Abstract
Purposes
To compare the effects of aflibercept and other anti-vascular endothelial growth factor (anti-VEGF) medications on both functional and anatomical outcomes for treatment-naïve neovascular age-related macular degeneration (nAMD) in the real world.
Methods
A systematic review and meta-analysis of observational comparative studies.
Results
A total of 18 studies remained after literature selection and quality assessment of 1697 studies. The most common aflibercept treatment regimen was three monthly injections followed by pro re nata (PRN). Aflibercept and ranibizumab had similar effects in 2-year treatment. At 3, 6, 12, and 24 months, the differences in the logarithm of minimum angle of resolution (logMAR) decrease in aflibercept and ranibizumab groups were 0.00 (95% confidence interval [CI]: −0.03 to 0.02); 0.01 (95% CI: −0.02 to 0.05); −0.03 (95% CI: −0.07 to 0.01); and –0.06 (95% CI: −0.30 to 0.17), respectively; the differences in decrease of central retinal thickness (CRT) were 3.25 μm (95% CI: −15.03 to 21.53); 7.89 μm (95% CI: −31.91 to 47.69); 2.89 μm (95% CI: −18.33 to 24.11); and −2.42 μm (95% CI: −77.87 to 73.03), respectively. However, aflibercept was significantly more effective in patients with initial reduced visual acuity (logMAR >0.6 or <55 letters; P = 0.001). In the first year, treatment frequency was not significantly different for aflibercept and ranibizumab, but aflibercept required fewer injections than ranibizumab with PRN regimen (mean −0.90; 95% CI: −1.80 to 0.00).
Conclusions
Aflibercept has comparable effects with ranibizumab for treatment-naïve nAMD in the real world, and may be more effective for patients with initial lower visual acuity.
Keywords: aflibercept, age-related macular degeneration, comparative effectiveness
Visual impairment is a public health problem that affects patient functional status and quality of life.1,2 Blindness or vision impairment is among the top 10 disabilities in the United States according to Centers for Disease Control and Prevention (CDC).1,3
Among all causes of visual impairment, age-related macular degeneration (AMD) is a leading cause of adult blindness in industrialized countries.4 AMD is a degenerative disease of the central portion of the retina (the macula), and may cause loss of central vision.5 There are two types of AMD: atrophic (dry) and neovascular (wet or exudative) AMD (nAMD).5 nAMD is characterized by growth of abnormal vessels into the subretinal space, and consequent fluid and/or blood beneath the retina.5–7 Although less common than dry AMD, nAMD usually progresses rapidly, can cause severe vision loss or even blindness and accounts for 80% of all cases of severe visual loss or blindness due to AMD.5,7 Therefore, it is essential to determine efficient solutions to prevent and treat nAMD.
Vascular endothelial growth factor (VEGF) is a protein that plays a role in stimulating abnormal blood vessel growth and neovascularization.8 For this reason, anti-VEGF medications can limit progression of nAMD or reverse visual loss.9 Currently, the most commonly used anti-VEGF drugs are intravitreal ranibizumab (Lucentis; Genentech, Inc., San Francisco, CA, USA); bevacizumab (Avastin, Genentech, Inc.); and aflibercept (Eylea; Bayer HealthCare, Inc., and Regeneron Pharmaceutical, Inc.). Both ranibizumab and bevacizumab inhibit VEGF-A,10,11 and were introduced in the mid-2000s.12 However, bevacizumab has not been approved by the Food and Drug Administration (FDA) for AMD treatment.12 Aflibercept was approved to treat nAMD by FDA in 2011 and by the European Commission in 2012.13,14 In contrast to ranibizumab and bevacizumab, aflibercept is a recombinant fusion protein that inhibits VEGF-A, VEGF-B, and placental growth factor (PlGF).15,16 Furthermore, while both ranibizumab and bevacizumab usually require monthly injections, or as needed based on a monthly assessment of disease activity, aflibercept usually requires less-frequent injections.17,18 Currently, there are three regimens for aflibercept injections in clinical practice: fixed dosing according to the labelling, pro re nata (PRN), and treat-and-extend. PRN implies patients are only treated when deemed necessary by the clinician; and “treat and extend” means the interval between treatments is lengthened iteratively if there is no recurrent exudation.19,20 The labeled regimen for aflibercept in nAMD treatment is three initial 4-weekly (monthly) loading doses, followed by injections once every 8 weeks (2 months).21
Systematic reviews and meta-analyses of randomized clinical trials (RCTs) have shown comparable efficacy between aflibercept and other anti-VEGFs (e.g., ranibizumab) for nAMD.22–24 However, treatment outcomes in routine practice may be different from that in RCTs. The first reason is that study populations in RCTs are highly selected, and may not be representative of real-world patients.25 Additionally, patients in the community clinical setting may receive dosing and/or regimens that are different from the ones recommended in the product's label.26 Therefore, it is not automatically clear to what extent the study results from RCTs can be replicated in routine clinical practice.25 The objective of this study was to provide synthesized evidence about the effects of aflibercept on visual acuity and central retinal thickness (CRT) compared to other anti-VEGFs in routine practice based on observational studies.
Methods
Search Strategy
A systematic literature search in PubMed and Embase databases was conducted by one reviewer (YZ) in July 2017. The combined use of keywords and medical subject headings (MeSH) were applied to find relevant studies. Detailed information about search terms is in Supplementary Table S1. Available studies from January 2011 through July 2017 were examined. Reference lists of related articles were hand-searched for additional studies.
Inclusion and Exclusion Criteria
This review and meta-analysis was based on evidence from observational studies—studies in which researchers simply observe treatments and outcomes rather than influencing or interfering with treatment assignment; RCTs were excluded. Only published peer-reviewed journal articles with full text available were considered. Conference papers/abstracts, proposals, editorials, letters to authors, reviews, notes, commentaries, and news were not included in the review.
Most systematic reviews and meta-analyses of RCTs on this topic have been conducted among treatment-naïve patients with comparison group(s). In this study, articles were considered only if they compared the functional or anatomic outcomes between aflibercept monotherapy and other anti-VEGF(s) (ranibizumab or/and bevacizumab) among nAMD patients with no previous therapy. In addition, journal articles were included if they met the following criteria: 1) published in English; 2) conducted among humans; and 3) had relatively large sample size (>20 eyes); 4) were not case reports; and 5) patient follow-up was at least 3 months.
A stepwise review process (title review, abstract review, and full text review) was conducted to exclude irrelevant studies. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and Meta-analysis of Observational Studies in Epidemiology (MOOSE) criteria.27,28
Outcome Measures
This study evaluated both functional and anatomic effects of aflibercept compared to other anti-VEGF(s) as the primary outcome. The functional outcome was assessed by mean changes in best-corrected visual acuity (BCVA) from baseline to different time points of follow-up, as measured in logarithm of minimum angle of resolution (logMAR). Visual acuity measured in other units, such as Early Treatment Diabetic Retinopathy Study (ETDRS) letters or Snellen fraction, were converted into logMAR based on validated conversion methods.29,30 The anatomic outcome was estimated based on mean changes in CRT on spectral-domain ocular coherence tomography (SD-OCT). The number of injections was also compared based on available information from original articles. Additionally, this study also had the effects of aflibercept compared to pretreatment as the secondary outcome.
Quality Assessments
The Newcastle-Ottawa quality assessment scale was used to assess the quality of studies that met the inclusion criteria.31 One study could achieve a maximum of nine points based on the eight items on the scale. Additionally, baseline characteristics; study population (inclusion/exclusion criteria); possible differences in treatment assignments; differences in outcome measurements, and time of follow-up were also compared among studies. Two reviewers (YZ, CC) performed the quality assessments independently. Thereafter differences in quality assessment were resolved via discussion. Another author (MS) helped with the clarification and resolutions of the disagreement in quality assessment of studies.
Data Abstraction
Study title, author(s), publication year, country(s)/region(s) of study, indication(s) for medications, study design, regimen and dosage, sample size, characteristics of study population, main outcome measure(s), and key findings were extracted by one reviewer (YZ). Authors of articles were contacted if there was unclear or incomplete information.
Data Synthesis and Analysis
Reported mean change in BCVA and CRT before and after treatment with standard deviation (SD) or 95% confidence intervals (CIs) was used in the meta-analysis. If one study did not report SD or 95% CI, P values were used to calculate SD if the sample size was large enough (n ≥ 60).32 Otherwise, for studies with relatively small sample size (n < 60), and both SD and 95% CI missed, correlation coefficients before and after treatments calculated based on other included studies were used to estimate the SDs of mean change in this study.33 Sensitivity analyses with different values of correlation coefficients were performed.
Heterogeneity of studies were evaluated by the I2 test. Models (fixed effect or random effect) were selected based on the I2 test results. Publication bias was evaluated with funnel plots. We used RStudio (version 0.98.1091; https://www.rstudio.com/products/RStudio, in the public domain) and Review Manager (version 5.3.; Cochrane, London, UK) were used for data imputation and analysis, respectively.
Results
Literature Search
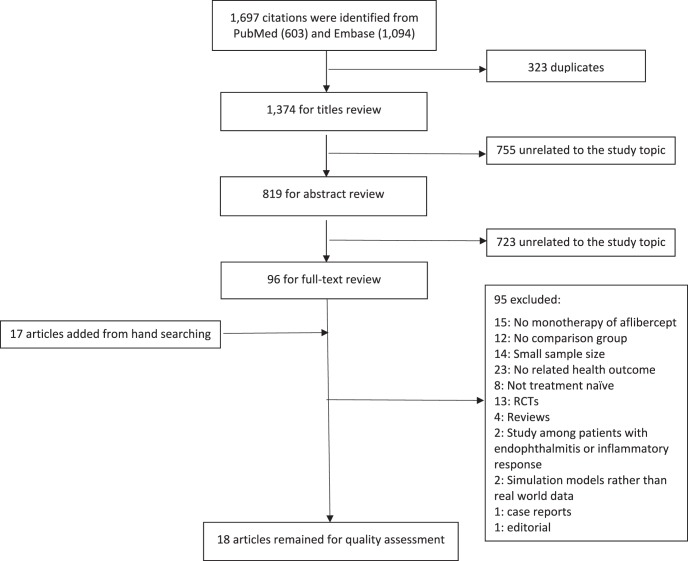
In the literature search, 603 and 1094 studies published in English since January 2011 were identified in PubMed and Embase, respectively. After checking for duplications, 1374 studies were kept for stepwise review. Of these studies, 96 articles that were relevant to the study topic remained for full-text review. An additional 17 studies were identified via manual search. Finally, after full-text review of these 113 articles, 18 studies met inclusion criteria.11,34–47 The literature search process is summarized in Figure 1.
Figure 1.
PRISMA flow diagram of study selection.
The quality score of the 18 remaining articles ranged from 5 to 9. One study (Dirani et al.39) was graded a score of 5, which was a relatively lower score compared to the other studies. In that study, there was a lack of baseline comparison across study groups and no detailed description about data source, even though anatomic outcomes were measured based on SD-OCT imaging. After contacting the author, it was clarified that the data came from “hospital files and multimodal imaging.” Quality assessments of these 18 studies are available in Supplementary Table S2.
Study Characteristics
All included studies were retrospective observational cohort studies. In total, there were 6779, 6054, and 217 eyes included in aflibercept, ranibizumab, and bevacizumab groups, respectively. Eight studies were conducted in Asia (four in Korea and four in Japan); six were conducted in Europe (United Kingdom, Switzerland, and Denmark); three in the United States; and another one in Australia. The treatment indications varied, although nAMD was the common main indication. Fifteen studies compared the effects of aflibercept and ranibizumab, two studies had both ranibizumab and bevacizumab as comparison drugs, and one study had bevacizumab as comparison. The mean age in aflibercept group ranged between 65 and 91 years, and the proportion of females varied from 6.9% to 73.6%. Most studies demonstrated comparable baseline characteristics in terms of BCVA and/or CRT among comparison groups.11,34,36,37,40,42–45,48–50 Study characteristics are summarized in Tables 1 and 2. Follow-up time varied from 3 to 24 months, and a majority of studies (12, 66.7%) had a 1-year follow up after drug initiations. The most common treatment regimen was initial monthly injections of aflibercept for 3 months followed by PRN. Detailed information about follow-up and regimen is summarized in Table 3.
Table 1.
Summary of Studies Included in the Systematic Review and Meta-Analysis
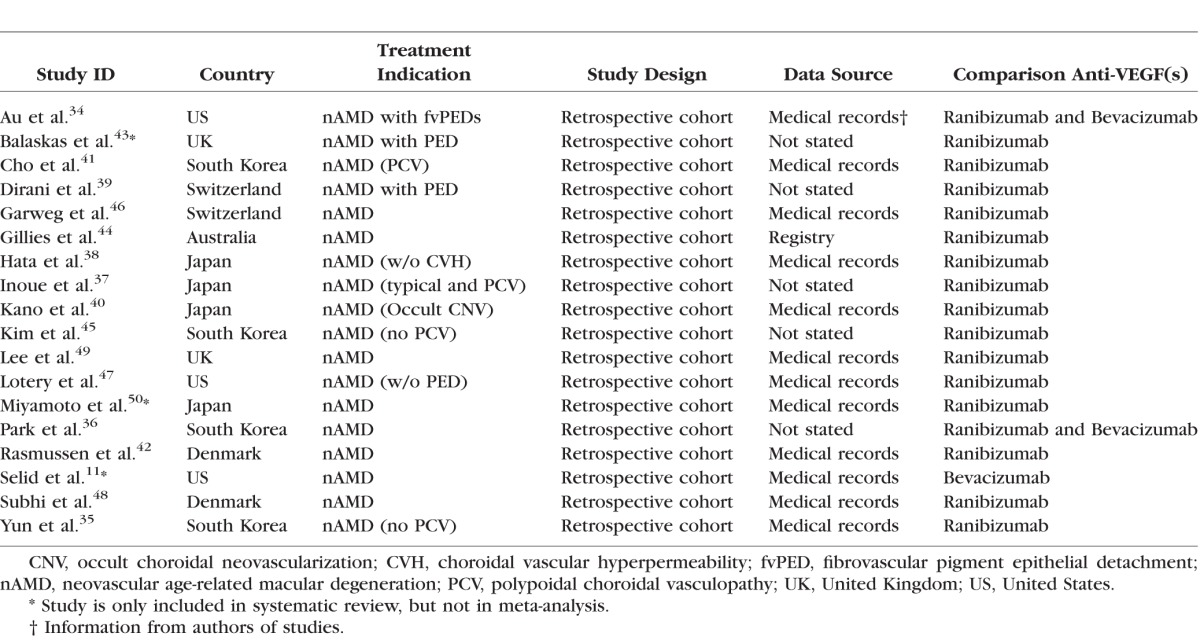
Table 2.
Baseline Characteristics of Aflibercept and Ranibizumab Treatment Groups
Table 3.
Treatment Regimens and Follow-Up Periods
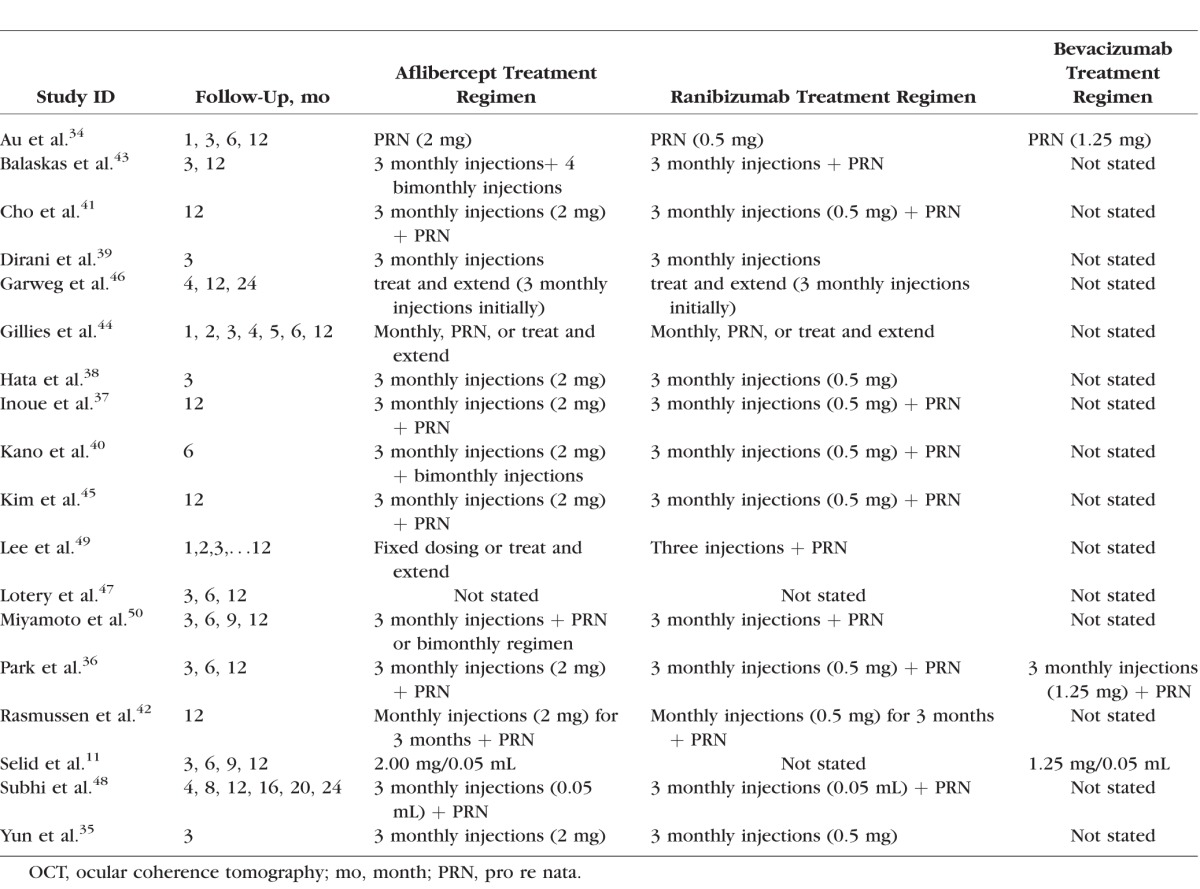
Some necessary data for meta-analysis was missing in comparing the effects of aflibercept and bevacizumab in two articles.11,50 Consequently, these two studies were included in the systematic review but not in the meta-analysis. For the same reason, we did not compare the effects of aflibercept and bevacizumab in the meta-analysis because only two studies36,34 provided complete information. Another study43 had clinical data reported in median and interquartile range because of nonnormal distribution. Therefore, this study was also only included in the systematic review rather than in the meta-analysis.
Visual Acuity
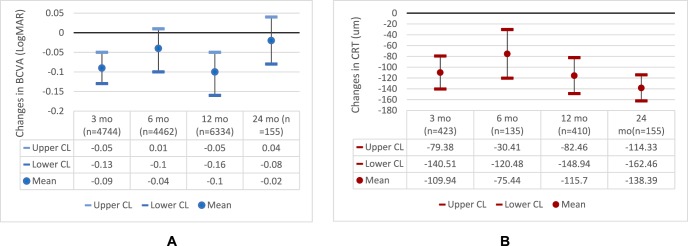
Seventeen studies reported changes in BCVA before and after injections, and 16 studies compared changes in BCVA between aflibercept and ranibizumab treatment groups. All studies had consistent findings of improved visual acuity for patients after initial aflibercept injections, but the treatment effects were not consistent in the later phase of follow-up (after 1 year). Figure 2A shows the estimated changes in BCVA at different time points of follow-up.
Figure 2.
Changes in (A) BCVA (logMAR) and (B) CRT after aflibercept treatment. BCVA, best-corrected visual acuity; CL, confidence limit; CRT, central retinal thickness; logMAR, logarithm of minimum angle of resolution; mo, months.
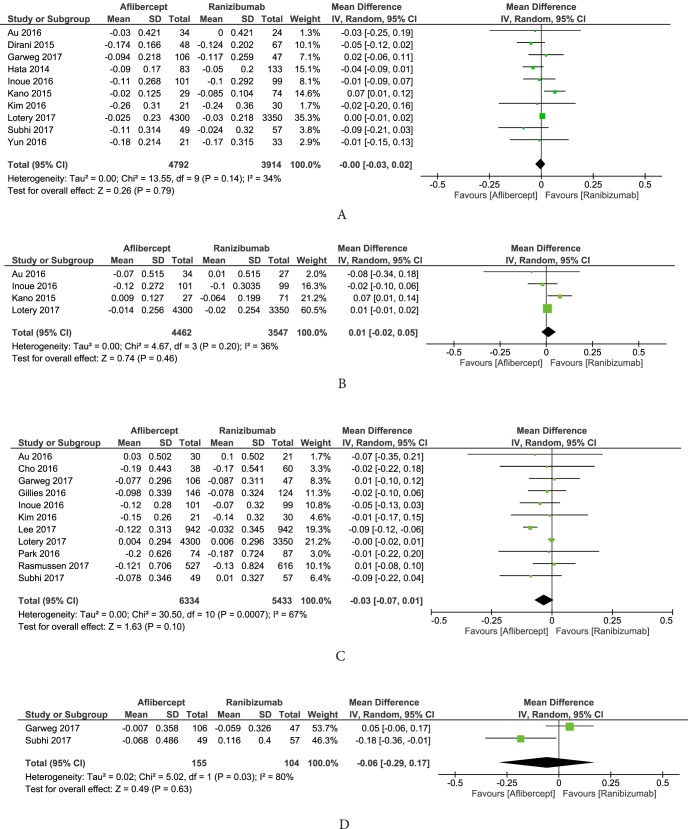
Effects of aflibercept and ranibizumab on visual acuity were compared at different time points of follow-up. Figure 3 shows the two anti-VEGF drugs had similar effects at 3, 6, 12, and 24 months of follow-up (mean differences in logMAR change of 0.00, 95% CI: −0.03 to 0.02; 0.01, 95% CI: −0.02 to 0.05; −0.03, 95% CI: −0.07 to 0.01; and −0.06, 95% CI: −0.29 to 0.17, respectively).
Figure 3.
Differences in BCVA (logMAR) changes between aflibercept and ranibizumab treatment at (A) 3, (B) 6, (C) 12, and (D) 24 months. BCVA, best-corrected visual acuity; logMAR, logarithm of minimum angle of resolution.
To detect the possible impact of age, baseline BCVA, region or ethnicity, and treatment regimen on the treatment effects of visual acuity, subgroup analyses based on 12-month treatment outcomes were performed. The results in Table 4 demonstrated that aflibercept and ranibizumab had comparable effects in spite of having different age groups, study areas, or treatment regimens. However, aflibercept was superior to ranibizumab in the worse BCVA group (logMAR >0.6, equivalent to approximately <55 ETDRS letters), with a mean difference in logMAR change of −0.06 (95% CI: −0.10 to −0.03). In addition, although comparable to ranibizumab, aflibercept received in routine practice was not helpful in improving visual acuity for older patients (≥75 years old), with relatively better baseline BCVA (logMAR ≤0.6), or in the United States.
Table 4.
Subgroup Analysis at 12 Months of Aflibercept Treatment
There was variation in the subtypes of nAMD (e.g., polypoidal choroidal vasculopathy [PCV]) that patients in the studies were diagnosed with. Hence, subgroup analysis was also performed to better understand the treatment effects for these subtypes. There was no significant difference between aflibercept and ranibizumab for typical nAMD, PCV, or nAMD with pigment epithelial detachment (PED; Table 4). Only one study40 explored the treatment effects of aflibercept and ranibizumab on occult choroidal neovascularization (CNV), and concluded that the improvement in BCVA was significantly higher in the ranibizumab group compared to that in the aflibercept group after 3-month treatments (P = 0.02).
According to the three studies comparing treatment outcomes of aflibercept and bevacizumab, both medications helped improve visual acuity, and their treatment effects were similar through the treatment course from 3 to 12 months. However, one study11 identified that the proportion of patients getting improvement in visual acuity (logMAR gain ≥0.3) was greater in the aflibercept treatment group at 6 months follow-up (P = 0.05).
Central Retinal Thickness
Of all included studies, 12 studies reported the treatment outcomes of CRT, 11 compared the effects of aflibercept and ranibizumab, and 3 compared the effects of aflibercept and bevacizumab. All studies reported decreased CRT after aflibercept injections. Figure 2B shows the estimated changes in CRT across follow-up of 2 years.
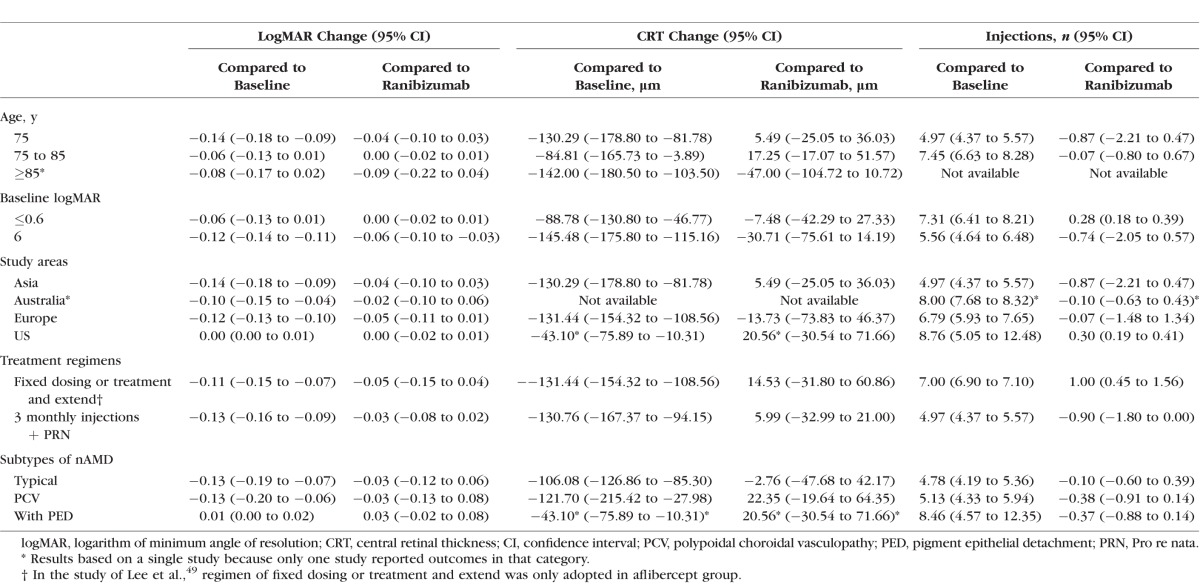
Aflibercept and ranibizumab had comparable effects in reducing CRT during a treatment course of 24 months. Figure 4 shows that the differences in CRT decrease at 3, 6, 12, and 24 months after aflibercept and ranibizumab injections were 3.25 μm (95% CI: −15.03 to 21.53); 7.89 μm (95% CI: −31.91 to 47.69); 2.89 μm (95% CI: −18.33 to 24.11); and −2.42 μm (95% CI: −77.87 to 73.03), respectively.
Figure 4.
Differences in CRT changes between aflibercept and ranibizumab treatment at (A) 3, (B) 6, (C) 12, and (D) 24 months. CRT, central retinal thickness.
Subgroup analysis in Table 4 shows that aflibercept was effective in reducing CRT based on the meta-analysis, regardless of age, baseline visual acuity, study areas, treatment regimens, or subgroups of nAMD. The two drugs were also demonstrated to have comparable treatment effects across these different subgroups. However, one study with patients with occult CNV concluded that aflibercept decreased CRT more significantly than ranibizumab after 3 months of initial treatments.40
According to the three studies comparing the treatment effects of aflibercept and bevacizumab, the two medications had no significant differences in reducing CRT overall. However, aflibercept appeared to reduce CRT more quickly than bevacizumab in one study.36
Number of Injections
Included studies had different treatment frequency because of diverse treatment regimens. In this review, only two studies used a labeled regimen of aflibercept, and six studies adopted PRN after loading doses for both aflibercept and ranibizumab treatments. In 1 year, the synthesized number of injections for aflibercept and ranibizumab were 6.39 (95% CI: 5.76–7.03) and 6.56 (95% CI: 5.97–7.14). Five studies with three monthly injections followed by PRN regimen provided number of drug injections at 12 months.36,37,41,42,45 The number of aflibercept injections during a 1-year treatment course varied from 4.3 to 5.9, and ranibizumab required 4.5 to 8.37 injections. Aflibercept required fewer injections than ranibizumab, with a mean difference of −0.90 (95% CI: −1.80 to 0.00; Table 4).
Besides treatment regimens, number of injections also differed across other subgroups. One study conducted among a very old patient population (≥90 years) reported a smaller number of aflibercept injections during 24 months (−1.20, 95% CI: −2.33 to 0.00).48 However, slightly more aflibercept injections were used compared to ranibizumab among patients with better baseline BCVA (logMAR ≤0.6) and patients in the United States, with differences in number of injections of 0.28 (95% CI: 0.18–0.39) and 0.30 (95% CI: 0.19–0.41), respectively (Table 4).
Discussion
Aflibercept has been a treatment option for nAMD since 2011. To our knowledge, this is the first meta-analysis analyzing the comparative effectiveness of aflibercept and other anti-VEGFs for treatment-naïve nAMD based on observational comparative studies.
The results showed that aflibercept helped improve BCVA and decrease CRT in routine clinical practice. Treatment indications, follow-up time, and regimens varied across studies. However, the included studies consistently reported the effectiveness of aflibercept in clinical practice. Compared to ranibizumab and bevacizumab, aflibercept had similar treatment effects on functional and anatomical outcomes. These finding were consistent with previously published meta-analyses of RCTs.22,24
Interestingly, in patients with lower BCVA (logMAR > 0.6), aflibercept may lead to significantly better visual outcome than ranibizumab (P = 0.001). In the large comparative Diabetic Retinopathy Clinical Research Network protocol T study of patients with treatment-naïve diabetic macular edema, aflibercept was also shown to be superior to ranibizumab and bevacizumab in the subgroup of patients with lower baseline visual acuity.51 Potentially a similarly differential effect of aflibercept is at play in CNV.
Aflibercept is expected to have a long duration of effect, and consequently reduce treatment burden. Based on the five included studies with a PRN regimen in this meta-analysis, aflibercept required 0.9 fewer injections than ranibizumab during one year. Given the comparative effect of the two drugs with a PRN regimen on BCVA and CRT, this reduced number of injections may indicate that similar treatment effects can be obtained with less-frequent aflibercept injections. However, to better understand the treatment patterns of anti-VEGFs in the real world, the healthcare system, patient treatment preferences, and metrics need to be taken into account in order to determine the number of injections.
First, anti-VEGF choice and treatment frequency may vary across countries. For example, different from study results of Rasmussen et al.42 that aflibercept was associated with fewer reinjections, studies in the United States and Switzerland found that aflibercept and ranibizumab had similar treatment patterns and number of injections.52–54
Second, some patients may prefer frequent physician visits and reinjections to control their symptoms. Mueller et al.,55 found out that patients in their study did not have strong preference toward any treatment scheme. The authors concluded that in order to improve their visual acuity, patients were receptive to receiving high treatment burden with regular intravitreal injections at short intervals.55 Obviously, the real-world management of patients with nAMD originates from shared decision making between physician and patient preferences.56 Consequently, the expected difference in injection frequency between aflibercept and other anti-VEGFs might not be reflected in routine practice.
Third, there were inconsistencies in the recording method of number of injections across studies. Studies provided number of injections but did not make clear explanations about what these numbers meant. Patients may be lost to follow-up because of lack of treatment response, disease inactivity, and death or other severe disease. It was difficult to determine what these reported numbers actually specified. Therefore, the estimated number of injections in our meta-analysis may be inaccurate.
We detected possible publication bias of the comparative treatment effects with funnel plots (Supplementary Figs. S3, S4). Supplementary Figure S3C shows that there could be some publication bias for the estimates of aflibercept effects on BCVA compared to ranibizumab at 12 months. Thus, more high-quality studies need to be performed to validate our findings.
Among the 18 included studies, BCVA was not confirmed in two studies, and best uncorrected or pinhole visual acuity were used for analysis.44,47 In another study, patients with discontinued treatment during 1 year were included into analysis.42 Therefore, sensitivity analysis about changes in visual acuity and treatment frequency were conducted with the exclusion of these three studies. According to the sensitivity analysis results, aflibercept had more effective functional outcomes than ranibizumab in 12 months (difference in logMAR changes: −0.08; 95% CI: −0.10 to −0.05) with similar treatment frequency (difference in injection numbers: −0.24; 95% CI: −1.46 to 0.98). Additionally, the findings in Supplementary Table S5 indicate aflibercept had effective functional outcome for patients with better baseline BCVA (logMAR ≤0.6).
There were two main limitations to this study. First, a single reviewer performed the literature search and data abstraction, resulting in a potential for subjective bias. However, all steps in this process can be reproduced from the PRISMA flow diagram. Second, only studies that included comparison treatment group(s) were included in our review and analysis. The inclusion criteria and control-matching algorithms that were used in the comparative studies have the potential to induce bias while comparing outcomes to baseline, as the control-matching algorithms may try to balance pretreatment characteristics (e.g., age and baseline BCVA). Thus, we emphasize that the primary outcome of this meta-analysis is the comparison of aflibercept to other anti-VEGF agents, and that the comparison of aflibercept to baseline is a secondary outcome that may have the limitations mentioned. This study only provides evidence about anti-VEGF selection, and does not demonstrate the treatment effects of aflibercept versus no treatment. However, anti-VEGFs have been the mainstay of treatment for nAMD since the mid-2000s, so the main clinical concern is which initial anti-VEGF drug to select. Previous reviews and analyses57–59 have verified the effects of aflibercept for persistent or resistant nAMD, yet, our study provides additional evidence about the effects for newly diagnosed patients.
In summary, this review and meta-analysis provides synthesized evidence about effects of aflibercept for treatment-naïve nAMD in the real world. The study results revealed that aflibercept is a treatment option given its comparable effects as other anti-VEGFs, and may be superior in patients with lower visual acuity. Future study is needed to evaluate the long-term effects of aflibercept.
Supplementary Material
Acknowledgments
The authors thank Paul Petraro, Claudia Tueckmantel, Zdravko P. Vassilev, and Montse S. Gabarro for their collaboration and manuscript editing, and Xiaomei Gu for her help in literature search. We also thank the study authors who provided additional data or information for our analysis.
Supported in part by the Department of Veterans Affairs and a VA Health Services Research and Development Career Development Award 11-215 (MLS); M.D. Abràmoff is the Robert C. Watzke, MD Professor of Ophthalmology and Visual Sciences.
Disclosure: Y. Zhang, None; C. Chioreso, None; M.L. Schweizer, None; M.D. Abràmoff, None
References
- 1. Centers for Disease Control and Prevention (CDC). The burden of vision loss. Available at: https://www.cdc.gov/visionhealth/basic_information/vision_loss_burden.htm.Accessed June 19, 2016.
- 2. Centers for Disease Control and Prevention (CDC). Why is Vision loss a public health problem? Available at: https://www.cdc.gov/visionhealth/basic_information/vision_loss.htm.Accessed June 19, 2016.
- 3. Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults --- United States, 1999. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5007a3.htm.Accessed June 19, 2016.
- 4. Hyman L. . Epidemiology of eye disease in the elderly. Eye (London). 1987; 1 Pt 2: 330– 341. [DOI] [PubMed] [Google Scholar]
- 5. Arroyo JG. . Age-related macular degeneration: Clinical presentation, etiology, and diagnosis. Available at: http://www.uptodate.com/contents/age-related-macular-degeneration-clinical-presentation-etiology-and-diagnosis.Accessed Jun 19, 2016.
- 6. Campochiaro PA, Soloway P, Ryan SJ,et al. The pathogenesis of choroidal neovascularization in patients with age-related macular degeneration. Mol Vis. 1999; 5: 34– 38. [PubMed] [Google Scholar]
- 7. Jager RD, Mieler WF, Miller JW. . Age-related macular degeneration. N Engl J Med. 2008; 358: 2606– 2617. [DOI] [PubMed] [Google Scholar]
- 8. Arroyo JG. . Age-related macular degeneration: Treatment and prevention. Available at: http://www.uptodate.com/contents/age-related-macular-degeneration-treatment-and-prevention.Accessed June 19, 2016.
- 9. Solomon SD, Lindsley K, Vedula SS,et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014; 8: CD005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrara N, Damico L, Shams N,et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26: 859– 870. [DOI] [PubMed] [Google Scholar]
- 11. Selid PD, Jundt MC, Fortney AC,et al. Intravitreal bevacizumab and aflibercept for the treatment of exudative age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2014; 45: 275– 281. [DOI] [PubMed] [Google Scholar]
- 12. Schmucker C, Ehlken C, Agostini HT,et al. A safety review and meta-analyses of bevacizumab and ranibizumab: off-label versus goldstandard. PLoS One. 2012; 7: e42701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regeneron Pharmaceuticals I. EYLEA® (Aflibercept) injection approved for the treatment of wet age-related macular degeneration in Europe, 2012. Available at: http://investor.regeneron.com/releasedetail.cfm?releaseid=723255. Accessed June 21, 2016.
- 14. U.S. Food & Drug Administration. FDA approves EYLEA for eye disorder in older people. Available at: http://www.prnewswire.com/news-releases/fda-approves-eylea-for-eyedisorder-in-older-people-134147948.html. Accessed January 31, 2017.
- 15. Calvo P, Abadia B, Ferreras A,et al. Diabetic macular edema: options for adjunct therapy. Drugs. 2015; 75: 1461– 1469. [DOI] [PubMed] [Google Scholar]
- 16. Stewart MW. . Aflibercept (VEGF trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012; 96: 1157– 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verner-Cole EA, Davis SJ, Lauer AK. . Drug treatment. Aflibercept for the treatment of neovascular age-related macular degeneration. Drugs Today (Barc). 2012; 48: 317– 329. [DOI] [PubMed] [Google Scholar]
- 18. Heier JS, Brown DM, Chong V,et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119: 2537– 2548. [DOI] [PubMed] [Google Scholar]
- 19. Heier JS, Clark WL, Boyer DS,et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014; 121: 1414– 1420.e1411. [DOI] [PubMed] [Google Scholar]
- 20. Gupta OP, Shienbaum G, Patel AH,et al. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration: clinical and economic impact. Ophthalmology. 2010; 117: 2134– 2140. [DOI] [PubMed] [Google Scholar]
- 21. U.S. Food & Drug Administration (FDA). EYLEA (aflibercept) injection label. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125387s004lbl.pdf. Accessed Aug 1, 2017.
- 22. Sarwar S, Clearfield E, Soliman MK,et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016; 2: CD011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Szabo SM, Hedegaard M, Chan K,et al. Ranibizumab vs. aflibercept for wet age-related macular degeneration: network meta-analysis to understand the value of reduced frequency dosing. Curr Med Res Opin. 2015; 31: 2031– 2042. [DOI] [PubMed] [Google Scholar]
- 24. Ba J, Peng R-S, Xu D,et al. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des Dev Ther. 2015; 9: 5397– 5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkes MM, Navickis RJ, Chan WW,et al. Bisphosphonates and osteoporotic fractures: a cross-design synthesis of results among compliant/persistent postmenopausal women in clinical practice versus randomized controlled trials. Osteoporos Int. 2010; 21: 679– 688. [DOI] [PubMed] [Google Scholar]
- 26. Singh RP, Srivastava SK, Ehlers JP,et al. A single-arm, investigator-initiated study of the efficacy, safety, and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration previously treated with ranibizumab or bevacizumab (ASSESS study): 12-month analysis. Clin Ophthalmol. 2015; 9: 1759– 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J,et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stroup DF, Berlin JA, Morton SC,et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000; 283: 2008– 2012. [DOI] [PubMed] [Google Scholar]
- 29. Gregori NZ, Feuer W, Rosenfeld PJ. . Novel method for analyzing Snellen visual acuity measurements. Retina. 2010; 30: 1046– 1050. [DOI] [PubMed] [Google Scholar]
- 30. Cotter SA, Chu RH, Chandler DL,et al. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to <13 years old. Am J Ophthalmol. 2003; 136: 655– 661. [DOI] [PubMed] [Google Scholar]
- 31. Wells GA, Shea B, O'Connell D,et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.Accessed June 19, 2016.
- 32. Altman DG, Bland JM. . How to obtain the confidence interval from a P value. BMJ. 2011; 343: d2090. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Green S. . Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 34. Au A, Parikh VS, Singh RP,et al. Comparison of anti-VEGF therapies on fibrovascular pigment epithelial detachments in age-related macular degeneration. Br J Ophthalmol. 2017; 101: 970– 975. [DOI] [PubMed] [Google Scholar]
- 35. Yun C, Oh J, Ahn J,et al. Comparison of intravitreal aflibercept and ranibizumab injections on subfoveal and peripapillary choroidal thickness in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 1693– 1702. [DOI] [PubMed] [Google Scholar]
- 36. Park DH, Sun HJ, Lee SJ. . A comparison of responses to intravitreal bevacizumab, ranibizumab, or aflibercept injections for neovascular age-related macular degeneration [published online ahead of print November 8, 2016] Int Ophthalmol. doi:http://dx.doi.org/10.1007/s10792-016-0391-4. [DOI] [PubMed]
- 37. Inoue M, Yamane S, Sato S,et al. Comparison of time to retreatment and visual function between ranibizumab and aflibercept in age-related macular degeneration. Am J Ophthalmol. 2016; 169: 95– 103. [DOI] [PubMed] [Google Scholar]
- 38. Hata M, Oishi A, Tsujikawa A,et al. Efficacy of intravitreal injection of aflibercept in neovascular age-related macular degeneration with or without choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014; 55: 7874– 7880. [DOI] [PubMed] [Google Scholar]
- 39. Dirani A, Ambresin A, Marchionno L,et al. Factors influencing the treatment response of pigment epithelium detachment in age-related macular degeneration. Am J Ophthalmol. 2015; 160: 732– 738.e2. [DOI] [PubMed] [Google Scholar]
- 40. Kano M, Sekiryu T, Sugano Y,et al. Foveal structure during the induction phase of anti-vascular endothelial growth factor therapy for occult choroidal neovascularization in age-related macular degeneration. Clinl Ophthalmol. 2015; 9: 2049– 2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cho HJ, Kim KM, Kim HS,et al. Intravitreal aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016; 165: 1– 6. [DOI] [PubMed] [Google Scholar]
- 42. Rasmussen A, Sander B, Larsen M,et al. Neovascular age-related macular degeneration treated with ranibizumab or aflibercept in the same large clinical setting: visual outcome and number of injections. Acta Ophthalmol. 2017; 95: 128– 132. [DOI] [PubMed] [Google Scholar]
- 43. Balaskas K, Karampelas M, Horani M,et al. Quantitative analysis of pigment epithelial detachment response to different anti–vascular endothelial growth factor agents in wet age-related macular degeneration. Retina. 2017.37: 1297– 1304. [DOI] [PubMed]
- 44. Gillies MC, Nguyen V, Daien V,et al. Twelve-month outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2016; 123: 2545– 2553. [DOI] [PubMed] [Google Scholar]
- 45. Kim JH, Lee DW, Chang YS,et al. Twelve-month outcomes of treatment using ranibizumab or aflibercept for neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 2101– 2109. [DOI] [PubMed] [Google Scholar]
- 46. Garweg JG, Gerhardt C, Kodjikian L,et al. Real-life experience with aflibercept and ranibizumab in the treatment of newly diagnosed neovascular age-related macular degeneration over 24 months. J Ocul Pharmacol Ther. 2017; 33: 567– 572. [DOI] [PubMed] [Google Scholar]
- 47. Lotery A, Griner R, Ferreira A,et al. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set [published online ahead of print July 21, 2017] Eye (Lond). doi:http://dx.doi.org/10.1038/eye.2017.143. [DOI] [PMC free article] [PubMed]
- 48. Subhi Y, Sorensen TL. . Neovascular age-related macular degeneration in the very old (≥90 years): epidemiology, adherence to treatment, and comparison of efficacy. J Ophthalmol. 2017: 2017: 7194927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee AY, Lee CS, Egan CA,et al. UK AMD/DR EMR REPORT IX: comparative effectiveness of predominantly as needed (PRN) ranibizumab versus continuous aflibercept in UK clinical practice [published online ahead of print May 6, 2017] Br J Ophthalmol. doi:http://dx.doi.org/10.1136/bjophthalmol-2016-309818. [DOI] [PMC free article] [PubMed]
- 50. Miyamoto N, Mandai M, Kojima H,et al. Response of eyes with age-related macular degeneration to anti-VEGF drugs and implications for therapy planning. Clinl Ophthalmol. 2017; 11: 809– 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wells JA, Glassman AR, Ayala AR,et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. New Engl J Med. 2015; 372: 1193– 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnston SS, Wilson K, Huang A,et al. Retrospective analysis of first-line anti-vascular endothelial growth factor treatment patterns in wet age-related macular degeneration. Adv Ther. 2013; 30: 1111– 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferreira A, Sagkriotis A, Olson M,et al. Treatment frequency and dosing interval of ranibizumab and aflibercept for neovascular age-related macular degeneration in routine clinical practice in the USA. PLoS One. 2015; 10: e0133968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reich O, Bachmann LM, Faes L,et al. Anti-VEGF treatment patterns and associated health care costs in Switzerland: findings using real-world claims data. Risk Manag Healthc Policy. 2015; 8: 55– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mueller S, Wilke T, Agostini H,et al. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016; 123: 876– 883. [DOI] [PubMed] [Google Scholar]
- 56. Legare F, Turcotte S, Stacey D,et al. Patients' perceptions of sharing in decisions: a systematic review of interventions to enhance shared decision making in routine clinical practice. Patient. 2012; 5: 1– 19. [DOI] [PubMed] [Google Scholar]
- 57. Spooner K, Hong T, Wijeyakumar W,et al. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: a systematic review with meta-analysis. Clin Ophthalmol. 2017; 11: 161– 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lazzeri S, Ripandelli G, Sartini MS,et al. Aflibercept administration in neovascular age-related macular degeneration refractory to previous anti-vascular endothelial growth factor drugs: a critical review and new possible approaches to move forward. Angiogenesis. 2015; 18: 397– 432. [DOI] [PubMed] [Google Scholar]
- 59. Seguin-Greenstein S, Lightman S, Tomkins-Netzer O. . A meta-analysis of studies evaluating visual and anatomical outcomes in patients with treatment resistant neovascular age-related macular degeneration following switching to treatment with aflibercept. J Ophthalmol. 2016; 2016: 4095852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.