Abstract
Introduction
In a developing country like Algeria, such expensive therapy is not available. Alternative approaches are needed to help these adult. In Algeria ‘imatib’ (CIPLA-India) was introduced in 2006; but no study has been published yet in the North Africa region regarding response and outcome of this copy in CML patients. The goal of this multicenter study is to characterize newly adult CML in the western region of Algeria and to assess the effectiveness and safety of imatib (IM, copy) as frontline therapy for patients with CML.
Patients and Methods
The study was carried out in 7 hematology centers in the western Algeria. Patients, who were diagnosed to be suffering from CML between January 1st, 2007 and December 31st, 2014 were selected for data analysis. All patients received a copy preparation, consisting of the alpha crystal form of imatinib, (IM, copy) at an oral dose of 400 mg daily and monitored for tolerance and side effects while on therapy.
Results
Between January 2007 and December 2014, 355 patients with CML were treated with imatib (Copy). The median follow- up of the study was 46 months (range: 13–107 months). Complete hematological response (CHR) was seen in 83% of patients within 3 months. According to the Sokal score, 72% patients with low, 78% with intermediate and 69% with high risk disease achieved a CHR in 3 months (p=0.26) and according to the EUTOS score, 81% of patients with low and 70% with high risk disease achieved a CHR in 3 months (p=0.08). The major molecular response (MMR) at six months (M6), M9, M12, M18 and M24 was 21%, 38%, 35%, 51% and 67% respectively and 34% of patients achieved a complete molecular response (CMR). The projected 5-year overall survival (OS) rate was 83%. Side effects of imatib (copy) in this study were similar to those reported previously for the entire imatinib mesylate treatment study and only 8% of patients were intolerant to imatib (copy) and treated with a second generation of BCR-ABL inhibitor.
Conclusion
This study reflects real world experience treating patients with CML in a developing country and thus sheds light on differences in this population compared to Western countries. In conclusion, imatib (copy) is effective and safe in treating patients with CML in chronic phase and proves to have a durable outcome. To our knowledge this is the first study reporting the response to imatib (copy) in an Algerian population.
Keywords: Chronic Myeloid, Leukemia, Imatinib, Generic Imatib
Introduction
Chronic myeloid leukemia (CML) is a malignant disorder of the stem cell due to reciprocal balanced translocation of genetic material between the long arms of chromosomes 9 and 22 t (9; 22) (q34; q11).1 The translocation causes the production of a new hybrid gene (BCR/ABL) that codes for a 210 Kb cytoplasmic protein (P210), which by autophosphorylation activates some signaling pathways involved in cell proliferation, maturation, apoptosis, and adhesion, leading to the malignant cell transformation.2 Imatinib mesylate (Glivec®/Gleevec®) is the standard first-line therapy for the treatment of CML due to its high hematologic, cytogenetic, and molecular response rates and favorable long-term safety profile.3 It has been some years since TKI were introduced in CML therapeutic strategy either in case of intolerance or resistance to Imatinib or as a first line drug when it was observed that it has a more rapid and profound molecular response when compared to Imatinib.4 More recently a third generation of TKI has been available in case of intolerance or resistance to TKI of the second generation and especially for patients who developed the resistance with a mutation T315I.5 Due to the fundamental role of Imatinib in first-line therapy of CML, as well as the high cost of Imatinib, which is not affordable in some countries, a copy preparation, consisting of the alpha crystal form of imatinib, has become commercially available under the name ‘imatib’ (CIPLA-India). It is currently available at a markedly reduced price in several countries. The manufacturer of imatib (IM, copy) lists the product as being ‘comparable’ to imatinib mesylate.6 However, the safety and efficacy of these molecules have not been widely assessed and for which patient data are limited, and the safety and efficiency of this drug have not been demonstrated yet in a randomized clinical trial.
In Algeria ‘imatib’ (CIPLA-India) was introduced in 2006, but no study has been published yet in the North Africa region regarding the response and the outcome of this copy in CML patients. The goal of this multicenter study is to characterize newly adult CML in the western region of Algeria and to assess the effectiveness and safety of imatib as frontline therapy for patients with CML.
Patients and Methods
In Algeria, the incidence of CML was 0.53/100,000 inhabitants with a prevalence of 1030 cases in 2014. The median age is 48 years (03–90) with a peak incidence in the age group (45–49 years) and slight male predominance (sex ratio: 1,2).
Patients’ characteristics
The study was carried out in all the 7 hematological services of university hospitals, in the western Algeria and data were collected using electronic medical records from patients’ clinic visits. All patients aged over 15 years with de novo CML were included.
It is a longitudinal study, multicentric and retrospective. Patients, who were diagnosed to be suffering from CML between 1st January 2007 and 31st December 2014, were selected for data analysis. In all patients, the diagnosis of CML was confirmed by morphologic review of peripheral blood (PB) and by RT-PCR based BCR-ABL analysis (Applied Biosystems 7500 Real-time PCR system).7 Staging and evaluations of response were determined according to the current European LeukemiaNet (ELN) recommendations.8 The prognosis of CML patients in chronic phase was determined by either Sokal and/or EUTOS prognostic scoring systems at initial presentation, using European LeukemiaNet calculator.9 Patients with acceleration and blastic phase were not included in our study. All patients received a copy preparation, consisting of the alpha crystal form of imatinib, (Imatib) at an oral dose of 400 mg daily and monitored for tolerance and side effects according to the ELN recommendations adapted to our conditions and capabilities in Algeria. Doses were reduced in the presence of grade 3–4 thrombocytopenia or grade 3–4 neutropenia. Wherever it was possible the dose was maintained above 300 mg/day. Patients were treated as long as they continued to respond.
Response assessment
All patients were assessed for response to treatment by weekly physical examination, full blood count, and biochemistry for the first 6-weeks of treatment and were assessed every 3 months during their follow-up. During the treatment, quantitative, real-time PCR (RQ-PCR) for the determination of BCR/ABL1 transcripts level using the international scale, has been performed every 3 months until achievement of an MMR, then every 3 to 6 months. The complete hematologic response (CHR) at 03 months and molecular response at 03, 06, 12, 18, 24 months and more according to capabilities. At 03 months and/or 6 months we looked for a BCR/ABL1 rate <10%. At 12 months we looked for a major molecular response (MMR), defined by BCR/ABL1 ratio lower than 0,1% according to the ELN. A ratio between 0,1 to 1% is considered a good response according to GAT-LMC (the CML study Algerian group), so the Imatinib treatment is continued.
Statistical Analysis
The statistical analysis used the calculation of averages and the Khi2 test. Overall survival (OS) was calculated from the time of diagnosis to the date of death or last follow-up. The Kaplan–Meier method was used to estimate survival rates and the log-rank test to determine differences between subgroups. For all tests, a p-value of less than 0.05 was considered statistically significant. Statistical calculations were performed using the software package SPSS 20 (SPSS, Inc., Chicago, IL). The median follow-up of patients in December 2014 is 46 months (13–107 months).
Results
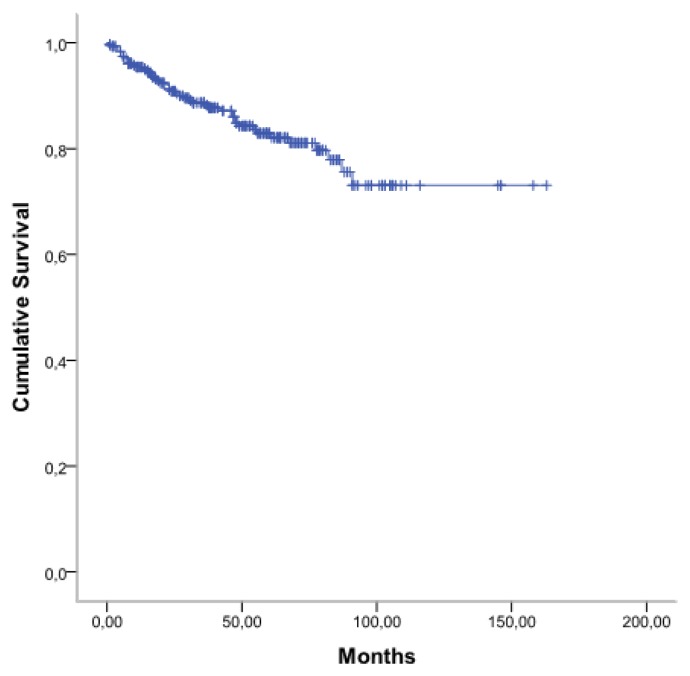
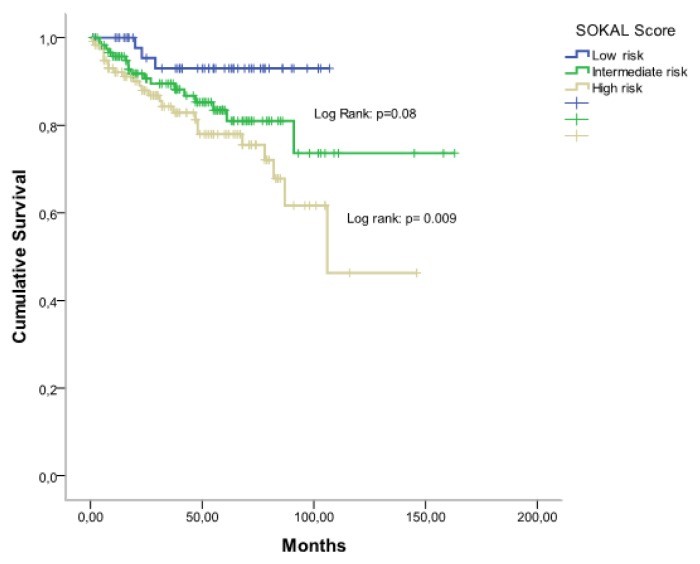
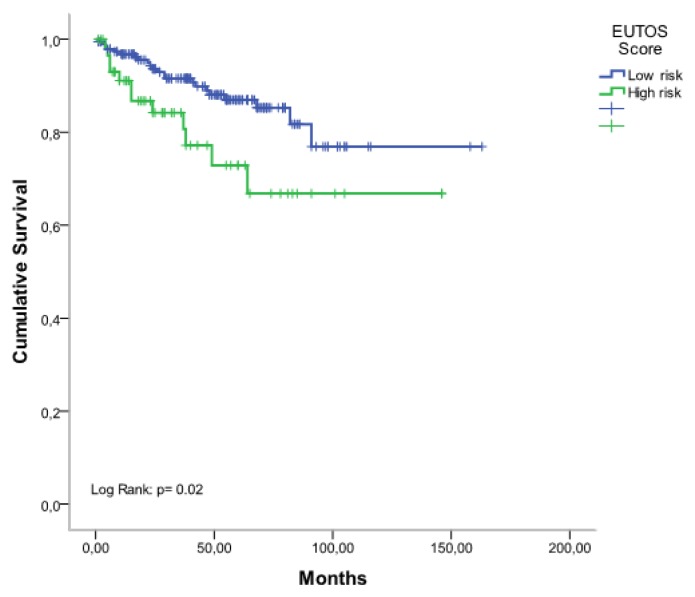
From January 2007 to December 2014, 387 patients with CML were treated with imatib. Among them 355 patients are evaluable. The clinical characteristics of patients are shown in Table 1. The median age of patients was 48 years (range: 16–85 years), 182 patients were male, and 173 were female (sex ratio= 1,05). The median time from diagnosis to treatment was 3 months (0.1–4). Splenomegaly was the most common clinical feature present in 86% of patients. At diagnosis, the median number of white blood cells (WBC) was 346 G/L (range: 78–588 G/L). The prognostic classification, according to the Sokal score, found a low risk in 18%, intermediate risk in 40% and a high risk in 42%. The Eutos score is less than 87 in 75,5% and more than 87 in 24,5%. A CHR at 03 months was found in 295 patients (83%) without any significant differences considering either the Sokal groups [72% in low-risk group, 78% in intermediate risk group, and 69% in high-risk group (p=0.26)] or the EUTOS score [81% in low-risk group, and 70% in high-risk group (p=0.08)]. A major molecular response (MMR) was achieved at six months (M6), M9, M12, M18, and M24 in 21%, 38%, 35%, 51% and 67% respectively and 34% of patients achieved a complete molecular response (CMR). The 5-year overall survival (OS) rate was 83% (Figure 1). According to Sokal groups, we observed a significant difference in OS with at 5-years, 93% for low-risk group, 84% for intermediate risk group and 78% in high-risk group (p = 0.009) (Figure 2). When patients were stratified according to EUTOS risk classification, a significant difference in OS rates was observed (p=0.02), and the 5-year OS rates for low, and high-risk groups were 90% and 74% respectively (Figure 3).
Table 1.
Pretreatment characteristics of the study population with CML.
| Patients number, N | 355 |
|---|---|
| Gender male, N (%) | 182 (51) |
| Age, years, median (range) | 48 (16–85) |
| Spleen, cma, median (range) | 13.0 (0–29.0) |
| Palpable spleen, N (%) | 305 (86) |
| Blastsb, %, median (range) | 3.0 (0–12.5) |
| Basophilsb, %, median (range) | 3.7 (0–13.5) |
| PLT count, G/L; median (range) | 393 (98–1.360) |
| Hemoglobin, g/dl, median (range) | 11.5 (6.0–16.8) |
| WBC count, G/L; median (range) | 346 (78–588) |
| Sokal score, (%) | |
| Low | (18) |
| Intermediate | (40) |
| High | (42) |
| EUTOS score, (%) | |
| Low | (75.5) |
| High | (24.5) |
Maximum distance below the costal margin, assessed by manual palpation.
Percentage in peripheral blood.
Figure 1.
Overall survival of patient with CML Imatib copy therapy.
Figure 2.
Overall survival according to Sokal scoring system.
Figure 3.
Overall survival according to EUTOS scoring system.
Side effects of imatib (copy) in this study are shown in Table 2, and no adverse effects related deaths have occurred. At a median follow-up duration of at least 48 months (12–84 months), 81% of patients are alive and are still taking imatib, 10% of patients relapsed among them 50% because of low adherence, 42% because of resistance and only 8% developed intolerance to imatib. 9% of patients died after developing blastic phase disease. 6% of patients discontinued their follow-up, and 4% of patients died due to other reasons.
Table 2.
Incidence of grade 3–4 side effects with imatib copy therapy.
| Toxicity | Grades 3–4 (%) |
|---|---|
| Non Hematologic toxicities | |
| Muscle cramps | (8) |
| Nausea and diarrhea | (6) |
| Skin rash | (13) |
| Bone or joint aches | (27) |
| Liver toxicity | (10) |
| Hematologic toxicities | |
| Neutropenia | (16) |
| Thrombocytopenia | (25) |
| Anemia | (3) |
Discussion
CML constitutes the most common myeloproliferative disorder in Algeria. The incidence of CML was 0.53/100,000 inhabitants with a prevalence of 1030 cases in 2014 in the country, with 472 newly cases/year.10 The median age is 48 years with a peak incidence in the age group (45–49 years) and slight male predominance (sex ratio: 1,2).10 The median age of patients with CML in Algeria and other African countries with a similar demographic pattern is over 40 years and lower than that reported in Europe and United States.11–12
Our multicenter study of patients with newly diagnosed CML is unique in Northern Africa. Regarding the clinical and biological feature, CML of Algerian patients seems to be frequently aggressive with anemia and a massive splenomegaly associated in a majority of cases with a high-risk Sokal.
After ITK therapy, results were very well known and described regarding Glivec®/Gleevec® used in multiple clinical trials showing cumulative OS rates of 86%, after 7-years of follow-up.3
In low-income countries like Algeria, such expensive treatment is not available, and physicians in charge of adult CML patients are obliged to use a copy form, which could appear as less effective therapy in this setting. To our knowledge, there are only a few data regarding generic forms or imatib for Algerian patients with CML. A.H. Goubran,13 Z. Chouffai,14 I.A. Asfour15 and M. Mattar16 published only case reports and showed outcomes failure with Imatib copy. Ostojic A, showed that when taken at equivalent doses, imatinib generics are bioequivalent, comparable in clinical efficacy and have the potential for substantial savings in the treatment cost for CML.18 In a prospective, multicenter clinical trial to evaluate the early clinical efficacy and safety of a generic imatinib in treating patients with CP of CML in China, the authors showed that among 107 patients at 3-month, the CHR rate was 98.1% (105/107). The BCR-ABL transcript was ≤10% in 77/106 patients (72, 6%), 11 of them (10, 4%) achieved MMR (BCR-ABL≤0,1%). At 6-month, the CHR rate was 100%; BCR-ABL was ≤1% in 68,5%, and 33% of them achieved MMR. Grade 3 leukopenia, thrombocytopenia and anemia rates were 19,5%, 23% and 13,8%, respectively. No patient experienced grade 4 non-hematologic toxicity. No adverse effects related deaths have occurred.18
Our study is the first study concerning a large cohort of patients receiving Imatib for CML and which has analyzed the safety, efficacy, OS at short, medium and long-term. With a median follow-up of 46 months (range: 13–107), we found 83% of CHR with 67% of MMR at 2 years, and 34% of CMR. The IRIS trial at 7-years has demonstrated better overall survival rate of 86%.19 At 5-years and 9-years, OS in our study was 83% and 67% respectively. Respect to literature data we observed a larger group of patients (17%) who have never achieved a CHR probably related to the existence of higher proportion (42%) with high-risk Sokal score and it was demonstrated that there was a relationship between prognosis and disease response. In parallel, the delayed diagnosis is one of the arguments in favor of the disease extension.
Side effects of imatib in our study were similar to those reported previously for the imatinib mesylate treatment study and only 8% of patients showed intolerance to imatib and switch to the second generation of ITK. There was a previous report from Canada with similar conclusions about the efficacy and tolerability of generic imatinib, although they used a different source of generic imatinib from our study.20
Scarce compliance in CML patients treated with BCR-ABL inhibitors is common and associated with critical outcomes. Poor adherence to therapy was associated with a negative impact on both clinical and economic outcomes.21 In our study, more than 15% of patients presented poor adherence to CML treatment, and they had a lower CHR response at 3 months and MMR at 6 months. The estimated 5-year OS in our patients was comparable to the 72% reported in the study from Côte d’Ivoire22 and the 80% in the study from Nepal,23 whom patients were treated with imatinib mesylate (Gliveec®).
Conclusions
This study reflects a real-world experience by treating patients with CML in a developing country and thus sheds light on differences in this population compared to Western countries. A higher proportion of patients were diagnosed at later stages of CP compared to reports from Western countries, and that may impact response rates seen with frontline imatib. Imatib (copy) is effective and safe in treating patients with CML in chronic phase and proves to have a durable outcome. To our knowledge, this is the first study reporting the response to imatib (copy) in an Algerian population.
Acknowledgments
We thank Pr Bouhass Rachid, Dr. Taibi Karima, Dr. Siali Nadia, Dr. Benlazar Mohamed, Dr. Benzineb Brahim, and Dr Yachkour Toufik for collection and assembly database.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–350. doi: 10.1016/S0140-6736(07)61165-9. https://doi.org/10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. https://doi.org/10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien SG, Guilhot F, Goldman JM, et al. International randomized study of interferon versus STI571 (IRIS) 7-year follow-up: sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib (IM) Blood (ASH, Annual Meeting Abstract) 2008 Abstract 186. [Google Scholar]
- 4.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. https://doi.org/10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 5.O’Hare T, Shakespeare WX, Zhu X, et al. AP24534, a pan-BCR- ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. https://doi.org/10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipla Imatib Product Website. [Accessed November, 2008]. www.cipla.com.
- 7.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17:2318–57. doi: 10.1038/sj.leu.2403135. https://doi.org/10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 8.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.25.0779. [published online ahead of print November 2, 2009]. https://doi.org/10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baccarani M. Calculation of relative risk of CML patients [online] 2010. [last changed 2010/11/08]. Available from: http://www.leukemia-net.org/content/leukemias/cml/cml_score/
- 10.Djouadi K, Abdennebi N, Harieche F, Nacer R Ahmed, Hamladji RM, Bouchakour A, et al. Epidemiological approach of chronic myeloid leukemia. Algerian-tunisian study. Blood. 2016;128:5440. http://www.bloodjournal.org/content/128/22/5440. [Google Scholar]
- 11.Fleming AF, Menendez C. Blood. In: Parry E, Godfrey R, Mabey D, Gill G, editors. Principles of Medicine in Africa. Vol. 78. Cambridge University Press; Cambridge, UK: 2004. [Google Scholar]
- 12.Groves FD, Linet MS, Devesa SS. Patterns of occurrence of the leukaemias. European Journal of Cancer Part A. 1995;31(6):941–949. doi: 10.1016/0959-8049(95)00024-0. https://doi.org/10.1016/0959-8049(95)00024-0 [DOI] [PubMed] [Google Scholar]
- 13.Goubran Hadi Alphonse. Failure of a non-authorized copy product to maintain response achieved with imatinib in a patient with chronic phase chronic myeloid leukemia: a case report. Journal of Medical Case Reports. 2009;3:7112. doi: 10.1186/1752-1947-3-7112. https://doi.org/10.1186/1752-1947-3-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chouffai Z. Hematologic Relapse after? 2 Years on a Non-Authorized Copy Version of Imatinib in a Patient with Chronic Myeloid Leukemia in Chronic Phase: A Case Report. Case Rep Oncol. 2010;3:27. doi: 10.1159/000319150. https://doi.org/10.1159/000319150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asfour IA, Elshazly SA. Changing therapy from Glivec® to a “copy” imatinib results in a worsening of chronic myeloid leukemia disease status: two case reports. Cases Journal. 2009;2:9342. doi: 10.1186/1757-1626-2-9342. https://doi.org/10.1186/1757-1626-2-9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattar M. Failure of copy Imatib (CIPLA, India) to maintain hematologic and cytogenetic responses in chronic myeloid leukemia in chronic phase. Int J Hematol. 2010 Jan;91(1):104–6. doi: 10.1007/s12185-009-0431-1. https://doi.org/10.1007/s12185-009-0431-1. [DOI] [PubMed] [Google Scholar]
- 17.Ostojic A, Sertic D, Roncevic P, Peric Z, Granic P, et al. Comparison of Branded and Generic Imatinib Plasma Concentrations in Patients With Chronic Myelogenous Leukemia: Unicentric Study. Clin Lymphoma Myeloma Leuk. 2016 Aug;16(8):472–6. doi: 10.1016/j.clml.2016.04.003. https://doi.org/10.1016/j.clml.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q, Zhao D, Jin J, Wu D, Meng F, Hu J, et al. A prospective, multi-centre clinical trial to evaluate the early clinical efficacy and safety of a generic imatinib in treating patients with chronic phase of chronic myelogenous leukemia. Zhonghua Xue Ye Xue Za Zhi. 2015 Aug;36(8):651–5. doi: 10.3760/cma.j.issn.0253-2727.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA. IRIS Investigators: Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. https://doi.org/10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 20.Kang M, Xenocostas A, LazoLangner A, ChinYee IH, Howson-Jan K, Abouzeenni M. Impact of Transition to Generic Imatinib in the Molecular Response Among Patients with Chronic Myeloid Leukemia. Blood. 2014;124:5527. [Google Scholar]
- 21.Kapoor J, Agrawal N, Ahmed R, Sharma SK, Gupta A, Bhurani D. Factors Influencing Adherence to Imatinib in Indian Chronic Myeloid Leukemia Patients: A Cross-Sectional Study. Mediterr J Hematol Infect Dis. 2015;7(1):e2015013. doi: 10.4084/MJHID.2015.013. DOI: https://doi.org/10.4084/mjhid.2015.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koffi KG, Nanho DC, N’dathz E, Kouehion P, Dissieka R, Attia A, et al. The Effect of Imatinib Mesylate for Newly Diagnosed Philadelphia Chromosome-Positive, Chronic-Phase Myeloid Leukemia in Sub-Saharan African Patients: The Experience of Côte d’Ivoire. Advances in Hematology. 2010:6. doi: 10.1155/2010/268921. Article ID 268921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kayastha Gyan K, Gurung Padma, Acharya Paras K, et al. Patan hospital experience in treating philadelphia chromosome/BCR-ABL1 positive chronic myeloid leukemia patients with gleevec (imatinib mesylate); the first generation specific tyrosine kinase inhibitor. BMC Blood Disorders. 2010;10:8. doi: 10.1186/1471-2326-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]