Abstract
Antihypertensive medication and low systolic and diastolic blood pressure (SBP and DBP) have been associated with an increased falls risk in some studies. Many older adults have indicators of frailty, which may increase their risk for falls. We contrasted the association of SBP, DBP, number of antihypertensive medication classes taken, and indicators of frailty with risk for serious fall injuries among 5,236 REasons for Geographic and Racial Difference in Stroke study participants ≥65 years taking antihypertensive medication at baseline with Medicare fee-for-service coverage. SBP and DBP were measured and antihypertensive medication classes being taken assessed through a pill bottle review during a study visit. Indicators of frailty included low body mass index, cognitive impairment, depressive symptoms, exhaustion, impaired mobility and history of falls. Serious fall injuries were defined as fall-related fractures, brain injuries or joint dislocations using Medicare claims through December 31, 2014. Over a median of 6.4 years, 802 (15.3%) participants had a serious fall injury. The multivariable-adjusted hazard ratio for a serious fall injury among participants with 1, 2 or ≥3 indicators of frailty versus no frailty indicators were 1.18 (95% confidence interval [CI], 0.99–1.40), 1.49 (95%CI, 1.19–1.87) and 2.04 (95%CI, 1.56–2.67), respectively. SBP, DBP, and number of antihypertensive medication classes being taken at baseline were not associated with risk for serious fall injuries after multivariable adjustment. In conclusion, indicators of frailty, but not BP or number of antihypertensive medication classes, were associated with increased risk for serious fall injuries among older adults taking antihypertensive medication.
Keywords: Hypertension, blood pressure, antihypertensive medication, frailty, falls
INTRODUCTION
Older adults have a high risk for falls, which often result in a fracture, serious injury, or death.1–3 Low blood pressure (BP) and use of antihypertensive medication have been associated with an increased risk for falls among older adults in some, but not all, studies.4–6 The recently completed Systolic Blood Pressure Intervention Trial (SPRINT) showed that a systolic BP (SBP) target goal of 120 mm Hg versus 140 mm Hg did not increase the risk for injurious falls, overall or in the pre-specified sub-group of participants ≥75 years of age.7,8 Some health care providers may remain concerned about low BP targets in older adults.
Indicators of frailty, including low body mass index (BMI), impaired cognition, depressive symptoms, exhaustion, limited mobility, and a history of falls, are common among older adults with hypertension and may increase the risk for falls.9 The objective of the current study was to contrast the association between SBP, diastolic BP (DBP), number of antihypertensive medication classes being taken, and indicators of frailty with risk for serious fall injuries among older adults with hypertension taking antihypertensive medication at baseline. To accomplish this objective, we used data from the REasons for Geographic And Racial Difference in Stroke (REGARDS) study, linked to Medicare claims. Findings from this study may identify sub-groups of older adults with hypertension at high risk of falling, with the overall goal of reducing serious fall injuries.
METHODS
Study Population
The REGARDS study enrolled a population-based cohort and was designed to evaluate black:white and regional disparities in stroke mortality in the United States.10 Black adults and residents of the Southeastern region of the United States were oversampled. Twenty-one percent of the sample was randomly selected from the stroke buckle, which includes the coastal plain region of North Carolina, South Carolina, and Georgia. Thirty-five percent was from the stroke belt, which includes the remainder of North Carolina, South Carolina, and Georgia in addition to Alabama, Mississippi, Tennessee, Arkansas, and Louisiana. The remaining 44% was selected from the other 40 contiguous states. Additional details on the design and conduct of the REGARDS study have been published previously.10 Overall, 30,239 participants 45 years of age and older were enrolled between January 1, 2003 and October 31, 2007.10 The current analysis was restricted to participants 65 years and older (n=14,961, Figure S1, please see http://hyper.ahajournals.org). We excluded 3,590 participants without Medicare Part A and B coverage on the date of their baseline study visit. Medicare is the US federal health insurance program for adults 65 years or older, and younger adults with disabilities or end-stage renal disease. Medicare Parts A and B provide insurance coverage for inpatient and outpatient care, respectively. Claims are not required to be submitted for Medicare beneficiaries with Part C coverage, also known as Medicare Advantage, potentially resulting in the incomplete ascertainment of serious fall injuries. Therefore, 1,843 REGARDS participants with Medicare Part C coverage on the date of their baseline study visit were excluded from the current analysis. We excluded 25 participants who did not have data available on SBP or DBP from their baseline study visit and 4,267 participants who were not taking antihypertensive medication at baseline. After these exclusion criteria were applied, data from 5,236 participants were available for analysis. The REGARDS study protocol was approved by the Institutional Review Boards at the participating institutions and all participants provided written informed consent including for the linkage with Medicare claims.
Data Collection
Trained staff conducted computer-assisted telephone interviews to obtain information on participants’ demographics, education, household income, cigarette smoking status, cognitive impairment, depressive symptoms, exhaustion, impaired mobility, history of falls, and self-report of prior physician-diagnosed co-morbid conditions including coronary heart disease (CHD) and stroke. Following the interview, trained technicians conducted an in-home visit that included the measurement of height, weight, and BP, a pill bottle review, the collection of blood and urine samples, and an electrocardiogram. For the pill bottle review, technicians recorded the names of all medications that participants had taken during the 2 weeks preceding the in-home study visit. Medication doses were not recorded. Use of statins, osteoporosis medication, and benzodiazepines were determined based on the pill bottle review.
History of CHD at baseline was defined by a self-reported history or electrocardiogram (ECG) evidence of myocardial infarction (MI) or a self-reported history of a coronary artery bypass graft or percutaneous coronary intervention. History of stroke was defined on the basis of self-report. Diabetes was defined by self-report of a prior diagnosis with current use of insulin or oral hypoglycemic agents, or a fasting blood glucose ≥ 126 mg/dL or a non-fasting blood glucose ≥ 200 mg/dL. Urinary albumin was measured using the BN ProSpec Nephelometer from Dade Behring (Marburg, Germany). Urinary creatinine was measured with a rate-blanked Jaffé procedure, using the Modular-P analyzer (Roche/Hitachi; Indianapolis, IN). Using urinary creatinine and albumin, we calculated the albumin-to-creatinine ratio (ACR) in milligrams per gram.
Blood Pressure
BP was measured two times during the in-home examination by trained health professionals using an aneroid sphygmomanometer following a standardized protocol. An appropriate sized cuff was selected after measuring each participant’s arm circumference. Participants were asked to sit for five minutes with both feet on the floor prior to the first measurement. A 30-second rest occurred between BP measurements. The two BP measurements were averaged for the current analyses. Quality control of BP was monitored by central examination of digit preference and retraining of technicians took place as necessary. Sphygmomanometers were returned to the manufacturer for calibration as needed.
Antihypertensive Medication Classes
Antihypertensive medication use at baseline was determined by self-report and during the pill bottle review. To be categorized as taking antihypertensive medication at baseline, participants had to both self-report taking antihypertensive medication and have one or more classes of antihypertensive medication identified during the pill bottle review. Antihypertensive medication classes included angiotensin converting enzyme inhibitors, aldosterone antagonists, alpha-blockers, angiotensin receptor blockers, beta-blockers, calcium channel blockers, central acting α2-agonists, thiazide diuretics, potassium-sparing diuretics, loop diuretics, and vasodilators.
Indicators of Frailty
We studied six indicators of frailty using data collected during the telephone interview and in-home study visit. Indicators of frailty included low BMI, cognitive impairment, depressive symptoms, exhaustion, impaired mobility, and history of falls. Each indicator of frailty is defined in Table 1.
Table 1.
Method of ascertainment and definition for each indicator of frailty.
| Indicator | Method of ascertainment | Definition |
|---|---|---|
| Low Body Mass Index | Height and weight were measured using standardized equipment and used to calculate BMI | <18.5 kg/m2 |
| Cognitive Impairment | Six-item Screener which evaluates global cognitive function.29 | Score of 4 or less |
| Depressive Symptoms | 4-item Center for Epidemiologic Studies Depression Scale (CES-D) which evaluates frequency of feelings of depression, loneliness, sadness, and crying spells.30 | Score of 4 or more |
| Exhaustion | Short Form-12 question, “How much of the time during the past 4 weeks did you have a lot of energy?” | Self-report “little of the time” or “none of the time” |
| Impaired Mobility | SF-12 question, “Does your health now limit you in climbing several flights of stairs?” | Self-report “limited a lot” |
| History of Falls | Question, “Have you experienced a fall within the past year?” | Self-report “yes” |
Serious Fall Injuries
We obtained data on serious fall injuries from Medicare claims. REGARDS participants were linked to the Medicare beneficiary summary file and claims data by social security number with confirmation assessed by matching sex and date of birth.11 Using a previously published algorithm, we defined serious fall injuries as an Emergency Department (ED) or an inpatient claim for fall-related fractures, brain injuries or joint dislocations.6,12 In the absence of a fall-related code, a serious fall injury was defined as an ED or inpatient claim for any of the above serious injuries without a motor vehicle accident.6,12,13 For the analysis of serious fall injuries, each participant was followed from their REGARDS in-home study visit to the first occurrence of a serious fall injury, loss of Medicare fee-for-service Parts A or B coverage, initiation of Part C coverage, death or December 31, 2014.
Statistical Analyses
Baseline characteristics and the cumulative incidence and incidence rates of serious fall injuries were calculated by level of SBP (<110 mmHg, 110–119 mmHg, 120–129 mmHg, 130–139 mmHg, and ≥140 mmHg), DBP (<60 mmHg, 60–69 mmHg, 70–79 mmHg, 80–89 mmHg, and ≥90 mmHg), number of classes of antihypertensive medication being taken at baseline, and number of frailty indicators. Cox proportional hazards models were used to calculate the hazard ratio for serious fall injuries associated with levels of SBP with 120–129 mmHg as the referent category, levels of DBP with 70–79 mmHg as the referent category, number of antihypertensive medication classes at baseline with use of a single class as the referent category, and the number of frailty indicators with 0 indicators as the referent category. The middle categories for SBP and DBP were used as the referent in order to evaluate the association between low and high BP with risk for serious fall injuries. Initial models included adjustment for age, sex, race, and region of residence (Model 1). A subsequent model included additional adjustment for education, household income, cigarette smoking, statin use, osteoporosis medication use, benzodiazepine use, ACR, diabetes, history of CHD, and history of stroke (Model 2). A third model for the association between SBP, DBP and number of antihypertensive medication classes with serious fall injuries included adjustment for indicators of frailty (Model 3). The third model evaluating the association between indicators of frailty with serious fall injuries included adjustment for SBP, DBP, and number of antihypertensive medication classes being taken at baseline (Model 3). We used linear and quadratic terms to assess trends across levels of SBP, DBP, number of antihypertensive medication classes being taken at baseline, and number of frailty indicators. We repeated the analysis of the association between SPB, DBP, number of antihypertensive medication classes being taking at baseline, and number of frailty indicators with serious fall injuries among the subgroup of REGARDS participants ≥75 years of age (n=1,912).
The association between SBP, DBP and number of antihypertensive medication classes being taken at baseline with serious fall injuries may differ by degree of frailty.14 Therefore, we calculated the hazard ratios for serious fall injuries associated with SBP, DBP and number of antihypertensive medication classes being taken at baseline for participants with 0, 1, or ≥ 2 indicators of frailty, separately. In a final analysis, we calculated the incidence rates and hazards ratios for serious fall injuries associated with each indicator of frailty, separately. To account for missing data (Table S1), we conducted multiple imputation with chained equations to generate 10 data sets, which were combined for all analyses.15 Analyses were conducted using STATA/IC 13 (Stata Corporation, College Station, Texas).
RESULTS
Participant Characteristics
The mean age of the 5,236 participants included in this analysis was 73 years, 53.7% were female and 39.3% were black. Of the study sample, 55.7% had no indicators of frailty and 26.6%, 11.8% and 5.9% had 1, 2, and 3 or more indicators of frailty, respectively. Participants with more indicators of frailty were older, more likely to be black, have less than a high school education, an annual household income < $20,000, be a current smoker, have history of CHD, stroke, and diabetes, have a higher ACR, and to be taking benzodiazepines (Table 2). Participant characteristics are presented by level of SBP, DBP, and number of antihypertensive medication classes being taken at baseline in Tables S2, S3 and S4, respectively.
Table 2.
Characteristics of REasons for Geographic And Racial Difference in Stroke (REGARDS)-Medicare linked study participants ≥ 65 years of age, taking antihypertensive medication at baseline by number of indicators of frailty.
| Characteristic | Number of Indicators of Frailty | |||
|---|---|---|---|---|
| 0 (n = 2,919) |
1 (n = 1,391) |
2 (n = 618) |
≥3 (n = 308) |
|
| Age, years (SD) | 72.2 (5.4) | 73.4 (5.9) | 74.4 (6.4) | 74.0 (6.4) |
| Women | 48.7 | 57.5 | 64.9 | 61.7 |
| Black | 35.0 | 43.7 | 46.5 | 46.1 |
| Less than a high school education | 13.0 | 20.4 | 26.1 | 31.8 |
| Household income < $20,000 | 20.0 | 32.3 | 41.4 | 48.6 |
| Region of residence Stroke belt Stroke buckle Non-belt |
34.2 23.3 42.5 |
36.0 21.9 42.1 |
39.4 23.0 37.6 |
38.1 25.4 36.6 |
| Current smoker | 7.3 | 10.3 | 11.4 | 12.0 |
| Mean SBP, mmHg | 132.2 (16.3) | 133.3 (18.8) | 133.4 (18.2) | 134.1 (19.1) |
| Mean DBP, mmHg | 76.0 (9.5) | 75.8 (9.6) | 75.8 (10.1) | 75.2 (10.8) |
| Classes of antihypertensive medications | 2 (1–3) |
2 (1–3) |
2 (2–3) |
2 (2–3) |
| Coronary heart disease | 26.2 | 31.2 | 33.8 | 44.5 |
| Stroke | 6.6 | 12.1 | 19.7 | 29.5 |
| Diabetes | 25.1 | 30.2 | 39.1 | 45.5 |
| ACR, mg/g | 12.6 (12.0–13.2) |
15.4 (14.2–16.7) |
19.6 (17.2–22.3) |
19.2 (16.1–22.9) |
| Osteoporosis medication use | 8.1 | 9.3 | 9.1 | 7.6 |
| Benzodiazepine use | 5.5 | 7.3 | 12.1 | 14.2 |
| Statin use | 45.3 | 45.5 | 43.6 | 46.1 |
| Indicators of frailty | ||||
| Low BMI | 0.0 | 1.9 | 1.9 | 2.7 |
| Cognitive impairment | 0.0 | 21.6 | 26.0 | 38.4 |
| Depressive symptoms | 0.0 | 11.2 | 29.3 | 60.4 |
| Exhaustion | 0.0 | 19.2 | 55.7 | 82.3 |
| Impaired mobility | 0.0 | 34.7 | 60.6 | 82.9 |
| History of falls | 0.0 | 11.6 | 26.5 | 52.4 |
Numbers in this table are percentage or mean (standard deviation) except for ACR, which is the geometric mean (95% confidence interval) and number of antihypertensive medications is the median (interquartile range).
ACR: Urine Albumin to Creatinine Ratio; BMI: Body mass index; DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure.
Systolic and Diastolic Blood Pressure, Number of Antihypertensive Medications, and Number of Indicators of Frailty
Over a median follow-up of 6.4 years (maximum 11.9 years), 802 (15.3%) participants had a serious fall injury. There were no linear or quadratic trends in the cumulative incidence of serious fall injuries across levels of SBP or number of classes of antihypertensive medication being taken at baseline (Figure 1). The cumulative incidence of serious fall injuries was increased at both low and high levels of DBP and among participants with more indicators of frailty. After multivariable adjustment, SBP, DBP and number of antihypertensive medication classes taken at baseline were not associated with risk for serious fall injuries (Figure 2, upper left and lower left panels and Table S5–S7). DBP was not associated with risk for serious fall injuries after multivariable adjustment (Figure 2, upper right panel and Table S6). The multivariable adjusted hazard ratio (95% CI) for serious fall injuries among participants with 1, 2 and ≥ 3, versus 0, indicators of frailty was 1.18 (0.99–1.40), 1.49 (1.19–1.87) and 2.04 (1.56–2.67), respectively (Figure 2, lower right panel and Table S8). Results were consistent when the analysis was restricted to participants ≥75 years of age (Table S9). SBP, DBP, and number of antihypertensive medication classes being taken at baseline were not associated with serious fall injuries among participants with 0, 1, and ≥2 indicators of frailty, analyzed separately (Table S10).
Figure 1.
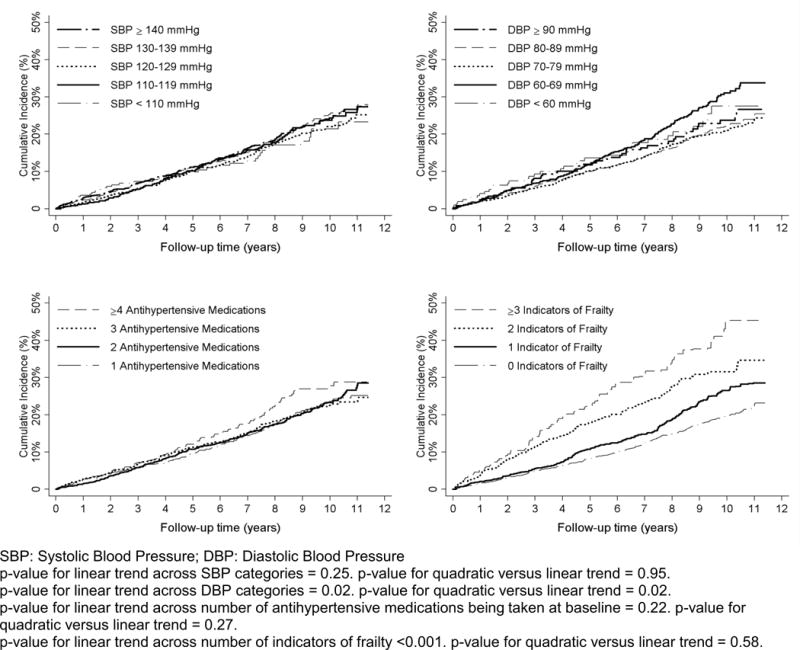
Cumulative incidence of serious fall injuries by level of systolic blood pressure (upper left panel), diastolic blood pressure (upper right panel), number of antihypertensive medications classes being taken at baseline (bottom left panel), and number of indicators of frailty (bottom right panel).
Figure 2.
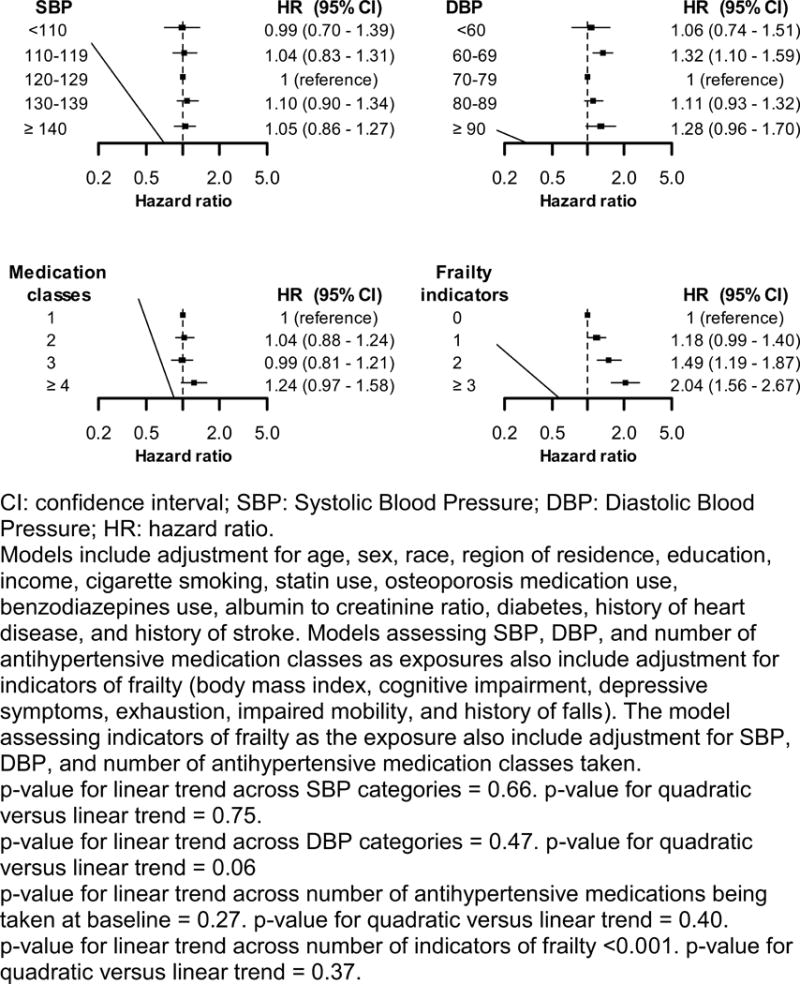
Hazard ratios for serious fall injuries associated with systolic blood pressure (upper left panel), diastolic blood pressure (upper right panel), number of antihypertensive medications classes being taken at baseline (lower left panel), and numbers of indicators of frailty (lower right panel).
Individual Indicators of Frailty
After each level of adjustment, having cognitive impairment, depressive symptoms, exhaustion, and a history of falls were each associated with an increased risk for serious fall injuries (Table S11). Having a low BMI and impaired mobility were associated with serious fall injuries after age, sex, race, and region of residence adjustment. These associations were not statistically significant after additional adjustment.
DISCUSSION
In the current study of older adults with hypertension taking antihypertensive medication at baseline, SBP, DBP, and the number of antihypertensive medications being taken were not associated with an increased risk for serious fall injuries after multivariable adjustment. In contrast, having 2 or more indicators of frailty was associated with a substantially increased risk for serious fall injuries. When indicators of frailty were investigated separately, having cognitive impairment, depressive symptoms, exhaustion, and a history of falls were each associated with an increased risk for serious fall injuries. These data suggest that interventions to reduce risk for serious fall injuries among older adults taking antihypertensive medication may be directed towards those with multiple indicators of frailty.
The association between BP and risk for falls among older adults has been evaluated previously.4 Among 3,544 community-dwelling Austrian adults ≥ 60 years of age, there was a lower odds for falls among women with SBP ≥ 140 compared to < 140 mmHg (odds ratio: 0.71, 95% CI: 0.51–0.99) and with DBP ≥ 90 compared to < 90 mmHg (odds ratio: 0.62, 95% CI: 0.43–0.89). Additionally, SBP < 120 mmHg compared to 120–139 mmHg and DBP < 80 mmHg compared to 80–89 mmHg were associated with a higher odds for falls among men (odds ratio: 2.46, 95% CI: 1.10–5.54 and 1.77, 95% CI: 1.02–3.07, respectively). This study was limited by its retrospective design as participants were asked to self-report falls in the prior three months and BP was measured after the falls occurred. The current study used a prospective study design and indicates that low SBP is not associated with an increased risk for serious fall injuries. Both low and high DBP levels were associated with a higher risk for serious fall injuries in crude analyses and after adjustment for age, sex, race and region of residence. However, this association was no longer present after multivariable adjustment. These findings are consistent with a previous study we conducted on the association between SBP and falls among older adults.16
Prior studies have reported conflicting results on the association between antihypertensive medication use and risk for falls or fall-related injuries.5,6,13,17–19 A meta-analysis of 22 studies that evaluated the association between several drug classes with falls in adults ≥ 60 years of age reported a pooled odds ratio of 1.24 (95% CI: 1.01–1.50) for those taking versus not taking antihypertensive medication.5 However, the risk for falls by the number of antihypertensive medication classes that people were taking was not reported. In a retrospective cohort study of US adults >70 years of age with health insurance through Medicare, the number of antihypertensive medication classes being taken was not associated with risk for falls and related injuries.6 In the Hypertension in the Very Elderly Trial (HYVET), being randomized to antihypertensive medication compared to placebo was associated with a lower fracture rate (hazard ratio: 0.58, 95% CI: 0.33–1.00).18 Consistent with several of the aforementioned studies, the current study suggests taking more classes of antihypertensive medication is not associated with increased risk for a serious fall injury.
In SPRINT, intensive treatment of SBP with a target goal of <120 mmHg lowered the risk for cardiovascular events and mortality compared to the standard target goal of <140 mmHg.7 There was no difference in the risk for injurious falls between the intensive and standard treatment groups. Also, in an a priori specified sub-group analysis of SPRINT participants ≥75 years of age, those randomly assigned to the intensive versus standard treatment group did not have an increased risk for falls (hazard ratio: 0.91, 95% CI: 0.65–1.29).8 Findings from the current study support the results of SPRINT in a general population sample of older adults with hypertension. A separate analysis of SPRINT data reported frailty, as measured by a 36-item index, to be associated with self-reported falls (hazard ratio: 1.72, 95% CI: 1.55–1.91) and injurious falls (hazard ratio: 1.75, 95% CI: 1.35–2.28).20 The association between individual indicators of frailty and falls was not reported.
In a systematic review and meta-analysis of 74 studies on risk factors for falls, several indicators of frailty were associated with an increased risk for falls in older adults.21 Cognitive impairment (odds ratio: 1.36, 95% CI: 1.12–1.65), depression (odds ratio: 1.63, 95% CI: 1.36–1.94), and history of falls (odds ratio: 2.77, 95% CI: 2.37–3.25), were each associated with a higher odds of falls. Low BMI was not associated with falls risk (odds ratio: 1.17, 95% CI: 0.93–1.46). The associations of exhaustion and impaired mobility, as defined in the current study, with falls risk were not reported. Other mobility factors including having gait problems or vision impairment, which can limit mobility, were associated with an increased odds for falls. Findings from the current study were consistent with the results of this meta-analysis. Fall risk assessment for older adults with multiple indicators of frailty may identify those with a high risk for serious fall injuries.
The current study suggests that cognitive impairment, depressive symptoms, exhaustion, and a history of falls may be predisposing factors for serious fall injuries in older adults who are taking antihypertensive medication. Additionally, several other potential mechanisms may increase the risk for serious fall injuries in this population. For example, postural hypotension is common in older adults and may lead to balance and gait impairments that result in a fall or fall-related injury.22,23 Incorporating a multifactorial fall prevention strategy which may include a combination of behavioral and physical modifications into the management and care of older adults taking antihypertensive medication may prevent falls and fall-related injuries.24
The effect of low BP and antihypertensive treatment on falls risk in older adults has been a long-held concern of clinicians.25–27 Initiating and titrating antihypertensive medication often triggers healthcare providers to think about their patients’ falls risk. Combined with the results from SPRINT, the current study suggests that BP can be intensively lowered to reduce cardiovascular and mortality risk without increasing the risk for falls.7 However, serious fall injuries are common among older patients taking antihypertensive medication. Conducting frailty assessments when antihypertensive treatment is initiated or titrated among older adults may be a practical approach to identify high-risk individuals.
This study has several strengths including a large population-based sample of US adults. BP was measured following a standardized protocol, antihypertensive medication classes were identified through a pill bottle review by trained staff and indicators of frailty were identified by standardized questionnaires and examination measurements. Data on serious fall injuries over up to 10 years of follow-up were available through Medicare claims. The REGARDS population with Medicare coverage has been shown to have a high degree of representation of all US adults ≥ 65 years of age with Medicare fee-for-service coverage.11 Despite these strengths, the findings from this study should be interpreted within the context of known and potential imitations. Findings may not be generalizable to older adults residing in nursing homes as the REGARDS study only enrolled community dwelling adults. BP was measured and information on antihypertensive medication use was obtained during a single visit at baseline and was not available during follow-up, resulting in potential misclassification and preventing the examination of the initiation and intensification of antihypertensive medication on risk for serious fall injuries. A recent study reported a short-term, but not long-term, increased risk for serious fall injuries after initiation and intensification of antihypertensive medication.25 Aneroid sphygmomanometers were used to measure BP. These devices are susceptible to calibration issues. Device calibration was monitored by staff and returned to the manufacturer when they needed to be recalibrated. The questionnaire on cognitive impairment was not implemented in the REGARDS study until December 2003, which resulted in a substantial percentage of missing data for cognition. Additionally, we relied on a claims-based algorithm for ascertainment of serious fall injuries and were not able to identify falls that did not result in an emergency room visit or hospitalization.6 Information was not available on other risk factors for falls including low standing BP, visual or hearing impairment, and environmental hazards. Other indicators of frailty such as weight loss, gait speed, and grip strength were not available.28
PERSPECTIVES
In the current study among community-dwelling adults ≥ 65 years of age taking antihypertensive medication at baseline, those with two or more indicators of frailty had an increased risk for serious fall injuries. In contrast, SBP, DBP, and taking more classes of antihypertensive medication at baseline were not independently associated with risk for serious fall injuries. These data support the safety of intensively lowering BP to reduce cardiovascular and mortality risk without increasing the risk for serious fall injuries among older adults taking antihypertensive medication. Assessment of frailty should be considered to identify older adults taking antihypertensive medication who may have a high falls risk.
Supplementary Material
Novelty and Significance.
What Is New?
There are population-based studies that have examined the association between BP and risk for falls.
We contrasted the association between SBP, DBP, number of antihypertensive medication classes being taken, and indicators of frailty with the risk for serious fall injuries among older hypertensive individuals taking antihypertensive medication at baseline.
What is Relevant?
BP and the number of antihypertensive medications were not associated with an increased risk for serious fall injuries.
There was a strong association of multiple indicators of frailty with serious fall injuries among individuals taking antihypertensive medications at baseline.
There is a need to identify indicators of frailty in individuals taking antihypertensive medications and consider interventions to prevent falls.
Summary.
Having 2 or more indicators of frailty was associated with an increased risk for serious fall injuries among older adults taking antihypertensive medications at baseline. These results suggest the importance of assessing frailty in older adults taking antihypertensive medication.
Acknowledgments
The authors thank the investigators, staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org).
Sources of Funding
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. Additional support was provided by grants R01-HL080477 and K24-HL111154 from the National Heart, Lung, and Blood Institute and 15SFRN2390002 from the American Heart Association.
Footnotes
Conflict of Interest/Disclosures
Dr. Daichi Shimbo is a consultant for Abbott Vascular and Novartis Pharmaceuticals Corporation.
References
- 1.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Kumar C. The patient who falls: “It’s always a trade-off”. JAMA. 2010;303:258–266. doi: 10.1001/jama.2009.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein D, Nagel G, Kleiner A, et al. Blood pressure and falls in community-dwelling people aged 60 years and older in the VHM&PP cohort. BMC Geriatr. 2013;13:50. doi: 10.1186/1471-2318-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 6.Tinetti ME, Han L, Lee DSH, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588–595. doi: 10.1001/jamainternmed.2013.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright JT, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries OJ, Peeters GMEE, Lips P, Deeg DJH. Does frailty predict increased risk of falls and fractures? A prospective population-based study. Osteoporos Int. 2013;24:2397–2403. doi: 10.1007/s00198-013-2303-z. [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 11.Xie F, Colantonio LD, Curtis JR, et al. Linkage of a Population-Based Cohort With Primary Data Collection to Medicare Claims: The Reasons for Geographic and Racial Differences in Stroke Study. Am J Epidemiol. 2016;184:532–544. doi: 10.1093/aje/kww077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowling CB, Bromfield SG, Colantonio LD, et al. Association of Reduced eGFR and Albuminuria with Serious Fall Injuries among Older Adults. Clin J Am Soc Nephrol. 2016;11:1236–1243. doi: 10.2215/CJN.11111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch Intern Med. 2012;172:1739–1744. doi: 10.1001/2013.jamainternmed.469. [DOI] [PubMed] [Google Scholar]
- 14.Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med. 2012;172:1162–1168. doi: 10.1001/archinternmed.2012.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 16.Banach M, Bromfield S, Howard G, et al. Association of systolic blood pressure levels with cardiovascular events and all-cause mortality among older adults taking antihypertensive medication. Int J Cardiol. 2014;176:219–226. doi: 10.1016/j.ijcard.2014.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med. 2014;29:1599–1606. doi: 10.1007/s11606-014-2961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters R, Beckett N, Burch L, et al. The effect of treatment based on a diuretic (indapamide) +/- ACE inhibitor (perindopril) on fractures in the Hypertension in the Very Elderly Trial (HYVET) Age Ageing. 2010;39:609–616. doi: 10.1093/ageing/afq071. [DOI] [PubMed] [Google Scholar]
- 19.Berry SD, Zhu Y, Choi H, Kiel DP, Zhang Y. Diuretic initiation and the acute risk of hip fracture. Osteoporos Int. 2013;24:689–695. doi: 10.1007/s00198-012-2053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing Frailty Status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi: 10.1093/gerona/glv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk Factors for Falls in Community-dwelling Older People. Epidemiology. 2010;21:658–668. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- 22.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120:841–847. doi: 10.1016/j.amjmed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Masuo K, Mikami H, Ogihara T, Tuck ML. Changes in frequency of orthostatic hypotension in elderly hypertensive patients under medications. Am J Hypertens. 1996;9:263–268. doi: 10.1016/0895-7061(95)00348-7. [DOI] [PubMed] [Google Scholar]
- 24.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 25.Shimbo D, Barrett Bowling C, Levitan EB, et al. Short-Term Risk of Serious Fall Injuries in Older Adults Initiating and Intensifying Treatment With Antihypertensive Medication. Circ Cardiovasc Qual Outcomes. 2016;9:222–229. doi: 10.1161/CIRCOUTCOMES.115.002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramov D, Cheng H. Controversy in treating the oldest old with hypertension: is the hypertension in the very elderly trial the final answer? J Am Geriatr Soc. 2009;57:570–571. doi: 10.1111/j.1532-5415.2009.02148.x. [DOI] [PubMed] [Google Scholar]
- 27.Berry SD, Kiel DP. Treating hypertension in the elderly: should the risk of falls be part of the equation? JAMA Intern Med. 2014;174:596–597. doi: 10.1001/jamainternmed.2013.13746. [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 29.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


