Abstract
Introduction
Fatal cervical spinal cord injury (SCI) associated with collapse of the circulatory and/or respiratory system usually consists of high cervical SCI caused by high‐energy trauma. As the elderly population grows, however, the etiology of fatal SCI may be changing. The aim of the present study was to understand the current clinical features of fatal SCI.
Methods
Retrospective analysis was performed on 73 consecutive patients with acute cervical SCI between 2007 and 2013. Fourteen (19%) of them presented in a state of coma due to severe circulatory collapse after cardiac arrest (CA, n = 11) or respiratory arrest (RA, n = 3), and were resuscitated at the scene or hospital. The clinical features and radiological findings of these 14 patients were compared with those of the other 59.
Results
Eleven of the 14 fatal SCI patients were injured by minor traumas. Computed tomography revealed C1‐2 fractures or dislocations in 11 patients and subaxial injuries in three patients. Eleven patients showed some kind of ossification of the spinal column ligaments. In a univariate analysis, the predictive factors associated with fatal circulatory collapse were age (P = 0.02), estimated C1/2 injury (P < 0.0001), and complete tetraplegia (P < 0.0001). In a multiple regression model for fatality, the odds ratios (OR) for C1/2 injury and ASIA impairment score (AIS) A were 20.58 (P = 0.006) and 151.97 (P = 0.002).
Conclusions
A state of unconsciousness with fatal circulatory collapse was significantly associated with C1/2 injury and AIS A. Moreover, our data show that fatal SCI can occur due to minor trauma in elderly people.
Keywords: Cardiopulmonary arrest, cervical spinal cord injury, elderly, resuscitation, trauma
Introduction
Circulatory collapse due to high cervical spinal cord injury (SCI) is a life‐threatening condition associated with high‐energy trauma mechanisms.1, 2 Cervical SCI in the elderly, on the other hand, is likely to occur in more minor accidents, and is sometimes overlooked or diagnosed after a delay because there is no apparent bruise or contusion on the body surface.3, 4 For example, when an elderly patient is found unconscious and without traumatic bruising, so that no history of trauma is known, her unconsciousness may be interpreted as being due to some endogenous pathology, and the possibility of cervical spine fracture may not be taken into account. In such cases, the signs of cervical spine fracture may be missed because it can be difficult to determine the neurological status of comatose patients and because diagnostic testing for SCI might not be performed. Thus it is worthwhile to investigate the characteristics of severe cervical spinal cord injury in a state of coma. Recently, there have been some reports of survival among SCI patients after resuscitation from cardiac arrest.5 Advances in pre‐hospital emergency systems, trauma protocols, and diagnostic imaging tools are largely responsible for these improved outcomes.6 In addition, the etiology of fatal SCI may be changing as the elderly population grows. In this study, we retrospectively investigated the patients treated at our hospital for acute cervical SCI with severe cardio‐respiratory collapse. The purpose of this study was to identify the current clinical features of fatal spinal cord injuries and to provide a clinical basis for further exploration of the management of this devastating condition.
Materials and Methods
Data collection
We reviewed the records of 355 patients who were admitted to the Emergency Department (ED) at Sapporo Medical University Hospital between January 2007 and November 2013 with traumatic head and neck injuries and an anatomic severity grade of 3 to 6 (classified according to the Abbreviated Injury Scale, Head and Neck7). Among the 77 patients diagnosed with cervical SCI in ED, four patients that arrived with no vital signs and died in the emergency room were excluded from this study. Fourteen (19%) of the cervical SCI patients were in a state of profound unconsciousness (coma) on arrival at ED; 11 of the comatose patients were in cardiac arrest (CA) and three were in respiratory arrest (RA), but all were immediately resuscitated. The first electrocardiogram (ECG) finding recorded by emergency medical service personnel was reviewed in CA patients. Although the latter three RA patients exhibited signs of severe shock, ultrasound cardiograms (UCG) and peripheral arterial pressure showed evidence of cardiac contraction. Whole‐body radiological and physiological examination suggested that the severe collapses of their circulatory and respiratory systems were due to high cervical SCI. These 14 comatose patients who were resuscitated from severe circulatory collapse, including cardiac arrest and spontaneous respiratory arrest, were defined as the C group. The other 59 patients were defined as the N group (Fig. 1).
Figure 1.
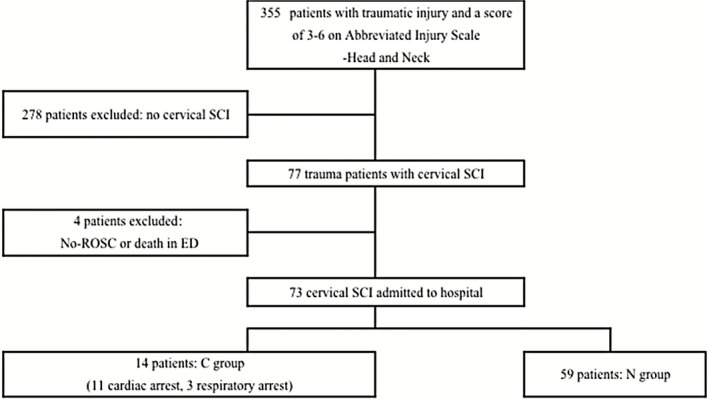
Study population flow diagram. Summary of patients with cervical spinal cord injury who were treated in our hospital. CA, cardiac arrest; ED, Emergency department; RA, respiratory arrest; ROSC, return of spontaneous circulation; SCI, spinal cord injury.
The clinical characteristics of the C group were reviewed and compared with those of the N group. Each patient's neurological state was evaluated after resuscitation. The severity of SCI was classified according to the American Spinal Injury Association Impairment Scale (AIS).8 The estimated duration of CA was the time from trauma to return of spontaneous circulation (ROSC) in cases where a witness to the accident could establish the time. When there was no witness, the estimated duration was the time from the initial emergency report to ROSC. Thus the estimated duration of cardiac arrest may be shorter than the actual duration in cases with no witnesses. The post‐resuscitation neurological outcome was evaluated using the Glasgow‐Pittsburg Cerebral Performance Categories (CPCs) at discharge from our hospital: CPC1 = full recovery; CPC2 = moderate disability; CPC3 = severe disability; CPC4 = comatose or in a persistent vegetative state; CPC5 = death or brain death.9
Statistical analysis
Data are expressed as means ± standard deviation. Statistical analyses were performed to compare the C and N groups using Student's t‐test. χ2 tests and Fisher's exact tests were then used for non‐parametric data analyses. Simple logistic regression was used for univariate analysis concerning coma due to circulatory collapse. Multivariate analysis was performed for all potential predictive factors associated with the event in the univariate analyses. A value of P < 0.05 was considered statistically significant. SPSS software (version 19; SPSS, Chicago, IL, USA) was used to perform the analysis.
Results
Clinical features (Table 1)
Table 1.
Characteristics of 14 resuscitated patients with fatal cervical spinal cord injury
| No | Age/Sex | Cause of injury | Possible mechanism | CT diagnosis | MRI T2 finding | First ECG finding | CA time (min) | CPC |
|---|---|---|---|---|---|---|---|---|
| 1 | 71/M | Motor vehicle crash | Distraction, HE, HF | Hangman Fracture (type 2) | C1‐2 intramedullary hyperintensity | PEA | 5 | 4 |
| 2 | 82/M | Sports | HE, Vertical compression | Hangman Fracture (type 1) | — | Asystole | 35 | 5 |
| 3 | 90/F | Pedestrian | HE | Odontoid Fracture (type 2) | C2 spinal cord discontinuity | Unknown | 8 | 5 |
| 4 | 71/M | Fall (1.5 m) | Distraction, HE | Odontoid Fracture (type 2) | C2 spinal cord discontinuity | Unknown | 10 | 4 |
| 5 | 62/M | Bicycle crash | Distraction, HE | Odontoid Fracture (type 2) | — | Asystole | 28 | 5 |
| 6 | 87/F | Fall (ground level) | HE, Vertical compression | Odontoid Fracture (type 2) | C2 spinal cord discontinuity | — | RA | 5 |
| 7 | 52/M | Fall (3 m) | HE | Odontoid Fracture (type 3) | C2 spinal cord discontinuity | — | RA | 2 |
| 8 | 78/M | Fall (staircase) | Distraction, HF | Atlanto‐axial dislocation (vertical) | — | PEA | 18 | 3 |
| 9 | 72/M | Motor vehicle crash | Distraction, HF | Atlanto‐axial dislocation (lateral) | — | PEA | 20 | 5 |
| 10 | 69/M | Fall (3 m) | HE | Atlanto‐axial subluxation | C1‐3 intramedullary hyperintensity | Asystole | 10 | 2 |
| 11 | 71/M | Motor vehicle crash | Distraction, HE, HF | Atlanto‐axial subluxation | C1 intramedullary hyperintensity | PEA | 16 | 5 |
| 12 | 67/M | Fall (staircase) | HE | C3/4, C4/5 subluxation | C2‐5 intramedullary hyperintensity | PEA | 38 | 4 |
| 13 | 92/M | Fall (ground level) | HE | C4/5 posterior dislocation | — | Asystole | 6 | 5 |
| 14 | 75/M | Fall (staircase) | HE | C4/5 anterior dislocation | C2‐6 intramedullary hyperintensity | — | RA | 2 |
Clinical characteristics and radiological findings of the 14 patients with cardiac or respiratory arrest caused by cervical SCI. CA time, cardiac arrest time; CPC, Glasgow‐Pittsburg Cerebral Performance Categories; ECG, electrocardiogram; HE, hyperextension; HF, hyperflexion; PEA, pulseless electric activity; RA, respiratory arrest.
The 14 patients in the C group included 12 men and two women aged from 52 to 92 years (mean 74, median 72). Causes of injury were simple falls on level ground (n = 2), falls from stairs (n = 3), falls from ladders (n = 1; height of 1.5 m), falls from roofs (n = 2; height of 3 m), traffic accidents (n = 5), and sports accidents (n = 1; skiing). Three of the patients involved in traffic accidents had experienced high‐energy trauma, defined as trauma caused by high‐speed impacts such as motor vehicle collisions or pedestrian‐automobile impacts.
The traumatic injury was witnessed in seven cases and was soon noticed due to the sound of the impact in four patients. In the other three patients, there were no witnesses. Asystole was documented in four cases, Pulseless Electrical Activity (PEA) in five by the first recorded ECG, and unknown in two. The duration of CA was estimated as ranging from 5 to 38 min or more. Head bruises were observed in nine patients. Polytrauma including limb or pelvic fractures was observed in three patients. One patient exhibited signs of traumatic brain injury. Cerebral infarction due to vertebral artery injury was seen in one patient.
Radiological findings
The radiological findings of the C group are summarized in Table 1. Computed tomography (CT) examinations revealed that 11 patients had C1‐C2 fractures or dislocations, while three patients showed subaxial injuries. Five patients had odontoid fractures, and type II was the most frequent of these (Fig. 2A). Three of them were associated with C1 fractures (two Jefferson fractures and one posterior arch fracture). Two patients had hangman fractures (one type I and two type II). One of these had a C3 spinous process fracture. Vertical (Fig. 2C) or lateral (Fig. 2D) atlanto‐axial dislocations with C1 posterior arch fractures were noted in two patients. The remaining two patients showed no fractures or dislocations, but magenetic resonance imaging (MRI) showed SCI, presumably due to atlanto‐axial subluxation (Fig. 3A,B). The subaxial injury group included one patient with C4/5 anterior dislocation and two with posterior subluxation at C3/4‐C4/5 (Fig. 3C,D) or at C4/5.
Figure 2.
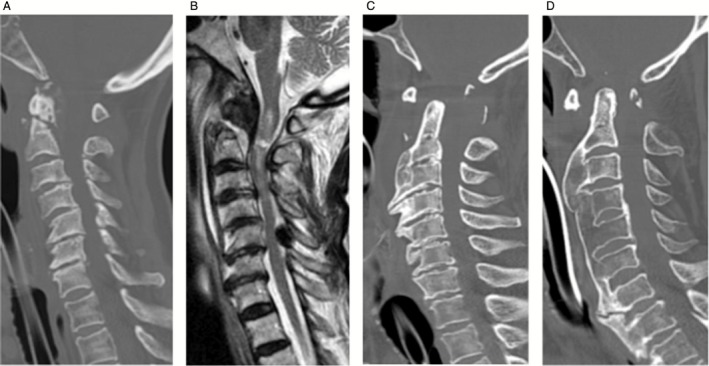
(A) Computed tomography (CT) scan shows atlanto‐odontoid joint degenerative change and type 2 odontoid fracture in case 3. (B) Magnetic resonance imaging (MRI) shows upper cervical laceration in case 3. (C) CT scans show vertical type atlanto‐axial dislocation associated with DISH in case 8. (D) CT scans show lateral type atlanto‐axial dislocation associated with diffuse idiopathic skeletal hyperostosis (DISH) in case 9. OPLL, ossification of the posterior longitudinal ligament; SCI, spinal cord injury.
Figure 3.
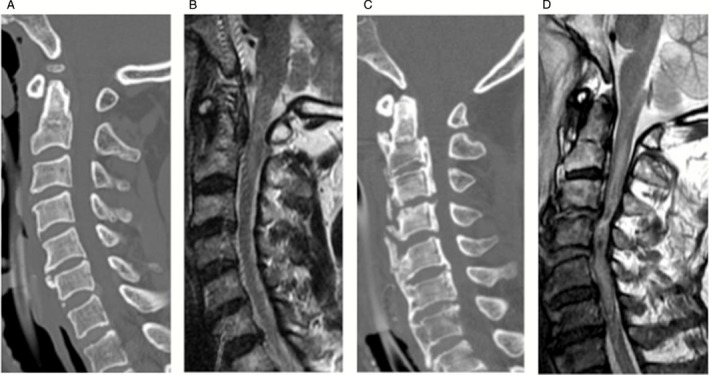
(A) Computed tomography (CT) scan showed no apparent fractures or dislocations in case 10. (B) Magnetic resonance imaging (MRI) showed intramedullary hyperintensity from the lower medulla to C3. The spinal cord injury (SCI) was presumably due to atlanto‐axial subluxation in Case 10. (C) CT scan shows diffuse idiopathic skeletal hyperostosis (DISH) and C3/4 C4/5 narrow canal stenosis due to ossification of posterior longitudinal ligament (OPLL) in case 12. (D) MRI shows C3/4 and C4/5 posterior subluxation and diffuse high‐intensity signal in upper cervical spine in case 12.
Computed tomography scans demonstrated various degenerative changes of the cervical spine. Calcification or ossification of the transverse ligament of the atlas (five patients) or the apical ligament of the odontoid process (two patients) was referred to as atlanto‐odontoid joint degeneration (AOJD) (Fig. 2A). Ossification of posterior longitudinal ligament (OPLL) was recognized in five patients (Fig. 3C). Six patients showed extensive ossification of the anterior longitudinal ligament and fused vertebrae compatible with diffuse idiopathic skeletal hyperostosis (DISH) (Figs 2C,D, 3C). Four patients showed multiple lesions of the spinal ligaments. Thus, 11 (79%) of the 14 patients had one or more degenerative spinal ligament lesions. Six of the seven patients with AOJD showed C1‐2 fracture or subluxation.
Magenetic resonance imaging examination was performed in nine out of 14 patients (four with odontoid fracture, one with hangman fracture, two with atlanto‐axial subluxation, and two with subaxial injuries). In the four patients with odontoid fractures, MRI revealed spinal cord discontinuities at C2 (Fig. 2B). The other three patients with C1‐2 lesions showed intramedullary hyperintensity from the upper cervical spinal cord to the medulla (Fig. 3B). In the two patients with subaxial lesions, MRI revealed extensive intramedullary hyperintensity from the lower cervical spinal cord to the C2 level (Fig. 3D).
Based on CT and MRI findings, the primary spinal cord injury levels in the C group were estimated to be C1/2 in 10, C2/3 in one, C3/4 in two and C4/5 in one. Radiological examination of the spinal column injuries indicated that 79% (11/14) of SCI were related to hyperextension injuries.
Treatment and clinical outcome
After resuscitation, only two patients regained spontaneous respiration. The other 12 patients remained in mechanical ventilation. In four patients, mild hypothermia therapy was performed for hypoxic brain. Halo vest was used for external fixation in five patients. The clinical outcomes at discharge were CPC 2 in three, CPC 3 in one and CPC 4 in three patients. Seven patients died of hypoxic brain swelling in the early phase (one to six days after trauma, CPC5). The period from admission to our department to discharge ranged from 1 to 112 days (mean ± standard deviation [SD]: 19 ± 30 days). Neurological examination revealed that all survivors had AIS A at discharge.
Comparison to SCI without cardio‐respiratory arrest
Clinical characteristics of the C and N groups are shown in Table 2. Compared to the N group, the C group had a significantly older mean age (P = 0.01) and higher prevalences of estimated SCI level of C1,2 (P < 0.0001) and AIS A (P < 0.0001). There were no significant differences in cause of injury between the two groups. Associated degenerative spinal column ligaments (OPLL, DISH, or AOJD) were present in 79% of the C group and 68% of the N group (data not shown). Although the prevalence of DISH was higher in the C group, there was no significant difference (P = 0.2).
Table 2.
Clinical characteristics of 14 patients resuscitated from cardiac arrest or respiratory arrest (C group) and 59 patients without arrest (N group)
| Characteristics | Overall | C group | N group | P‐value |
|---|---|---|---|---|
| Number of patients | 73 | 14 | 59 | |
| Male/Female | 67/6 | 12/2 | 55/4 | 0.4 |
| Age, years (mean ± SD) | 64 ± 17 | 74 ± 11 | 62 ± 17 | 0.01† |
| Cause of injury (%) | ||||
| Fall | 40 (55) | 8 (57) | 32 (54) | 0.8 |
| Pedestrian/Bicycle | 5 (7) | 2 (14) | 3 (5) | 0.5 |
| 4w/2w motor vehicle crashes | 18 (25) | 3 (21) | 15 (35) | 1.0 |
| Sports | 7 (10) | 1 (7) | 6 (10) | 0.9 |
| Others | 3 (4) | 0 (0) | 3 (5) | |
| High‐Energy Trauma (%) | 21 (29) | 3 (21) | 18 (30) | 0.7 |
| Brain injury (%) | 9 (12) | 1 (7) | 8 (14) | 1.0 |
| AIS (%) | <0.0001‡ | |||
| Complete: A | 23 (32) | 14 (100) | 9 (15) | |
| Incomplete: B/C/D | 29 (68) | 0 (0) | 50 (85) | |
| Estimated SCI level (%) | <0.0001‡ | |||
| C1/2 | 19 (26) | 11 (79) | 8 (14) | |
| below C3 | 54 (74) | 3 (21) | 51 (86) | |
| Ossified spinal column ligaments (%) | ||||
| DISH | 21 (29) | 6 (43) | 15 (25) | 0.2 |
| OPLL | 28 (38) | 5 (36) | 23 (39) | 0.8 |
| AOJD | 34 (47) | 7 (50) | 27 (46) | 0.8 |
AIS, American Spinal Injury Association (ASIA) Impairment Scale; AOJD, atlanto‐odontoid joint degeneration; DISH, diffuse idiopathic skeletal hyperostosis; OPLL, ossification of the posterior longitudinal ligament; SCI, spinal cord injury. †Student's t‐test, ‡χ2 test.
Predictive factors for coma due to fatal circulatory collapse among the 73 SCI patients are shown in Table 3. In our univariate analysis of data, significant differences were noted, including an increase in age of 10 years (P = 0.02) and greater likelihoods of estimated SCI level of C1,2 (P < 0.0001) and AIS A (P < 0.0001). Multiple regression analysis indicated that estimated SCI level of C1,2 and AIS A were the predictive factors.
Table 3.
Univariate analysis and multivariate analysis of predictors for cardiac or respiratory arrest associated with cervical spinal cord injury
| All data sources | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | P‐value | OR (95%CI) | P‐value | |
| Sex (Female) | 2.29 (0.38–13.99) | 0.37 | ||
| Polytrauma | 0.57 (0.064–5.06) | 0.62 | ||
| High‐energy trauma | 0.62 (0.15–2.50) | 0.50 | ||
| Brain injury | 0.49 (0.056–4.28) | 0.52 | ||
| Estimated SCI level C1,2 | 23.38 (5.33–102.50) | <0.0001 | 20.58 (2.43–174.50) | 0.006 |
| AIS A | <0.0001 | 151.97 (6.50‐> 999.99) | 0.002 | |
| OSCL lesions: | ||||
| DISH | 2.20 (0.66–7.38) | 0.20 | ||
| OPLL | 0.87 (0.26–2.92) | 0.82 | ||
| AOJD | 1.19 (0.37–3.80) | 0.78 | ||
| Number of lesions | 1.12 (0.63–2.00) | 0.70 | ||
| Age increase of 10 years | 1.94 (1.13–3.35) | 0.02 | ||
95%CI, 95% confidence interval; AIS, American Spinal Injury Association (ASIA) Impairment Scale; AOJD, atlanto‐odontoid joint degeneration; DISH, diffuse idiopathic skeletal hyperostosis; OPLL, ossification of the posterior longitudinal ligament; OR, Odds ratio; OSCL, ossification of the spinal column ligaments; SCI, spinal cord injury.
Discussion
Etiological features
In the present study, the majority (11/14 patients: 79%) of fatal cervical SCI cases were caused by minor or low‐energy trauma events. There was no significant difference in etiological factors between the C and N groups, with the exception of age: mean age was significantly older in the C group. This may be closely related to the fact that, as the Japanese population continues to age, an ever greater proportion of SCI are caused by minor or low‐energy trauma events such as falls on level ground.10, 11 Indeed, the incidence of falls in the geriatric population is high, and minor falls are recognized as a significant cause of mortality and morbidity among the elderly, as they cause 25% of injury deaths among persons 65 years of age and older.12 Wang et al. reported that 7% of all geriatric trauma patients who had suffered ground‐level falls had sustained cervical spine fractures; C1/C2 fractures were especially common, accounting for approximately 60% of fractures in these patients.13
In our series, MRI revealed that the patients in the C group had devastating high cervical cord damage even in the subaxial injury cases. The acute high cervical cord damage produced profound neurogenic shock leading to life‐threatening cardiovascular events. Prolonged respiratory arrest can result in cardiac arrest due to hypoxia in some cases. Seven patients (50%) died of hypoxic brain damage and recovery of consciousness was achieved in only four patients (29%). Long‐term care for survivors of such injuries is an important socioeconomic issue.
Degenerative changes of the spinal column
There may be a relationship between the severity of a fracture and the presence of pre‐existing age‐related degeneration of the cervical spine. In our series, degenerative changes of the spinal ligaments at the C1‐C2 levels (AOJD) were seen in six of the 11 patients with C1‐2 injuries. Lakshmanan et al.14 reported that there is a significant relationship between upper cervical‐spine osteoarthritis and the incidence of type II odontoid fractures. The presence of osteoarthritis of the upper cervical spine may lead to devastating high cervical SCI induced by minor trauma.
Diffuse idiopathic skeletal hyperostosis is recognized radiographically by the presence of “flowing” ossification along the anterolateral margins of at least four contiguous vertebrae.15 It should be noted that minor trauma events such as falls on level ground may result in severe neurological symptoms in DISH patients.16 MacMillan et al.17 stated that traumatic fractures in patients with partially fused spines tend to occur adjacent to the fused regions. In patients with DISH, the markedly limited mobility in the subaxial spinal column allows traumatic force to produce disruption of the atlanto‐axial joint or upper cervical fractures upon even low‐energy trauma.18, 19 In our study, six patients in the C group showed radiological evidence of DISH; in these patients, SCI occurred at the upper cervical levels.
Ossification of posterior longitudinal ligament also contributed to the limited mobility of the subaxial spine. The literature reveals that OPLL is found in 6.5–23% of acute cervical SCI patients10, 20 and in 38% of SCI patients without fracture or dislocations of the cervical spine in Japan.21 The prevalence of OPLL was higher in our study (36% in the C group, 39% in the N group). The age factor (elderly population) may account for the higher prevalence of OPLL.
Conclusion
The present study demonstrates the current clinical features of acute cervical SCI presenting with fatal circulatory collapse. AIS A and SCI level of C1,2 are thought to be the predictive factors. Age‐related degenerative changes at the upper cervical spine increase the risk of devastating upper cervical SCI due to minor trauma. The possibility of SCI should be considered in elderly patients exhibiting unconsciousness with cardiac or respiratory arrest and some evidence of minor trauma.
Author Contributions
KM carried out the evaluation, performed the statistical analysis and drafted the manuscript. TM and IK were involved in and supervised the evaluation and helped to draft the manuscript. NM and EN supervised the evaluation and corrected the manuscript. All authors read and approved the final manuscript.
Competing Interests
All authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to thank Hideto Irifune and Suguru Hirayama for their support in performing surgical procedures. We would like to thank the statistician Toshihito Furukawa for his support in analyzing our data.
References
- 1. Huelke DF, O'Day J, Mendelsohn RA. Cervical injuries suffered in automobile crashes. J. Neurosurg. 1981; 54: 316–322. [DOI] [PubMed] [Google Scholar]
- 2. Hadley MN, Sonntag VK, Grahm TW, Masferrer R, Browner C. Axis fractures resulting from motor vehicle accidents. The need for occupant restraints. Spine (Phila Pa 1976) 1986; 11: 861–864. [DOI] [PubMed] [Google Scholar]
- 3. Platzer P, Hauswirth N, Jaindl M, Chatwani S, Vecsei V, Gaebler C. Delayed or missed diagnosis of cervical spine injuries. J. Trauma 2006; 61: 150–155. [DOI] [PubMed] [Google Scholar]
- 4. Reid DC, Henderson R, Saboe L, Miller JD. Etiology and clinical course of missed spine fractures. J. Trauma 1987; 27: 980–986. [DOI] [PubMed] [Google Scholar]
- 5. Gautschi OP, Woodland PR, Zellweger R. Complete medulla/cervical spinal cord transection after atlanto‐occipital dislocation: an extraordinary case. Spinal Cord 2007; 45: 387–393. [DOI] [PubMed] [Google Scholar]
- 6. Kleweno CP, Zampini JM, White AP, Kasper EM, McGuire KJ. Survival after concurrent traumatic dislocation of the atlanto‐occipital and atlanto‐axial joints: a case report and review of the literature. Spine (Phila Pa 1976) 2008; 33: E659–662. [DOI] [PubMed] [Google Scholar]
- 7. Garthe E, States JD, Mango NK. Abbreviated injury scale unification: the case for a unified injury system for global use. J. Trauma 1999; 47: 309–323. [DOI] [PubMed] [Google Scholar]
- 8. Steeves JD, Lammertse D, Curt A et al Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–221. [DOI] [PubMed] [Google Scholar]
- 9. Edgren E, Hedstrand U, Kelsey S, Sutton‐Tyrrell K, Safar P. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet 1994; 343: 1055–1059. [DOI] [PubMed] [Google Scholar]
- 10. Chikuda H, Seichi A, Takeshita K et al Acute cervical spinal cord injury complicated by preexisting ossification of the posterior longitudinal ligament: a multicenter study. Spine (Phila Pa 1976) 2011; 36: 1453–1458. [DOI] [PubMed] [Google Scholar]
- 11. Chieregato A. High cervical spinal cord complete transection. Arch. Neurol. 2008; 65: 1126. [DOI] [PubMed] [Google Scholar]
- 12. Sterling DA, O'Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J. Trauma 2001; 50: 116–119. [DOI] [PubMed] [Google Scholar]
- 13. Wang H, Coppola M, Robinson RD et al Geriatric trauma patients with cervical spine fractures due to ground level fall: five years experience in a level one trauma center. J. Clin. Med. Res. 2013; 5: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lakshmanan P, Jones A, Howes J, Lyons K. CT evaluation of the pattern of odontoid fractures in the elderly—relationship to upper cervical spine osteoarthritis. Eur. Spine J. 2005; 14: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976; 119: 559–568. [DOI] [PubMed] [Google Scholar]
- 16. Berlot G, Viviani M, Gullo A, Magnaldi S. Delayed traumatic cervical cord transection. Am. J. Emerg. Med. 1995; 13: 101–103. [DOI] [PubMed] [Google Scholar]
- 17. Nunnally ME, Jaeschke R, Bellingan GJ et al Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit. Care Med. 2011; 39: 1113–1125. [DOI] [PubMed] [Google Scholar]
- 18. Paley D, Schwartz M, Cooper P, Harris WR, Levine AM. Fractures of the spine in diffuse idiopathic skeletal hyperostosis. Clin. Orthop. Relat. Res. 1991; 267: 22–32. [PubMed] [Google Scholar]
- 19. Liang CL, Lu K, Lee TC, Lin YC, Chen HJ. Dissociation of atlantoaxial junction in ankylosing spondylitis: case report. J. Trauma 2002; 53: 1173–1175. [DOI] [PubMed] [Google Scholar]
- 20. Endo S, Shimamura T, Nakae H et al Cervical spinal cord injury associated with ossification of the posterior longitudinal ligament. Arch. Orthop. Trauma Surg. 1994; 113: 218–221. [DOI] [PubMed] [Google Scholar]
- 21. Koyanagi I, Iwasaki Y, Hida K, Akino M, Imamura H, Abe H. Acute cervical cord injury without fracture or dislocation of the spinal column. J. Neurosurg. 2000; 93: 15–20. [DOI] [PubMed] [Google Scholar]


