Abstract
Background
Transfusion-transmitted malaria is undoubtedly a potential health hazard for blood recipients. Egypt is still on the prevention of reintroduction phase of malaria control program. Fayoum Governorate is considered one of the high-risk foci in Egypt due to its geology. However, no studies have been reported to evaluate the current status of subclinical Plasmodium infection based on sensitive molecular techniques. Moreover, screening of malaria is not listed within screening protocols of blood-borne pathogens in Fayoum blood banks.
Objective
To assess the current prevalence of subclinical Plasmodium infection among blood donors of Fayoum inhabitants for transfusion biosafety. To predict any possibility of the reemergence of malaria in the governorate and the effectiveness of malaria control measures.
Methods
A cross-sectional survey was conducted on 400 apparently healthy blood-donors in blood transfusion center of Fayoum University hospital from Jun 2012 to Jan 2013. Conventional PCR was used to detect the 18 S ssrRNA Plasmodium gene.
Results
All Fayoum inhabitants’ blood donors’ samples were negative for Plasmodium infection.
Conclusions
Current applied control, and preventive measures are valid in the context of blood transfusion biosafety in Fayoum blood banks and, therefore, the implementation of a routine malaria screening test in Fayoum blood banks is not merited at this time.
Keywords: Malaria, Fayoum, Transfusion biosafety, Plasmodium
Introduction
Malaria caused by Plasmodium parasites is one of health burden blood diseases worldwide, particularly in Africa.1,2,3 As according to the latest report from the World Health Organization (WHO) it is estimated that approximately 212 million people globally were having malaria. In 2015, most malaria cases (88%) and deaths (90%) occurred in sub-Saharan Africa.4 In Egypt, malaria is a well-known endemic infection and has been reported since ancient times. Investigators confirmed its presence in Egyptian mummies from Fayoum Governorate using the highly sensitive PCR tests.5 As reported by the WHO, Egypt is one of the countries that has interrupted malaria transmission and is still in the prevention of reintroduction stage of malaria control program.6 Although malaria has been eliminated successfully from most foci in Egypt due to an efficient national control program applied by the Ministry of Health in cooperation with the WHO,7 there is a high probability of re-emergence of the disease.8
Fayoum Governorate was assigned as a high risk of malaria foci in Egypt during the last two decades,9 due to its geology,1,10,11 the nature of its irrigation system which depends on a network of small canals,5 the presence of Quarun Lake, as well as the current changes in climate all over the country.9 All these conditions may have a direct impact on malaria transmission, facilitating the re-invasion of Anopheles arabiensis into the region which may come from other endemic areas of Africa such Sudan.12
Although no autochthonous cases of Plasmodium infection have been reported officially in Fayoum Governorate or elsewhere in Egypt since 1998, some sporadic cases from Al-Fayoum, Siwa Oasis, and Cairo governorate were reported by Zaher et al., 200713 and El-Bahnasawy et al., 2010.14 Moreover, a recent outbreak occurred in southern Upper Egypt; Aswan Governorate during May 2014.15 Consequently, this supports the idea that malaria is reemerging in the country as reported by WHO-EMRO, 2016 and other researchers.2,5,12–17
Transfusion-transmitted malaria, although uncommon, is considered a potential hazard for blood recipients.18 As post-transfusion clinical manifestations of malaria usually simulate other post-transfusion reactions, as a result of this simulation the most cases may be misdiagnosed. Moreover, donors who are subclinical carriers of Plasmodium become a reservoir for the parasite, maintaining its transmission in the community through blood donation3. Hence, high sensitivity screening tests should be done to ensure blood transfusion biosafety for the recipient.19
As far as we know, no previous studies have been reported based on sensitive molecular (PCR) techniques to assess the prevalence of Plasmodium infection in Fayoum inhabitants. Therefore, the current status of malaria is unclear in this high risk area of Egypt.20 In Egyptian blood transfusion centers, including Fayoum Governorate, screening of malaria parasite is not currently validated and not listed within screening protocols of blood-borne pathogens,21 although the risk of transfusion-transmitted malaria was still poorly documented in this high risk foci of Egypt.3
Definitive determination of the prevalence of asymptomatic Plasmodium infection in a general population and blood-donors, in particular, is like to help in updating the screening operating policy and procedures (SOP) for blood recipient safety as well as reviewing health policy decisions.21
Undoubtedly, molecular-based assays are about 100 fold greater sensitivity compared to microscopy or RDTs at detecting malaria parasite, especially in subclinical infections with detection limits as low as 5 parasites/μl.22 Our study was conducted to detect the 18S rRNA gene of Plasmodium parasite. This study aimed to assess the current prevalence of subclinical Plasmodium infection in blood donors at a public blood bank of the University hospital in Fayoum Governorate, Egypt. Furthermore, this study could provide to give accurate, current epidemiological data on malaria in Fayoum Governorate considering the changes in climate, so evaluating the effectiveness of national malaria control measures.
Material and Method
Participants and study area
A cross-sectional study aimed to estimate the prevalence of subclinical Plasmodium infections among blood donors of Fayoum resident during the expected malaria transmission season from Jun 2012 to Jan 2013. The study sample included four hundred asymptomatic apparently healthy volunteers who enrolled for blood donation in blood transfusion center of Fayoum University hospital, which is the main transfusion center in Fayoum Governorate. Donated blood was processed either in the transfusion center or in mobile blood donation campaigns which targeted different destination of the governorate. Standard donor history questionnaire was fulfilled to exclude the potential possibility of the previous infection with malaria or traveling to endemic regions in the last six months of donation. Written informed consent for the study was taken from each participant. The sample size was calculated based on the prevalence of subclinical malaria infection among blood donors (50%) and the power of study 0.8 considering alpha 0.05. The study was committed to ethical committee guidelines approved by the College of Medicine, Fayoum University.
Sample collection
Blood samples were obtained from each donor as a whole venous blood on EDTA, and as a dried blood sample by finger prick, where 2 drops of capillary blood were spotted onto Whatman®31ETCHR filter paper (Whatman, Piscataway, NJ). Filtered papers were air dried at room temperature and kept in separate clean zipper bags until further processing. All blood samples were screened as routine for ABO grouping and serological screening tests, including HCV, HBV, HIV, and Syphilis.
Malaria testing
A) Microscopic examination
Was done using thick and thin blood films stained with Giemsa according to WHO competency assessment protocol.23 Immunological rapid diagnostic test (BinaxNOW Malaria Test; Binax, Inc., Scarborough, ME, USA), was used for primary detection of malaria antigen positive cases.
B) DNA extraction
DNeasy Blood and Tissue kit, Cat no. 69504 (Qiagen, Valencia, CA, USA) was used as per manufacturer’s instructions with some modifications. Whatman filter paper was cut into strips (almost 1cm2) and put into a 1.5 mL Eppendorf tube. The concentration of extracted DNA was measured using a Nanodrop 2000 (Thermo Fisher Scientific Inc, CA, USA).
C) PCR amplification
Conventional PCR amplification was performed using 10 ng of extracted DNA with purity 1.7 to 2 in a total volume of 25 μL reaction volume using master mix (Go Taq green 2x master mix, Promega, USA) with primers (Bioneer, Korea) rPLU6 (5′-TTA AAA TTG TTG CAG TTA AAA CG 3′) and rPLU5 (5′-CCT GTT GTT GCC TTA AAC TTC-3); as described by Gal et al., 2001.24 Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with accession number NG 007073, GAPDH-F (5′-AGG TGG TCT CCT CTG ACT TCA AC-3′), GAPDH-R (5′-CGC CAG ACC CTG CAC TTT T-3′) was designed in this study with amplicon size 150 bp.
As the cycle conditions began with an initial denaturing period at 95°C for 5 min, followed by 35 cycles of 94°C for 30 s, 58°C for 1 min, and 72°C for 1 min, with a final extension for 5 min at 72°C.23 PCR amplicon was separated on 1% agarose gel (Agarose, LE, Analytical Grade, Promega, USA). The presence of the 1200 amplicon was detected in the gel under UV light after its staining with Invitrogen TM SYBER TM Safe.
D) Statistical methods
Data were collected, coded and analyzed using IBM SPSS software, version 23 software (IBM, Armonk, NY, USA). The result of simple descriptive analysis of numbers and percentages for qualitative data were tabulated. Graphs were produced using Excel software.
Results
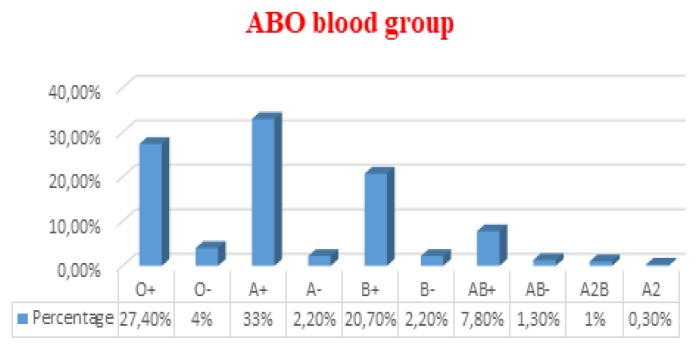
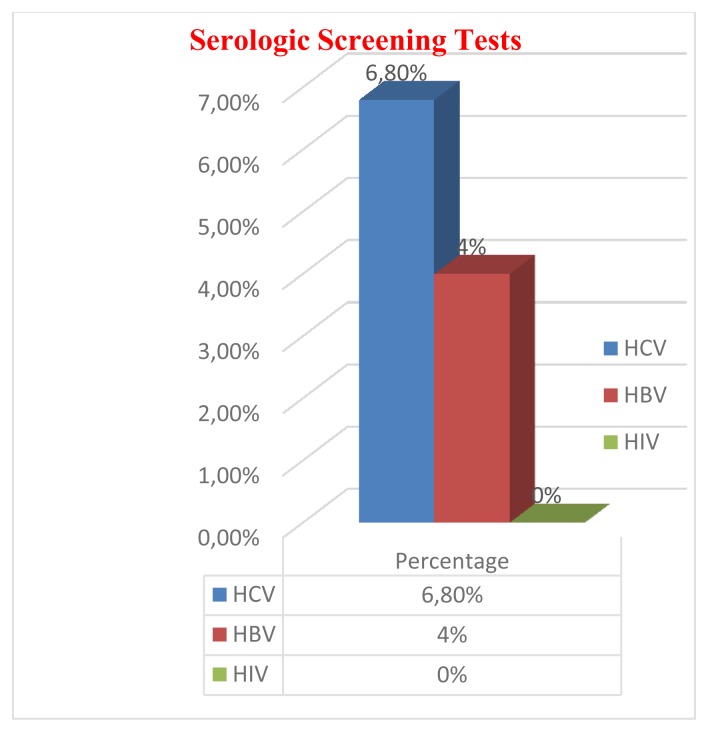
The descriptive analysis of data revealed that mean age of participants was 29.88 ±7.6 years. The general proportion for male and female was (91.7%) and (8.3%) respectively. The most common blood group was A+ (33%) and least common was A2 (0.3%). In the Rh blood group, Rh positive donors (90.3%) were much more than Rh-negative donors (9.7%) (Figure 1). Prevalence of HCV, HBV, and HIV among blood donors was presented in Figure 2. Both microscopically and rapid immunological assays were negative for malaria in all blood samples.
Figure 1.
Distribution of ABO blood groups among blood donors.
Figure 2.
Prevalence of HCV, HBV and HIV among blood donors.
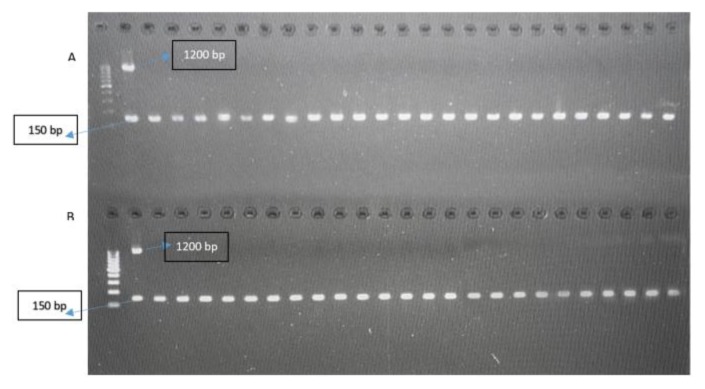
PCR results on Agarose gel showed that all samples were negative for 18 S ssrRNA Plasmodium gene. For PCR validation, malaria positive control was used for each run which gives band at 1200 bp. In addition, a housekeeping gene (GAPDH), which was used as an internal control for PCR negative samples for testing the integrity of DNA, revealed clear positive PCR band at 150 bp (Figure 3 and 4).
Figure 3.
PCR results on Agarose gel (Gel A and B) showed; (Lane 1): 100 bp ladder, (EZ Load TM 100 bp Molecular Ruler-BioRad). (Lane 2): Malaria positive control, (1200 bp). All the samples were malaria negative as shown from lane 3 up to lane 26 in both gels, but were positive (150 bp) GAPDH internal control.
Figure 4.
PCR results on Agarose gel (Gel A and B) showed; (Lane 1): 100 bp ladder, (EZ Load TM 100 bp Molecular Ruler-BioRad). (Lane 2): Malaria positive control, (1200 bp). All the samples were malaria negative as shown from lane 3 up to lane 26 in both gels, but were positive (150 bp) GAPDH internal control.
Discussion
Blood bank biosafety, especially in endemic malaria regions, is one of the most important targets for controlling maintenance of the Plasmodium reservoir of malaria transmission.19
Many recent reports seemed to suggest that malaria is reemerging in Egypt,16 due to current climate changes,9 and the recently discovered malaria outbreak in a specific region of the country.2 Recently, the Military Fever Hospital research group recorded that 12.5% out of 36 malaria-infected cases were from Fayoum governorate.25 Similarly, many studies have reported sporadic malaria-infected cases in Fayoum.5,13–17,25
In accordance, Kamel et al. who assessed the prevalence of malaria in Fayoum governorate among patients admitted Fayoum fever hospitals using rapid diagnostic tests (RDTs- ABON PLUS), pointed out that malaria control program in South Egypt – including the Fayoum Governorate – should be strengthened to prevent reintroduction of malaria. Kamel et al. further recommended the need to evaluate the validity of the RDT results of their study using more sensitive technique.8
Several studies support the higher sensitivity of PCR assay compared to microscopy and RDTs to roll out infection and to monitor the success of control programs in low transmission areas.26 Hence, a study highlighted the urgency to use a sensitive PCR based molecular assay to determine the current malaria status in the high risk areas of Egypt.20
Many molecular assays are available for detection of Plasmodium pathogens. However, it is challenging to select the most ideal and appropriate method for screening of blood donors, that can screen a large number of samples with a higher sensitivity and easily performed technique.27 Since using PCR based pooling strategy reduces the number of assays needed when screening for infectious diseases,10 individual PCR testing of dried blood spot samples allows pathogen detection despite its higher efforts and cost.27 Therefore, in the present study, PCR of dried blood spot samples was used, due to its sensitivity, to validate the results obtained from microscopic evaluation and rapid immunologic assay for malaria antigen.
Regarding the sensitivity of detection, Bharti et al. (2009) who assessed PCR based pooling strategy with a pool size of 10, reported six positives cases out of 200 ‘negative’ malaria samples using microscopic detection.22 Furthermore, Taylor et al. (2010) reported 35 positive samples out of 1,092 negative cases with microscopy.28 Lima GF et al. (2016) reported in a study conducted to assess four different molecular assays in detecting Plasmodium pathogens that the sensitivity of pooled samples is not ideal (86.7%) mainly when used for blood transfusion screening, and more efforts still need to improve the assays used for detection low-density parasitemia27. Another study pointed out that PCR using dried blood spot from filter paper was able to detect almost one-fifth and one-third of Plasmodium presences that were missed by RDT and microscopy, respectively.29
In contrast to the previously mentioned studies which were claimed the high probability of reemergence of malaria in Upper Egypt (including Fayoum), the present study’s results made it clear by using PCR based assay for detecting 18S ssrRNA Plasmodium gene. The results demonstrated no subclinical malarial infection in the sample group as no Plasmodium DNA genome was detected in any of the donors’ blood samples. The results obtained in our study were confirmed by using an internal control gene (GAPDH) to check the quality of DNA extraction and to assure that there were not misleading results due to the poor DNA quality or no DNA at all.
In response to recent studies suggesting the possibility of re-emergence of malaria in the area,5,9,13–17 the results of our study support the idea that all cases of malaria previously reported in the Fayoum governorate8,25 are imported from outside.
In the present study, the prevalence of blood groups O+, O−, A+, A−, B+, B−, AB+, AB−, A2B, and A2 among donors was 27.4 %, 4%, 33%, 2.2%, 20.7%, 2.2%, 7.8%, 1.3%, 1%, and 0.3% respectively. Similar prevalence have been reported in another study carried out all over 26 governorates of Egypt,30 as the frequency of groups O+, O−, A+, A−, B+, B−, AB+, and AB− was 27.5%, 2.3%, 33.6%, 2.7%, 22%, 1.8%, 9.3%, 0.7% respectively.
Conclusions
Currently applied control and preventive measure are effective in the context of blood transfusion biosafety in Fayoum blood banks and, therefore, the implementation of a routine malaria screening test in Fayoum blood banks is not merited at this time.
In light of the results of our study, we may assume that the current control and preventive measures applied under the umbrella of the WHO and Egyptian MOH were able to control malaria, at least during the time of the study, in the Fayoum Governorate. However further comprehensive study is recommended to screen and stratify the results according to age to substantiate the assume eradication of the infection. Regular monitoring is still needed.
Acknowledgments
The authors thank all staffs of Fayoum University blood bank for data and sample collection. Sincere appreciation for Prof. Hala Elmorshedy, Professor of public health, hiph, Alexandria University for her valuable time and comments.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.WHO. Africa Malaria Report 2003. Geneva (Switzerland): World Health Organization; 2004. http://apps.who.int/iris/bitstream/10665/67869/1/WHO_CDS_MAL_2003.1093.pdf. [Google Scholar]
- 2.WHO- EMRO. Vector- borne diseases. Eastern Mediterranean Regional Office: World Health Organization; 2016. http://www.emro.who.int/egy/programmes/neglected-tropical-diseases.html. [Google Scholar]
- 3.Noubouossie D, Tagny CT, Same-Ekobo A, Mbanya D. Asymptomatic carriage of malaria parasites in blood donors in Yaoundé. Transfusion Medicine. 2012 Feb 1;22(1):63–7. doi: 10.1111/j.1365-3148.2011.01121.x. https://doi.org/10.1111/j.1365-3148.2011.01121.x. [DOI] [PubMed] [Google Scholar]
- 4.WHO. World Malaria Report 2016. Geneva (Switzerland): World Health Organization; 2016. http://www.who.int/mediacentre/factsheets/fs094/en/ [Google Scholar]
- 5.Lalremruata A, Ball M, Bianucci R, Welte B, Nerlich AG, Kun JF, Pusch CM. Molecular identification of falciparum malaria and human tuberculosis co-infections in mummies from the Fayum depression (Lower Egypt) PloS one. 2013 Apr 2;8(4):e60307. doi: 10.1371/journal.pone.0060307. https://doi.org/10.1371/journal.pone.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO-Global Malaria Programme. World Malaria Report 2012. Geneva (Switzerland): World Health Organization; 2012. http://www.who.int/malaria/publications/ [Google Scholar]
- 7.Hassan AN, Kenawy MA, Kamal H, Abdel Sattar AA, Sowilem MM. GIS-based prediction of malaria risk in Egypt. Eastern Mediterranean Health Journal. 2003;9:548–558. [PubMed] [Google Scholar]
- 8.Kamel MM, Attia SS, Emam GD, Al Sherbiny NA. The Validity of Rapid Malaria Test and Microscopy in Detecting Malaria in a Pre-elimination Region of Egypt. Scientifica. 2016 Mar;21:2016. doi: 10.1155/2016/4048032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotfy WM. Climate change and epidemiology of human parasitosis in Egypt: A review. Journal of advanced research. 2014 Nov 30;5(6):607–13. doi: 10.1016/j.jare.2013.06.009. https://doi.org/10.1016/j.jare.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenawy MA. Anopheles sergentii (Diptera: Culicidae): seasonal variation in the development rates of immatures from El Faiyum and Siwa Oasis, Egypt. J Egypt Soc Parasitol. 1995;25:257–68. [PubMed] [Google Scholar]
- 11.Morsy TA, El Kadry AA, Salama MMI, Sabry AA, El Sharkawy IMA. Studies on bionomics and vector competence of adult anopheline mosquitoes in El Faiyum Governorate, Egypt. J Egypt Soc Parasitol. 1995;25:213–44. [PubMed] [Google Scholar]
- 12.Fuller DO, Parenti MS, Hassan AN, Beier JC. Linking land cover and species distribution models to project potential ranges of malaria vectors: an example using Anopheles arabiensis in Sudan and Upper Egypt. Malaria journal. 2012 Aug 6;11(1):1. doi: 10.1186/1475-2875-11-264. https://doi.org/10.1186/1475-2875-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaher T, Ahmadi M, Ibrahim A, El-Bahnasawy M, Gouda H, Shahat SA. Malaria in Egypt, Saudi Arabia and Yemen: a clinical pilot study. Journal of the EgyptianSociety of Parasitology. 2007;37:969–976. [Google Scholar]
- 14.El-Bahnasawy MM, Saleh NM, Khalil MF, Morsy TA. The impact of three anopheline mosquito species in Toshka, on the introduction of chloroquine resistant P. falciparum to Egypt. J Egypt Soc Parasitol. 2011 Dec;41(3):573–92. [PubMed] [Google Scholar]
- 15.WHO. Egypt: vector-borne diseases. Geneva (Switzerland): World Health Organization; 2014. http://www.emro.who.int/egy/programmes/neglected-tropical-diseases.html. [Google Scholar]
- 16.Kenawy MA. Review of Anopheles Mosquitoes and Malaria in Ancient and Modern Egypt. Journal of Mosquito Research. 2015 Feb 15;5(4) https://doi.org/10.5376/jmr.2015.05.0004. [Google Scholar]
- 17.Dahesh SM, Bassiouny HK, El-Masry SA. Malariometric parasitological survey in El-Fayoum Governorate, Egypt. Journal of the Egyptian Society of Parasitology. 2009 Apr;39(1):213–25. [PubMed] [Google Scholar]
- 18.Kazemi B, Najari M, Saneimoghaddam E, Bandehpour M, Seyed N. Detection of Plasmodium parasites in healthy blood donors using polymerase chain reaction. Archives of Iranian Medicine. 2005 Apr 1;8(2):135–8. [Google Scholar]
- 19.Maselli LM, Levy D, Laporta GZ, Monteiro AM, Fukuya LA, Ferreira-da-Cruz MF, Daniel-Ribeiro CT, Dorlhiac-Llacer PE, Sallum MA, Bydlowski SP. Detection of Plasmodium falciparum and Plasmodium vivax subclinical infection in non-endemic region: implications for blood transfusion and malaria epidemiology. Malaria journal. 2014 Jun 6;13(1):1. doi: 10.1186/1475-2875-13-224. https://doi.org/10.1186/1475-2875-13-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdullah Malaria in Egypt:a perplexing behavior with no successful surveillance. Infectious diseases. 2015 [Google Scholar]
- 21.Gelaw B, Mengitsu Y. The prevalence of HBV, HCV and malaria parasites among blood donor in Amhara and Tigray regional states. Ethiopian Journal of Health Development. 2008;22(1):3–7. https://doi.org/10.4314/ejhd.v22i1.10056. [Google Scholar]
- 22.Bharti AR, Letendre SL, Patra KP, Vinetz JM, Smith DM. Malaria Diagnosis by a Polymerase Chain Reaction–Based Assay Using a Pooling Strategy. The American journal of tropical medicine and hygiene. 2009 Nov 1;81(5):754–7. doi: 10.4269/ajtmh.2009.09-0274. https://doi.org/10.4269/ajtmh.2009.09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doni NY, Zeyrek FY, Seyrek A. Detection of Plasmodium using filter paper and nested PCR for patients with malaria in Sanliurfa, in Turkey. Malaria journal. 2016 May 28;15(1):1. doi: 10.1186/s12936-016-1334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gal S, Fidler C, Turner SU, LO YD, Roberts DJ, Wainscoat JS. Detection of Plasmodium falciparum DNA in plasma. Annals of the New York Academy of Sciences. 2001 Sep 1;945(1):234–8. doi: 10.1111/j.1749-6632.2001.tb03891.x. https://doi.org/10.1111/j.1749-6632.2001.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 25.El-Bahnasawy MM, Dabbous H, Morsy TA. Imported malaria as a threat to Egypt. Journal of the Egyptian Society of Parasitology. 2010 Dec;40(3):773–88. [PubMed] [Google Scholar]
- 26.Hsiang MS, Lin M, Dokomajilar C, Kemere J, Pilcher CD, Dorsey G, Greenhouse B. PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children. Journal of clinical microbiology. 2010 Oct 1;48(10):3539–43. doi: 10.1128/JCM.00522-10. https://doi.org/10.1128/JCM.00522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Castro Lima GF, Lucchi NW, Silva-Flannery L, Macedo-de-Oliveira A, Hristov AD, Inoue J, de Jesus Costa-Nascimento M, Udhayakumar V, Di Santi SM. Still Searching for a Suitable Molecular Test to Detect Hidden Plasmodium Infection: A Proposal for Blood Donor Screening in Brazil. PloS one. 2016 Mar 9;11(3):e0150391. doi: 10.1371/journal.pone.0150391. https://doi.org/10.1371/journal.pone.0150391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor SM, Juliano JJ, Trottman PA, Griffin, Landis SH, Kitsa P, Tshefu AK, Meshnick SR. High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol. 2010;48:512–519. doi: 10.1128/JCM.01800-09. https://doi.org/10.1128/JCM.01800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matangila JR, Lufuluabo J, Ibalanky AL, da Luz RA, Lutumba P, Van Geertruyden JP. Asymptomatic Plasmodium falciparum infection is associated with anaemia in pregnancy and can be more cost-effectively detected by rapid diagnostic test than by microscopy in Kinshasa, Democratic Republic of the Congo. Malaria journal. 2014 Apr 2;13(1):1. doi: 10.1186/1475-2875-13-132. https://doi.org/10.1186/1475-2875-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eissa SA, Abdel Meguid LM, Ebeid SM, Abou Elfetouh RM, Abdel Moneim GM. National Cancer Institute experience in healthy Egyptian blood donors as regards blood group frequencies and seroprevalence of hepatitis b virus, hepatitis C Virus & HIV: 10 year evaluation. J Egypt Natl Canc Inst. 2007 Mar;19(1):71–6. [PubMed] [Google Scholar]