this high-risk population.6 In this manuscript, we report race and sex differences in BP phenotypes from a study assessing the feasibility of conducting ABPM among HIV+ adults.
Methods
Between February and August 2015, we conducted ABPM on 25 African American and 25 white adults attending the University of Alabama at Birmingham’s (UAB) 1917 HIV Clinic. The sample size was chosen to assess feasibility for conducting ABPM in HIV+ individuals at this clinic for a future large-scale research study. Participants were recruited during their regularly scheduled primary care visits or through referral by other study participants. Potential participants were required to meet the following eligibility requirements: non-Hispanic African American or white race, ≥ 19 years of age, HIV viral load ≤ 500 copies/ml and CD4 count ≥ 200 cells/mm3, currently receiving antiretroviral therapy (ART) but not antihypertensive medication, no previous diagnosis of CVD, and body mass index (BMI) < 35 kg/m2. Height and weight were measured and used to calculate BMI at the study visit. Education and current smoking status were determined by self-report. High ART adherence was determined by an answer of “excellent” to the question: “Thinking about the past 4 weeks, on average how would you rate your ability to take all of your HIV antiretroviral medications as your doctor prescribed?“ This validated single-item adherence question has been found to correlate with viral suppression.12 Values for CD4 T cell counts and HIV viral loads were determined from blood serum samples obtained during regular clinic visits and measured at the central UAB laboratory; most proximate lab values preceding their regular physician visit (to determine enrollment eligibility) and the first study visit (for analyses) were abstracted from the electronic medical record. This study was approved by the Institutional Review Boards at the University of Alabama at Birmingham and at Stony Brook University, which served as the ABPM reading center. All participants provided written informed consent.
BP Measures
Trained staff measured clinic BP three times in each participant’s non-dominant arm following a standardized protocol. Clinic BP was measured using an Omron 907XL, an oscillometric device (Omron Corp., Osaka, Japan). Prior to clinic BP measurements being taken, participants sat quietly for at least 5 minutes in an upright position with their back and arms supported, feet flat on the floor and legs uncrossed. An appropriately-sized cuff was used, as determined by measuring the mid-arm circumference. Three BP measurements were taken between one and two minutes apart. Clinic systolic BP (SBP) and diastolic BP (DBP) were defined as the average of the three readings. Clinic hypertension was defined as clinic SBP ≥ 140 mmHg or clinic DBP ≥ 90 mmHg.
Participants were fitted with an ABPM (Mobil-O-Graph 24h PWA monitor; IEM GmbH, Stolberg, Germany) that had an appropriately-sized cuff, and with an Actiwatch 2 wrist activity monitor (Phillips Respironics, Bend, OR, US) on the wrist of the non-dominant arm.13 The Actiwatch 2 activity monitor assesses the wearer’s movement intensity and duration and ambient white light illuminance exposure in units of lux, by integrating measurements over 15-second epochs. The ABPM device was programmed to measure BP every 30 minutes. Participants were asked to wear these devices for 24 hours. Awake and sleep periods for ABPM were determined using movement and light data from the activity monitor, combined with self-reported sleep onset and awakening times. Naps during the daytime were not included as part of either the awake or sleep BP periods. Consistent with the International Database on ABPM in relation to Cardiovascular Outcomes (IDACO) criteria, participants were required to have ≥ 10 daytime readings and ≥ 5 nighttime readings on the ABPM to be included in the statistical analyses.14 Out of 50 participants who underwent ABPM, 49 met these criteria. Participants who met these criteria had an average of 31.1 (standard deviation [SD] = 5.2) awake readings and 15.0 (SD = 4.1) sleep readings.
Mean SBP and DBP were calculated for the awake and sleep periods. Using all BP measurements from the awake period, awake hypertension was defined as a mean SBP ≥ 135 mmHg or mean DBP ≥ 85 mmHg.3 Using all BP measurements from the sleep period, sleep hypertension was defined as a mean SBP ≥ 120 mmHg or mean DBP ≥ 70 mmHg.3 The SBP dipping ratio was defined as 100%* [(awake SBP minus sleep SBP) divided by awake SBP] and the DBP dipping ratio as 100%* [(awake DBP minus sleep DBP) divided by awake DBP]. Non-dipping SBP and DBP were defined by dipping ratios < 10%.3 Examples of dipping and non-dipping BP patterns from ABPM are shown in Supplementary Figures 1 and 2, respectively.
Among participants who did not have clinic hypertension, those with awake and sleep hypertension were categorized as having awake and sleep masked hypertension, respectively. Also among participants without clinic hypertension, SBP and DBP masked effects were defined as mean awake SBP minus mean clinic SBP and mean awake DBP minus mean clinic DBP, respectively. Since only four participants had clinic hypertension, white coat hypertension (i.e., not having awake hypertension among those with clinic hypertension) and white coat effects (i.e., clinic BP minus awake BP among those with clinic hypertension) were not calculated.
Statistical analyses
Participant characteristics and clinic and ambulatory BP measures were calculated overall and by race and sex. The statistical significance of differences across race and sex were calculated using t-tests for age and BMI, Mann–Whitney U tests for CD4 counts, and Fisher’s exact tests for categorical variables. Linear regression was used to calculate differences in continuous BP measures including clinic, awake, sleep SBP and DBP, and dipping ratios for African Americans compared with whites and for men compared with women. Analyses were performed both unadjusted and adjusted for age, race, sex, and education. In addition to adjustment for these variables, fully adjusted models evaluating ambulatory SBP measures were adjusted for clinic SBP, and models evaluating ambulatory DBP measures were adjusted for clinic DBP. Regression models were not run for dichotomous measures (i.e., clinic hypertension, awake hypertension, sleep hypertension, non-dipping SBP and DBP, and awake and asleep masked hypertension) due to the low number of participants with these outcomes. Differences in SBP and DBP masked effects and the prevalence of awake, sleep, and any (i.e. awake or sleep) masked hypertension by race and sex were also calculated. All analyses were performed using SAS version 9.3 (SAS Institute, Cary NC). P-values < 0.05 were considered statistically significant.
Results
The mean age of study participants was 43.6 years; 49% were African American and 73% were men (Table 1). Compared with white participants, African Americans were less likely to be men (63% for African Americans versus 84% for whites) and report post-high school education (38% for African Americans versus 76% for whites).
Table 1.
Characteristics of HIV+ adults who completed ambulatory blood pressure monitoring, overall and by race and sex
| Characteristic | Overall (n=49) | African American (n=24) | White (n=25) | p-value for race | Men (n=36) | Women (n=13) | p-value for sex |
|---|---|---|---|---|---|---|---|
| Age, mean (SD) years | 43.6 (8.5) | 43.9 (8.3) | 43.3 (8.8) | 0.809 | 43.8 (8.9) | 43.0 (7.6) | 0.765 |
| African American, n (%) | 24 (49%) | – | – | – | 15 (42%) | 9 (69%) | 0.114 |
| Men, n (%) | 36 (73%) | 15 (63%) | 21 (84%) | 0.114 | – | – | – |
| Have ≥1 year post-high school education, n (%) | 28 (57%) | 9 (38%) | 19 (76%) | 0.001 | 22 (61%) | 6 (46%) | 0.515 |
| Body mass index, mean (SD) kg/m2 | 25.7 (4.2) | 26.4 (4.9) | 25.1 (3.4) | 0.255 | 25.0 (3.3) | 27.7 (5.6) | 0.131 |
| Current smoker, n (%) | 29 (59%) | 14 (58%) | 15 (60%) | >0.999 | 21 (58%) | 8 (62%) | >0.999 |
| Less than excellent ART adherence, n (%) | 14 (29%) | 8 (33%) | 6 (24%) | 0.538 | 11 (31%) | 3 (23%) | 0.731 |
| HIV load <20 copies/mL, n (%) | 44 (89.8%) | 20 (83.3%) | 24 (96.0%) | 0.190 | 34 (94.4%) | 10 (76.9%) | 0.109 |
| CD4 count, median (Q1, Q3) cell/mm3 | 627 (501, 962) | 649 (456, 824) | 627 (540, 992) | 0.399 | 643 (517, 979) | 586 (484, 903) | 0.797 |
ART = Antiretroviral Therapy; Q1 = 1st quartile; Q3 = 3rd quartile; SD = Standard deviation
P-values for age and body mass index are calculated using t-tests. P-values for CD4 counts are calculated using Mann–Whitney U tests. P-values for categorical variables are calculated using Fisher’s exact tests.
HIV loads <20 copies/mL are below the detectable limit.
Differences in clinic and ABPM phenotypes by race
Mean clinic SBP and DBP were 120 (SD = 10) and 72 (SD = 8) mmHg, respectively, among African Americans and 125 (SD = 10) and 77 (SD = 9) mmHg, respectively, among whites (Table 2). African Americans had higher sleep SBP and DBP and smaller dipping ratios compared with whites. After adjustment for age, sex, education, and mean clinic SBP, mean sleep SBP was 9.7 (95% CI: 4.7, 14.8) mmHg higher and the SBP dipping ratio was 5.2 (95% CI: 1.7, 8.7) percent smaller among African Americans compared with whites (Figure 1; Supplementary Table 1). After adjustment for age, sex, education, and mean clinic DBP, mean sleep DBP was 8.4 (95% CI: 4.3, 12.5) mmHg higher and the DBP dipping ratio was 6.1 (95% CI: 2.0, 10.3) percent smaller among African Americans compared with whites. The prevalence of sleep hypertension was 58% among African Americans and 24% among whites (p=0.021; Table 3). Additionally, the prevalence of non-dipping SBP among African Americans and whites was 42% and 20% (p=0.128), respectively, and the prevalence of non-dipping DBP was 29% and 0% (p=0.004), respectively.
Table 2.
Continuous clinic and ambulatory blood pressure measures in African American and white HIV+ adults
| Mean (SD) | |||
|---|---|---|---|
| BP measure | African American (n=24) | White (n=25) | p-value |
| Clinic SBP, mmHg | 120 (10) | 125 (10) | 0.131 |
| Clinic DBP, mmHg | 72 (8) | 77 (9) | 0.053 |
| Awake SBP, mean mmHg | 126 (11) | 124 (8) | 0.596 |
| Awake DBP, mean mmHg | 83 (7) | 82 (8) | 0.717 |
| Sleep SBP, mean mmHg | 114 (12) | 107 (7) | 0.009 |
| Sleep DBP, mean mmHg | 71 (9) | 65 (7) | 0.010 |
| SBP dipping ratio, mean percenta | 9.0 (5.7) | 14.0 (5.9) | 0.004 |
| DBP dipping ratio, mean percenta | 14.6 (5.8) | 21.1 (5.7) | 0.003 |
DBP = Diastolic blood pressure; SBP = Systolic blood pressure; SD = Standard deviation
P-values are calculated using t-tests.
SBP dipping ratio was defined as 100%* [(awake SBP minus sleep SBP) divided by awake SBP] and the DBP dipping ratio as 100%* [(awake DBP minus sleep DBP) divided by awake DBP].
Figure 1.
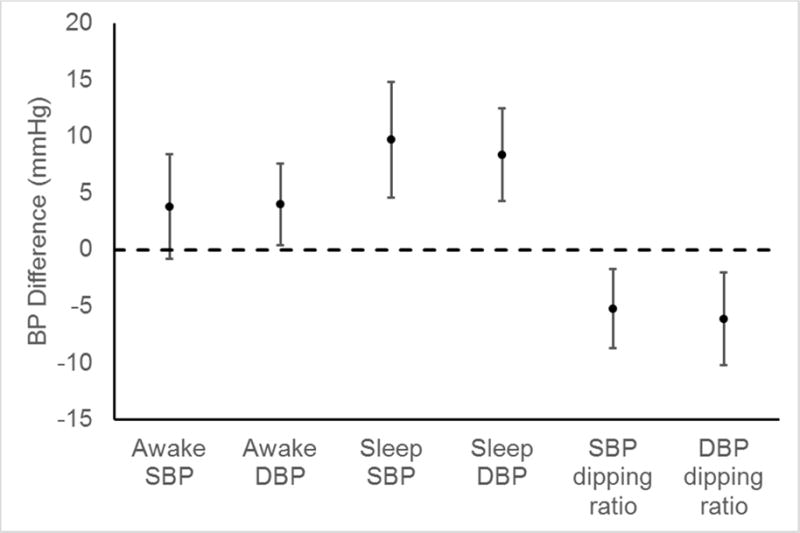
Differences in ambulatory blood pressure measures between African American and white† HIV+ adults adjusted for age, sex, education, and mean clinic blood pressure††
BP = Blood pressure; DBP = Diastolic blood pressure; SBP = Systolic blood pressure
†Difference is calculated as BP in African Americans minus BP in whites
††SBP measures are adjusted for clinic SBP and DBP measures are adjusted for clinic DBP
Table 3.
Dichotomous clinic and ambulatory blood pressure measures in African American and white HIV+ adults
| BP measure | African American (n=24) | White (n=25) | p-value |
|---|---|---|---|
| Clinic hypertension,a n (%) | 3 (12%) | 1 (4%) | 0.609 |
| Awake hypertension,b n (%) | 9 (38%) | 10 (40%) | >0.999 |
| Sleep hypertension,c n (%) | 14 (58%) | 6 (24%) | 0.021 |
| Non-dipping SBP,d n (%) | 10 (42%) | 5 (20%) | 0.128 |
| Non-dipping DBP,d n (%) | 7 (29%) | 0 (0%) | 0.004 |
DBP = Diastolic blood pressure; SBP = Systolic blood pressure
P-values are calculated using Fisher’s exact tests.
Clinic hypertension was defined as clinic SBP ≥ 140 mmHg or DBP ≥ 90 mmHg.
Awake hypertension was defined as a mean awake SBP ≥ 135 mmHg or mean awake DBP ≥ 85 mmHg.
Sleep hypertension was defined as a mean sleep SBP ≥ 120 mmHg or mean sleep DBP ≥ 70 mmHg.
Non-dipping SBP and DBP were defined by dipping ratios < 10%.
Differences in clinic and ABPM phenotypes by sex
Mean clinic SBP and DBP were 124 mmHg (SD = 9) and 75 mmHg (SD = 9), respectively, among men and 118 (SD = 12) and 73 (SD = 9) mmHg, respectively, among women (Table 4). After adjustment for age, race, education, and mean clinic SBP, awake SBP was 5.4 (95% CI: 0.5, 10.3) mmHg higher and sleep SBP was 6.0 (95% CI: 0.5, 11.4) mmHg higher in men compared with women (Figure 2; Supplementary Table 2). After adjustment for age, race, education, and mean clinic DBP, awake DBP was 7.6 (95% CI: 3.9, 11.3) mmHg higher and sleep DBP was 5.4 (95% CI: 1.3, 9.6) higher in men compared with women. The multivariable-adjusted differences in SBP and DBP dipping ratios comparing men to women were small and not statistically significant. The prevalence of awake hypertension was 47% in men and 15% in women (Table 5). The prevalence of sleep hypertension was 44% in men and 31% in women. Non-dipping among men and women were 28% and 38% (p=0.500), respectively, for SBP, and 8% and 31% (p=0.070), respectively, for DBP.
Table 4.
Continuous clinic and ambulatory blood pressure measures in men and women HIV+ adults
| Mean (SD) | |||
|---|---|---|---|
| BP measure | Men (n=36) | Women (n=13) | p-value |
| Clinic SBP, mmHg | 124 (9) | 118 (12) | 0.060 |
| Clinic DBP, mmHg | 75 (9) | 73 (9) | 0.466 |
| Awake SBP, mean mmHg | 127 (10) | 120 (8) | 0.026 |
| Awake DBP, mean mmHg | 84 (7) | 77 (7) | 0.002 |
| Sleep SBP, mean mmHg | 112 (10) | 107 (10) | 0.123 |
| Sleep DBP, mean mmHg | 69 (7) | 65 (11) | 0.278 |
| SBP dipping ratio, mean percenta | 11.7 (6.8) | 11.2 (4.8) | 0.783 |
| DBP dipping ratio, mean percenta | 18.5 (7.7) | 16.4 (8.6) | 0.414 |
BP = Blood pressure; DBP = Diastolic blood pressure; SBP = Systolic blood pressure; SD = Standard deviation
P-values are calculated using t-tests.
SBP dipping ratio was defined as 100%* [(awake SBP minus sleep SBP) divided by awake SBP] and the DBP dipping ratio as 100%* [(awake DBP minus sleep DBP) divided by awake DBP].
Figure 2.
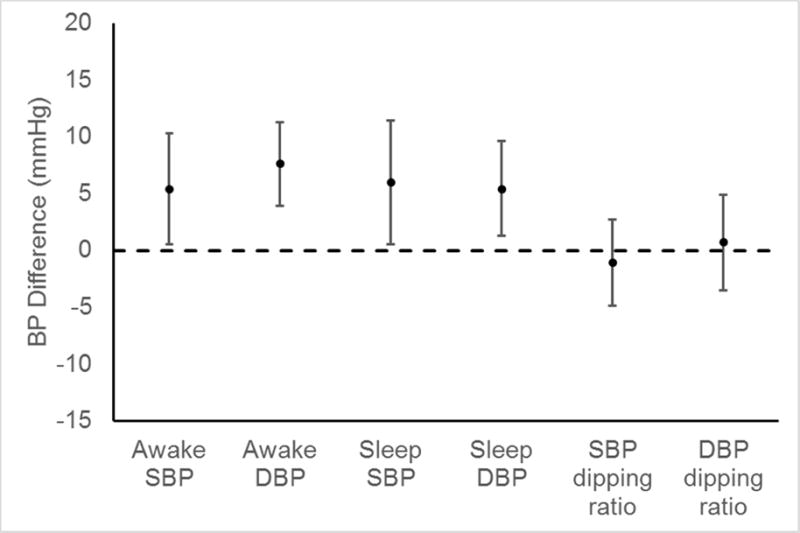
Differences in ambulatory blood pressure measures between men and women† with HIV+ adjusted for age, race, education, and mean clinic blood pressure††
BP = Blood pressure; DBP = Diastolic blood pressure; SBP = Systolic blood pressure
†Difference is calculated as BP in men minus BP in women
††SBP measures are adjusted for clinic SBP and DBP measures are adjusted for clinic DBP
Table 5.
Dichotomous clinic and ambulatory blood pressure measures in men and women with HIV+.
| BP measure | Men (n=36) | Women (n=13) | p-value |
|---|---|---|---|
| Clinic hypertension,a n (%) | 3 (8%) | 1 (8%) | >0.999 |
| Awake hypertension,b (%) | 17 (47%) | 2 (15%) | 0.054 |
| Sleep hypertension,c n (%) | 16 (44%) | 4 (31%) | 0.516 |
| Non-dipping SBP,d n (%) | 10 (28%) | 5 (38%) | 0.500 |
| Non-dipping DBP,d n (%) | 3 (8%) | 4 (31%) | 0.070 |
DBP = Diastolic blood pressure; SBP = Systolic blood pressure
P-values are calculated using Fisher’s exact tests.
Clinic hypertension was defined as clinic SBP ≥ 140 mmHg or DBP ≥ 90 mmHg.
Awake hypertension was defined as a mean awake SBP ≥ 135 mmHg or mean awake DBP ≥ 85 mmHg.
Sleep hypertension was defined as a mean sleep SBP ≥ 120 mmHg or mean sleep DBP ≥ 70 mmHg.
Non-dipping SBP and DBP were defined by dipping ratios < 10%.
Differences in masked effect and hypertension by race and sex
After adjustment for age, sex, education, and mean clinic SBP, SBP masked effect was 4.2 (95% CI: −0.6, 8.9) mmHg larger in African Americans compared with whites (Supplementary Table 3); after adjustment for age, sex, education, and mean clinic DBP, DBP masked effect was 3.9 (95% CI: 0.2, 7.6) mmHg larger in African Americans compared with whites. After multivariable adjustment, SBP and DBP masked effects were 4.8 (95% CI: −0.6, 8.9) mmHg and 7.7 (95% CI: 3.9, 11.5) mmHg larger, respectively, in men compared with women (Supplementary Table 4). The prevalence of awake masked hypertension was 39% among African Americans versus 32% among whites, and was 42% in men versus 17% in women (Figure 3). The prevalence of sleep masked hypertension was 57% among African Americans versus 18% among whites, and was 42% in men versus 25% in women.
Figure 3.
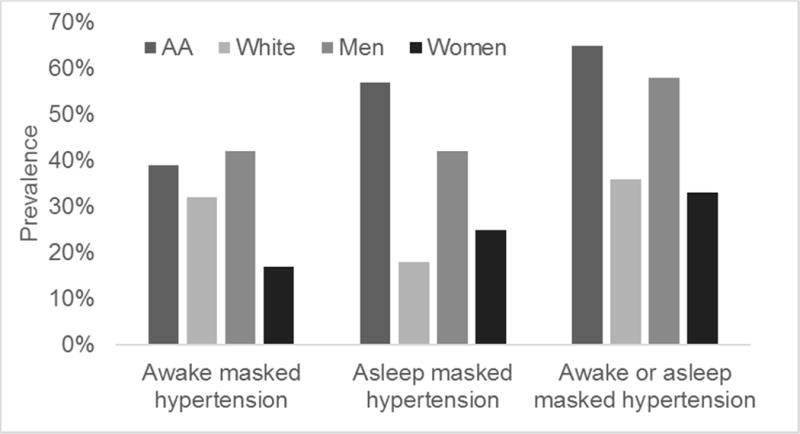
Prevalence of masked hypertension in HIV+ adults without clinic hypertension, by race and sex
AA= African American
Clinic hypertension was defined as clinic systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg. Among participants who did not have clinic hypertension, those with mean awake SBP ≥ 135 mmHg or mean awake DBP ≥ 85 mmHg were categorized as having awake masked hypertension and those with mean sleep SBP ≥ 120 mmHg or mean sleep DBP ≥ 70 mmHg were categorized as having sleep masked hypertension.
All differences are p>0.050.
DISCUSSION
In the current study of HIV+ adults, African Americans had higher sleep BP, smaller BP dipping ratios, and a higher prevalence of sleep masked hypertension, compared with whites. Men, compared with women, had higher awake and sleep BP and a higher prevalence of awake masked hypertension. BP dipping ratios did not differ between men and women. These data demonstrate that ABPM measures differ by race and sex in HIV+ adults.
The presence of HIV is associated with several factors that may contribute to abnormal ABPM phenotypes.6 For example, infection with the virus disrupts normal circadian rhythms and has been associated with poor sleep quality and sleep disturbances, all factors that have been associated with non-dipping BP.6, 15 HIV is associated with elevated systemic inflammatory biomarkers, which fail to fully return to normal levels with ART.5, 6 Excess inflammation has been associated with masked hypertension and non-dipping BP in the general population.5, 6 HIV+ individuals have a high prevalence of HIV-related stigma, stress, mood disorders, and autonomic dysfunction, which have been associated with masked hypertension and a higher prevalence of non-dipping BP.6, 16, 17 All of these factors may contribute to the development of hypertension and excess risk of CVD in the setting of HIV infection.
Similar to the current study in HIV+ adults, studies in the general population report that ABPM measures differ by race.7, 10, 18 In the Coronary Artery Risk Development in Young Adults (CARDIA) study (mean age = 30 years), nighttime SBP was 3.8 mmHg higher and the prevalence of non-dipping SBP was 2.44 times higher in African Americans compared with whites.10 Several mechanisms may contribute to the higher prevalence of nocturnal hypertension and non-dipping BP among African Americans compared with whites. High stress, discrimination, residence in neighborhoods with higher levels of noise and crime, and lack of social support are frequently reported to be more common in African Americans than whites and are associated with non-dipping BP.6, 15, 18–21 Worse sleep quality and sleep-disordered breathing in African Americans are additional mechanisms that may lead to racial differences in ABPM measures.6, 22, 23 A narrative review reported that African Americans compared to whites have shorter sleep duration, lower sleep efficiency, a smaller amount of restful slow-wave sleep, a greater prevalence of insomnia and sleep-disordered breathing, and more severe sleep-disordered breathing.22 In addition to psychosocial and sleep-related factors, inflammation and impaired autonomic and baroreflex function are more common in African Americans than whites and have each been associated with non-dipping BP and masked hypertension.6, 10, 24
Although racial differences in ABPM measures have been consistently reported for the general population, data on differences in ABPM measures by sex have not been consistent.11, 25–27 Recent studies have suggested that men have higher awake BP and prevalence of masked hypertension.28, 29 A higher prevalence of abnormal ABPM phenotypes among men may result from the higher prevalence of impaired sympathetic function and abnormal cardiac response to exercise, and lower estrogen levels, compared with women.25, 30, 31 Previous research has suggested that the prevalence of masked hypertension may be higher in men than women.11, 27 In an analysis of 7,320 participants not taking antihypertensive medication, the prevalence of non-dipping SBP was similar in men and women after multivariable adjustment.25
Some, but not all, studies have reported that HIV+ individuals have a higher prevalence of clinic hypertension than their HIV- counterparts.5, 32 A recent systematic review identified 8 studies that conducted ABPM in HIV+ individuals, 7 of which had HIV- comparison groups.7 In a meta-analysis containing 5 of these studies, HIV+ individuals had 2.72 times higher odds of non-dipping SBP, compared with HIV- controls.7 Of the 8 studies identified from the review, 7 of them consisted of European, mostly white individuals.7 One study was conducted in black South Africans, but no studies were conducted in the US.7 Examining racial disparities of CVD risk and risk factors among HIV+ adults is particularly important, since 44% of new HIV infections in the US occur in African Americans, and CVD and HIV are two of the leading causes of the African American-white mortality disparity.8, 33, 34
The current study has several strengths. There are scarce data available on ABPM in HIV+ adults, especially African Americans. Clinic BP and ABPM were collected following a standardized protocol and sleep and awake periods were determined using actigraphy combined with self-report. Despite these strengths, the current study has known and potential limitations. The sample size for the current study was small. Due to the relatively small sample size, caution should be taken in interpreting the prevalence estimates. Additionally, since all participants were taking ART we could not examine whether HIV treatment with ART was associated with any of the ABPM measures. Studies have found that some ART regimes are associated with higher clinic BP levels.6, 35, 36 However, this may not lead to racial differences in BP measures, since there is evidence that ART utilization is similar in African Americans and whites.37, 38 Additionally, studies have suggested that ART in the current era is not associated with hypertension.5, 6, 39 One study specifically reported that ABPM measures in HIV+ individuals did not change from before ART initiation to after 6 months of therapy.39 The current study only included HIV+ individuals and there was no HIV- comparison group. We also did not examine possible mechanisms for observed differences in ABPM measures.
In conclusion, in the current study of HIV+ individuals, African Americans compared to whites, and men compared to women, had a higher prevalence of ABPM phenotypes that are associated with increased CVD risk. Determining ABPM phenotypes in HIV+ individuals allows for more accurate BP assessment and the identification of race and sex CVD disparities in this high-risk population. Larger studies are needed to confirm the current findings and determine the reasons underlying these results.
Supplementary Material
Highlights.
Ambulatory blood pressure (BP) monitoring was assessed in 49 HIV+ individuals.
Awake BP was higher in men versus women, but did not differ by race.
Sleep BP was higher in African Americans versus whites and in men versus women.
The BP dipping ratio was smaller in African Americans versus whites.
Acknowledgments
This research was supported by the University of Alabama at Birmingham (UAB) Center For AIDS Research CFAR, a National Institutes of Health funded program (P30AI027767) that was made possible by the following institutes: NIAID, NCI, NICHD, NHLBI, NIDA, NIA, NIDDK, NIGMS, and OAR and the American Heart Association (15SFRN2390002). This work was supported by the NHLBI [T32HL00745733 to S.T.K., K24HL125704 to D.S.].
Abbreviations
- ABPM
Ambulatory blood pressure monitoring
- ART
Antiretroviral therapy
- BMI
Body mass index
- DBP
Diastolic blood pressure
- CVD
Cardiovascular disease
- IDACO
International Database on ABPM in relation to Cardiovascular Outcomes
- SBP
Systolic blood pressure
- SD
Standard deviation
- UAB
University of Alabama
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and Predictive Accuracy of Blood Pressure Screening Methods With Consideration of Rescreening Intervals: An Updated Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014 doi: 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 2.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163:691–700. doi: 10.7326/M15-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y, European Society of Hypertension Working Group on Blood Pressure M European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–68. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 4.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13:453–68. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 5.Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35:1373–81. doi: 10.1093/eurheartj/eht528. [DOI] [PubMed] [Google Scholar]
- 6.Kent ST, Burkholder GA, Tajeu GS, Overton ET, Muntner P. Mechanisms Influencing Circadian Blood Pressure Patterns Among Individuals with HIV. Curr Hypertens Rep. 2015;17:88. doi: 10.1007/s11906-015-0598-1. [DOI] [PubMed] [Google Scholar]
- 7.Kent ST, Bromfield SG, Burkholder GA, Falzon L, Oparil S, Overton ET, Mugavero MJ, Schwartz JE, Shimbo D, Muntner P. Ambulatory Blood Pressure Monitoring in Individuals with HIV: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0148920. doi: 10.1371/journal.pone.0148920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control. Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17 [Google Scholar]
- 9.Centers for Disease C and Prevention. Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence — 24 cities, United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2011;60:1045–9. [PubMed] [Google Scholar]
- 10.Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial Differences in Abnormal Ambulatory Blood Pressure Monitoring Measures: Results From the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. 2014;28:640–8. doi: 10.1093/ajh/hpu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viera AJ, Lin FC, Tuttle LA, Olsson E, Girdler SS, Hinderliter AL. Examination of Several Physiological and Psychosocial Factors Potentially Associated With Masked Hypertension Among Low-Risk Adults. J Clin Hypertens (Greenwich) 2015 doi: 10.1111/jch.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman BJ, Fredericksen RJ, Crane PK, Safren SA, Mugavero MJ, Willig JH, Simoni JM, Wilson IB, Saag MS, Kitahata MM, Crane HM. Evaluation of the single-item self-rating adherence scale for use in routine clinical care of people living with HIV. AIDS Behav. 2013;17:307–18. doi: 10.1007/s10461-012-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei W, Tolle M, Zidek W, van der Giet M. Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press Monit. 2010;15:225–8. doi: 10.1097/MBP.0b013e328338892f. [DOI] [PubMed] [Google Scholar]
- 14.Kikuya M, Hansen TW, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, investigators I Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Blood Press Monit. 2007;12:393–5. doi: 10.1097/MBP.0b013e3282f2b53d. [DOI] [PubMed] [Google Scholar]
- 15.Ulmer CS, Calhoun PS, Bosworth HB, Dennis MF, Beckham JC. Nocturnal blood pressure non-dipping, posttraumatic stress disorder, and sleep quality in women. Behav Med. 2013;39:111–21. doi: 10.1080/08964289.2013.813434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes M, Olfson M, Rabkin J, Hasin DS, Alegria AA, Lin KH, Grant BF, Blanco C. Gender, HIV status, and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2012;73:384–91. doi: 10.4088/JCP.10m06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trudel X, Brisson C, Milot A. Job strain and masked hypertension. Psychosom Med. 2010;72:786–93. doi: 10.1097/PSY.0b013e3181eaf327. [DOI] [PubMed] [Google Scholar]
- 18.Spruill TM, Gerin W, Ogedegbe G, Burg M, Schwartz JE, Pickering TG. Socioeconomic and psychosocial factors mediate race differences in nocturnal blood pressure dipping. Am J Hypertens. 2009;22:637–42. doi: 10.1038/ajh.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomfohr L, Cooper DC, Mills PJ, Nelesen RA, Dimsdale JE. Everyday discrimination and nocturnal blood pressure dipping in black and white americans. Psychosom Med. 2010;72:266–72. doi: 10.1097/PSY.0b013e3181d0d8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortmann AL, Gallo LC. Social support and nocturnal blood pressure dipping: a systematic review. Am J Hypertens. 2013;26:302–10. doi: 10.1093/ajh/hps041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang SC, Subramanian SV, Piccolo R, Yang M, Yaggi HK, Bliwise DL, Araujo AB. Geographic variations in sleep duration: a multilevel analysis from the Boston Area Community Health (BACH) Survey. J Epidemiol Community Health. 2015;69:63–9. doi: 10.1136/jech-2013-203256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med. 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dyke ME, Vaccarino V, Quyyumi AA, Lewis TT. Socioeconomic status discrimination is associated with poor sleep in African-Americans, but not Whites. Soc Sci Med. 2016;153:141–7. doi: 10.1016/j.socscimed.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A. How does race get “under the skin”?: inflammation, weathering, and metabolic problems in late life. Soc Sci Med. 2013;77:75–83. doi: 10.1016/j.socscimed.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staessen JA, Bieniaszewski L, O’Brien E, Gosse P, Hayashi H, Imai Y, Kawasaki T, Otsuka K, Palatini P, Thijs L, Fagard R. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The “Ad Hoc’ Working Group. Hypertension. 1997;29:30–9. doi: 10.1161/01.hyp.29.1.30. [DOI] [PubMed] [Google Scholar]
- 26.Pengo MF, Rossitto G, Bisogni V, Piazza D, Frigo AC, Seccia TM, Maiolino G, Rossi GP, Pessina AC, Calo LA. Systolic and diastolic short-term blood pressure variability and its determinants in patients with controlled and uncontrolled hypertension: a retrospective cohort study. Blood Press. 2015;24:124–9. doi: 10.3109/08037051.2014.992187. [DOI] [PubMed] [Google Scholar]
- 27.Banegas JR, Ruilope LM, de la Sierra A, de la Cruz JJ, Gorostidi M, Segura J, Martell N, Garcia-Puig J, Deanfield J, Williams B. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35:3304–12. doi: 10.1093/eurheartj/ehu016. [DOI] [PubMed] [Google Scholar]
- 28.Wang YC, Shimbo D, Muntner P, Moran AE, Krakoff LR, Schwartz JE. Prevalence of Masked Hypertension Among US Adults With Nonelevated Clinic Blood Pressure. Am J Epidemiol. 2017;185:194–202. doi: 10.1093/aje/kww237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz JE, Burg MM, Shimbo D, Broderick JE, Stone AA, Ishikawa J, Sloan R, Yurgel T, Grossman S, Pickering TG. Clinic Blood Pressure Underestimates Ambulatory Blood Pressure in an Untreated Employer-Based US Population: Results From the Masked Hypertension Study. Circulation. 2016;134:1794–1807. doi: 10.1161/CIRCULATIONAHA.116.023404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briant LJ, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol. 2016;101:219–29. doi: 10.1113/EP085368. [DOI] [PubMed] [Google Scholar]
- 31.Boggia J, Thijs L, Hansen TW, Li Y, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Olszanecka A, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Maestre G, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA, International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes I Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension. 2011;57:397–405. doi: 10.1161/HYPERTENSIONAHA.110.156828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonello VS, Antonello IC, Grossmann TK, Tovo CV, Pupo BB, Winckler Lde Q. Hypertension–an emerging cardiovascular risk factor in HIV infection. J Am Soc Hypertens. 2015;9:403–7. doi: 10.1016/j.jash.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C and Stroke Statistics S Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 34.Harper S, Lynch J, Burris S, Davey Smith G. Trends in the black-white life expectancy gap in the United States, 1983–2003. JAMA. 2007;297:1224–32. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- 35.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP, Multicenter ACS. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–60. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 36.Wilson SL, Scullard G, Fidler SJ, Weber JN, Poulter NR. Effects of HIV status and antiretroviral therapy on blood pressure. HIV Med. 2009;10:388–94. doi: 10.1111/j.1468-1293.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 37.Horberg MA, Hurley LB, Klein DB, Towner WJ, Kadlecik P, Antoniskis D, Mogyoros M, Brachman PS, Remmers CL, Gambatese RC, Blank J, Ellis CG, Silverberg MJ. The HIV Care Cascade Measured Over Time and by Age, Sex, and Race in a Large National Integrated Care System. AIDS Patient Care STDS. 2015;29:582–90. doi: 10.1089/apc.2015.0139. [DOI] [PubMed] [Google Scholar]
- 38.Barnes A, Nunn A, Karakala S, Sunesara I, Johnson K, Parham J, Mena L. State of the ART: Characteristics of HIV infected patients receiving care in Mississippi (MS), USA from the Medical Monitoring Project, 2009–2010. J Miss State Med Assoc. 2015;56:376–81. [PMC free article] [PubMed] [Google Scholar]
- 39.Borkum M, Wearne N, Alfred A, Dave JA, Levitt NS, Rayner B. Ambulatory blood pressure profiles in a subset of HIV-positive patients pre and post antiretroviral therapy. Cardiovasc J Afr. 2014;25:153–157. doi: 10.5830/CVJA-2014-029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


