Abstract
Background and objectives
The World Health Organization End tuberculosis (TB) Strategy, approved in 2014, aims at a 90% reduction in TB deaths and an 80% reduction in TB incidence rate by 2030. One of the suggested interventions is the systematic screening of people with suspected TB, belonging to specific risk groups. The Hospital Raoul Follereau (HRF) in Bissau, Guinea-Bissau, is the National Reference Hospital for Tuberculosis and Lung Disease of the country. We performed an active case-finding program among pediatric age family members and cohabitants of admitted adult TB patients, from January to December 2013.
Methods
Newly admitted adult patients with a diagnosis of TB were invited to bring their family members or cohabitants in childhood age for clinical evaluation in a dedicated outpatient setting within the hospital compound. All the children brought to our attention underwent a medical examination and chest x-ray. In children with clinical and/or radiologic finding consistent with pulmonary TB, a sputum-smear was requested.
Results
All admitted adult patients accepted to bring their children cohabitants. In total, 287 children were examined in 2013. Forty-four patients (15%) were diagnosed with TB. The number needed to screen (NNS) to detect one case of TB was 7. 35 patients (80%) had pulmonary TB; 2 of them were sputum smear-positive. No adjunctive personnel cost was necessary for the intervention.
Conclusions
A hospital-based TB active case-finding program targeted to high-risk groups like children households of severely ill admitted patients with TB can successfully be implemented in a country with limited resources.
Keywords: Tuberculosis, Child, Diagnosis, Case finding, Bissau
Introduction
According to World Health Organization, tuberculosis (TB) is one of the top 10 causes of death worldwide, having killed 1.4 million people in 2015. In the same year, there were an estimated 10.4 million new TB cases worldwide, of which 1.0 million (10%) among children.1 TB is considered a poverty-related disease; half of the deaths from TB are from the African region, where the problem of low resources is further compounded by the high prevalence of HIV co-infection and increasing mycobacterial drug resistance.2–4
One of the Sustainable Development Goals (SDGs) adopted by United Nations in 2015 is to end the epidemics of AIDS, TB, and malaria by 2030.5 The World Health Organization End TB Strategy, approved in 2014, aims at a 90% reduction in TB deaths and an 80% reduction in TB incidence rate by 2030, compared with 2015.1 One of the suggested interventions within the resolution is the active case-finding of affected people,6–7 meaning the systematic screening of people with suspected TB, belonging to specific risk groups. The diagnosis made early in the course of the disease allows initiating treatment earlier, when the illness is not widespread, and the general health is not heavily compromised, thereby reducing either morbidity and mortality, and the incidence and prevalence of the infection in the population. Under or late diagnosis contributes to sustained transmission of the disease, while active case finding can hasten the rate of decline. Dynamic models recently proved that this strategy could be both powerful and cost-effective in reducing TB incidence and mortality.6,8 Many screening and diagnostic algorithms have been proposed to date.6,9
A further major challenge is the diagnosis and cure of TB in children. Childhood TB was long neglected by organizations dedicated both to TB or child health at a global level. The main reasons for this phenomenon are both the fact that children with TB are rarely contagious and the many diagnostic difficulties of TB in childhood. Obtaining adequate sputum samples from children for laboratory diagnosis is often troublesome; the proportion of sputum smear-positive pediatric cases is low. Moreover, the availability of Xpert MTB/RIF assay is low in Africa outside of the research setting. Diagnosis often relies on clinical evaluation supported by diagnostic exams, chiefly chest X-ray.10–11 Since the majority of childhood TB cases are not diagnosed, the burden of the disease has been underestimated for a long time.
In the last few years, there has been increasing attention in childhood TB from international organizations and TB programs. In October 2013 WHO, UNICEF, CDC and other organizations released the Roadmap for Childhood Tuberculosis, with the goal of zero TB deaths in children.12
Guinea Bissau is a West African Country with a high TB burden,1,13–14 and a high mortality among children living with TB households.15
We performed an active case-finding program among pediatric age family members, and cohabitants of adult TB patients admitted to the Hospital Raoul Follereau (HRF) in Bissau, Guinea-Bissau, the National Reference Hospital for Tuberculosis and Lung Disease of the country,16 from January to December 2013.
Our objectives were to demonstrate the high burden of undiagnosed childhood TB and to show that a hospital-based active screening program could be both feasible and effective, even in a low resource context.
Material and Methods
Study setting
Guinea-Bissau is a West African country located on the Atlantic Coast with a population of approximately 1.8 million people.11 Since independence from Portugal in 1974, Guinea-Bissau has been the scene of considerable political upheaval, experiencing many military coups and civil war. It’s one of the least developed countries, with a poverty headcount ratio at national poverty lines of 69.3% in 2010, according to World Bank data. In 2012 life expectancy at birth was 54 years, while healthy life expectancy at birth was 7 years lower, being of 47 years, as reported by Global Health Observatory of WHO. Infrastructural problems typical of post-conflict countries still remain in Guinea Bissau and affect the health of the population.17–18
TB is one of the leading causes of morbidity and mortality, with an incidence rate of 373 cases per 100,000 persons per year. It is also one of the 30 countries with the highest estimated numbers of incident TB cases among people living with HIV.1
The Hospital Raoul Follereau (HRF), located in Bissau, capital of Guinea-Bissau, is the national reference center for the fight against TB. The services it provides (inpatient, outpatient clinic, radiology, and laboratory) are entirely free of charge for the patients, in a beneficial public-private partnership.16,19 Patients with poor clinical conditions or with severe disease are admitted to the HRF after referral from regional hospitals or health centers across the entire country, according to the National Guidelines for TB.20
Study Design
Our cross-sectional study describes the outcomes of the pediatric active case-finding program performed in the HRF from January to December 2013. All patients admitted to the HRF receive weekly health education sessions on Thursday afternoons. Moreover, a psychologist and social service person perform daily rounds to talk with patients and reinforce health education. During health education sessions the importance of early screening was explained throughout the year, and the active case finding project was presented. Every day, a nurse invited newly admitted adult patients with a diagnosis of TB to bring their family members or cohabitants in childhood age to clinically evaluate them in a dedicated outpatient setting within the hospital compound. Two mornings per week a physician and a nurse attended the children in the outpatient clinic of the hospital. After obtaining caregivers’ informed consent, all the children brought to our attention underwent a medical examination and chest x-ray. In children with clinical and/or radiologic finding consistent with pulmonary TB, a sputum-smear was requested.
Three samples of sputum analysis were collected and stained with Ziehl-Neelsen’s staining technique: if acid-fast bacilli were shown in at least two samples, children were considered smear positive. Diagnosis of TB was made according to the routinely used clinical protocol from the National Guidelines.20 Smear-positive children were diagnosed with confirmed pulmonary TB. In case of a negative smear, despite a clinical history and a chest x-ray suggestive of pulmonary TB, a course of antibiotics was administered for 10 days and, if no improvement was observed, the child was diagnosed as having presumptive TB and started on TB treatment. In case of Extra pulmonary TB involving the lymph nodes, an aspirate from the lymph nodes was analyzed for acid-fast bacilli; if the clinical suspicion was of bone TB, an x-ray of the involved skeletal parts was performed. Children with a diagnosis of TB were admitted while children with other conditions were given appropriate treatment according to need. Additional examinations were requested depending on the clinical findings.
Data were reported on a written medical record, including the name of the patient, gender, age, reported symptoms, test results, diagnosis, and prescriptions. All children diagnosed with TB during the outpatient clinic were admitted for therapy and observation in the HRF.
The study was approved by the local Institutional Review Board.
Data Analysis
Personal and clinical information was transferred from written medical records to Microsoft Excel for Mac 15.32 (Microsoft Corporation, Redmond, Washington, USA). The number needed to screen (NNS) to detect one case of TB, i.e., the ratio between the number of persons screened and the number of persons diagnosed with TB, was calculated.
Results
All admitted adult patients accepted to bring their children cohabitants. In total, 287 children were examined in 2013. The clinical characteristics of screened patients are detailed in Table 1. The children were 147 males (51%) and 140 females (49%). Mean age was 6.45 years (range: 1 month–16 years). The symptoms reported by the caregiver were recorded. Only 25 patients (9%) were reported healthy, while 262 (91%) had at least one symptom at home, mainly cough (205 patients, 71%), fever (203 patients, 71%), chest pain (90 patients, 31%) and rhinitis (70 patients, 24%).
Table 1.
Clinical characteristics of the 287 children, living in the household of adult patients admitted with Tuberculosis at the Raul Follereau Hospital (HRF), who were screened in the project.
| M (%) | F (%) | N (%) | |
|---|---|---|---|
| Children | |||
| Gender | 147 (51) | 140 (49) | 287 (100) |
| Symptoms | |||
| Cough | 110 (75) | 95 (68) | 205 (71) |
| Fever | 106 (72) | 97 (69) | 203 (71) |
| Chest pain | 40 (27) | 50 (36) | 90 (31) |
| Rhinitis | 38 (26) | 32 (23) | 70 (24) |
| Abdominal pain | 12 (8) | 20 (14) | 32 (11) |
| Headache | 13 (9) | 15 (11) | 28 (10) |
| Dyspnea | 8 (5) | 9 (6) | 17 (6) |
| Lymphadenopathy | 3 (2) | 2 (1) | 5 (2) |
| Diagnosis* | |||
| Tuberculosis | 18 (12) | 26 (19) | 44 (15) |
| Bronchitis | 22 (15) | 12 (9) | 34 (12) |
| Upper respiratory tract infections | 6 (4) | 13 (9) | 19 (7) |
| Anemia | 9 (6) | 7 (5) | 16 (6) |
| Pneumonia | 6 (4) | 3 (2) | 9 (3) |
| Malaria | 1 (1) | 4 (3) | 5 (2) |
| Tinea | 3 (2) | 1 (1) | 4 (1) |
| Parasitic disease | 2 (1) | 2 (1) | 4 (1) |
| Hospitalization | 27 (18) | 29 (21) | 56 (20) |
Patients could have more than 1 diagnosis at the same time
Overall, the number of patients with concomitant HIV infection or AIDS was 9 (3%), while the total number of hospitalized patients was 56 (20%).
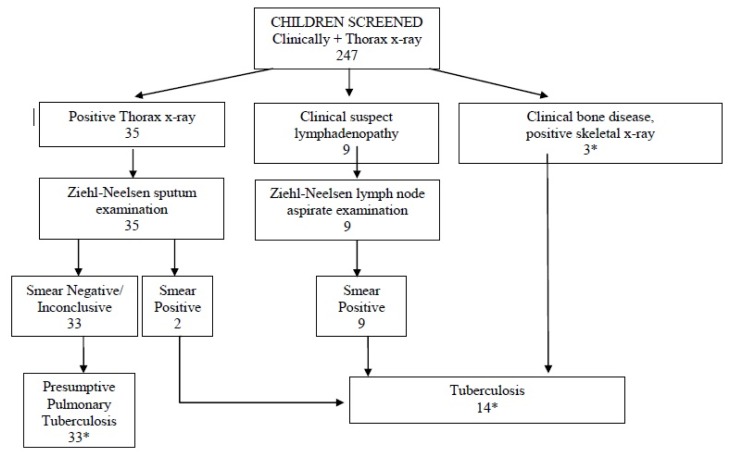
Forty-four patients (15%) were diagnosed with TB. The NNS was 7. The flowchart describing the diagnostic path is described in Figure 1, while clinical characteristics of TB patients are detailed in Table 2. The patients were 18 males (49%) and 26 females (51%). Mean age was 5.75 years (range: 1 month–16 years). 35 patients (80%) had pulmonary TB; 2 of them were sputum smear-positive (confirmed pulmonary TB), while 33 were smear-negative and therefore had presumptive pulmonary TB. Twelve patients had extra pulmonary TB, 3 of them having both pulmonary and extra pulmonary disease. Of the 12 extra pulmonary TB, 9 had lymph node TB, 3 bone TB, one of them having Pott’s disease. Three out of 44 patients with TB (7%) had concomitant HIV infection, although not all patients received HIV testing. All TB cases were admitted to HRF for therapy.
Figure 1.
Diagnostic pathway of children with Presumptive Pulmonary Tuberculosis and Tuberculosis. *Three children had multiple diagnosis: one girl with Smear Negative Pulmonary TB and Bone TB; one girl with Smear Negative Pulmonary TB and Smear positive Lymph node TB; one girl with Smear positive Lymph node TB and Bone TB (Pott’s disease).
Table 2.
Clinical and demographic characteristics of the screened children who were diagnosed with Tuberculosis (N=44).
| Patients with TB (N=44) | ||
|---|---|---|
| N | % | |
| Gender | ||
| M | 18 | 41 |
| F | 26 | 59 |
| Age (years) | ||
| 0–5 | 30 | 68 |
| 6–16 | 14 | 32 |
| Type of TB# | ||
| Pulmonary TB | 35 | |
| Pulmonary TB Smear + | 2 | 5 |
| Pulmonary TB Smear − | 6 | 14 |
| Pulmonary TB Smear inconclusive | 27 | 61 |
| Extrapulmonary TB | 12 | |
| Lymph node TB | 9 | 20 |
| Bone TB | 3 | 7 |
| Symptoms | ||
| Cough | 37 | 84 |
| Fever | 41 | 93 |
| Chest pain | 24 | 55 |
| Rhinitis | 4 | 9 |
| Abdominal pain | 5 | 11 |
| Headache | 5 | 11 |
| Dyspnea | 8 | 18 |
| Lymphadenopathy | 3 | 7 |
| Deaths | 3 | 7 |
Patients could have more than 1 type of TB at the same time.
As for the outcome of the hospitalized children, 3 patients (7%) died during treatment; the others (41 patients, 93%) were discharged with subsequent follow-up.
After TB the most common conditions diagnosed in the screened patients were bronchitis (34 patients, 12%), upper respiratory tract infection (19 patients, 7%), anemia (16 patients, 6%), pneumonia (9 patients, 3%) and malaria (5 patients, 2%).
Costs
A detailed cost analysis was beyond the scope of this study. Nevertheless, it is worth to notice that no adjunctive personnel cost was necessary for these interventions: the physician and the nurse twice a week were already working for the hospital and shifts were rearranged to perform the planned activity without extra-hours. X-rays are given to the HRF by the National Program against TB and did not require further funding. Nevertheless, it can be estimated from our previous experience19 that the diagnosis of a TB case at admission would have cost 65,11 USD.
Discussion
Our study shows that TB active case-finding could be a powerful tool when applied to a high-risk group of people like the pediatric co-inhabitants of severely ill TB patients admitted in hospitals, especially in a high TB burden country such as Guinea-Bissau.
The low NNS (7 patients) to diagnose one case of TB reflects the impact of the intervention on the target population, especially when compared to the one that was found in other contact investigation projects in high burden countries.6,21–23 It also confirms that children living with adult TB patients are at high risk of contracting the disease.23
Even if a 1 year period of active screening is too short to evaluate long-term indicators, the low mortality of admitted and treated patients (7%) demonstrates the value of a diagnosis performed early in the course of the disease. Adult patients are generally admitted in severe conditions, frequently with disseminated infections or comorbidities. Through active screening, patients can be treated earlier in the course of the disease.
Even though the active case finding screening program was addressed to assumingly asymptomatic children, not yet come to medical attention, only 25 patients (9%) were reported healthy by their caregivers. Most children had at least one sign or symptoms, such as cough, fever, rhinitis, and dyspnea. This may be due to the high burden of other diseases among African children, especially acute respiratory infections, which represent the third cause of death in children under 5 in the country, according to WHO.1 Children living in Guinea-Bissau are still in a precarious situation concerning water supply and housing and are often malnourished,17–18 although data regarding malnutrition are not homogeneous among the cohorts evaluated in different parts of the country.24 Thus, symptom screening had very low specificity, since pulmonary TB is a relatively rare cause of lower respiratory symptoms and signs, which are common in pediatric age. We also hypothesize that the presence of symptoms of any kind prompted caregivers to bring the children to our attention. Given the frequent presence of other diseases, medical history or physical examination alone were often insufficient to properly discriminate between TB cases and healthy subjects or patients having other conditions. Consequently, we performed a chest x-ray in every screened child.
The rate of HIV infection was higher in children with TB (7% vs. 3% of the whole sample). In fact, patients of all ages with a new diagnosis of TB are 19 times more likely to be co-infected with HIV than those without TB; equally, people with HIV are 20 to 30 times more likely to develop TB than those without HIV.25–27 Despite that, none of the observed deaths were among HIV infected children.
Our program was designed to involve children who could be infected by household contacts having TB, who represent a large proportion of the pool of undetected TB patients. This is particularly true for young children, for whom there is a clinical overlap between the features of TB and other frequent causes of morbidity and mortality, such as pneumonia. The prevalence of TB among children who are close contacts of a TB case is high.15,28–29 Therefore, screening of children who are contacts is already widely recommended, although rarely implemented systematically. TB in the pediatric age seems to be under-reported, so cases with active TB can spread the infection to their children who are underdiagnosed for TB.7 Our pediatric active case-finding intervention could contribute to the goal of zero TB deaths in children.
Our study was not designed to reach conclusive cost-effectiveness information. Nevertheless, the costs for our active hospital-based case finding intervention was low, compared to other active community-based case finding experiences.29 Early diagnosis could reduce diagnostic and treatment costs for health services and families while reducing mortality simply because less ill patients are taken in care. Isoniazid preventive therapy was not given to children contacting adult TB patients in this project, nor it is routinely performed in Guinea Bissau, except for few pilot projects,30 despite the WHO recommendations. A further phase of the active case finding program could be the implementation of a standardized isoniazid preventive therapy to all contacts and not only the treatment of children identified with confirmed or presumptive TB.
Our analysis has several limits. First, we could not detail the magnitude of the screening population and of the overall adult patients. The screening was offered to all family members and cohabitants of admitted adult TB patients, but extended families and house overcrowding have prevented us from determining the total number of children that could have been target. A joint project with the part of the TB National Program that is working on the field could allow home visits by nurses or social workers. Secondly, we could not compare the NNS of our study with alternative active case-finding interventions applied in the same setting, due to lack of comparable data. Moreover, local case notification rate was unavailable. Finally, no systematic HIV test was performed; therefore, the incidence of HIV co-infection could have been underestimated.
Conclusion
Our experience demonstrates that a simple TB active case-finding program targeted to high-risk groups like children households of severely ill admitted patients with TB, can successfully be implemented in a country with limited resources.
Despite the short timescale and notwithstanding the limitations of our study, our results show that a broad implementation of similar active screening could have rapid effects on TB transmission and disease in different low resource settings. In fact, WHO recommends such screening for contacts of bacteriologically confirmed cases to reduce the level of underdiagnosis. The limited resources required for the intervention makes it an attractive model that could produce savings in the long term, and the identified gaps and limitations should guide future interventions.
Acknowledgments
The Authors would like to thank the Ministry of Health, the National Program Against Tuberculosis, the staff of the Hospital Raoul Follereau and all the patients and their families.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.World Health Organization. Global Tuberculosis Report. 2016. [Last accessed June 14th 2017]. http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1.
- 2.Kirigia JM, Muthuri RD. Productivity losses associated with tuberculosis deaths in the World Health Organization African region. Infect Dis Poverty. 2016;5(1):43. doi: 10.1186/s40249-016-0138-5. https://doi.org/10.1186/s40249-016-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barter DM, Agboola SO, Murray MB, Bärnighausen T. Tuberculosis and poverty: the contribution of patient costs in sub-Saharan Africa--a systematic review. BMC Public Health. 2012;12:980. doi: 10.1186/1471-2458-12-980. https://doi.org/10.1186/1471-2458-12-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabna P, Ramos J, Ponce G, Sanca L, Mané M, Armada A, Machado D, Vieira F, Gomes VF, Martins E, Colombatti R, Riccardi F, Perdigão J, Sotero J, Portugal I, Couto I, Atouguia J, Rodrigues A, Viveiros M. Direct Detection by the Xpert MTB/RIF Assay and Characterization of Multi and Poly Drug-Resistant Tuberculosis in Guinea-Bissau, West Africa. PLoS One. 2015;10(5):e0127536. doi: 10.1371/journal.pone.0127536. https://doi.org/10.1371/journal.pone.0127536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nation General Assembly. Transforming our world: the 2030 Agenda for Sustainable Development Resolution A/70/L.1. [Last accessed June 14th 2017]. http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E.
- 6.Fox GJ1, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(1):140–56. doi: 10.1183/09031936.00070812. https://doi.org/10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshi DC, Chukwu JN, Nwafor CC, Meka AO, Madichie NO, Ogbudebe CL, Onyeonoro UU, Ikebudu JN, Ekeke N, Anyim MC, Ukwaja KN, Aguwa EN. Does intensified case finding increase tuberculosis case notification among children in resource-poor settings? A report from Nigeria. Int J Mycobacteriol. 2016;5(1):44–50. doi: 10.1016/j.ijmyco.2015.10.007. https://doi.org/10.1016/j.ijmyco.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Azman AS, Golub JE, Dowdy DW. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med. 2014;12:216. doi: 10.1186/s12916-014-0216-0. https://doi.org/10.1186/s12916-014-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van’t Hoog AH1, Onozaki I, Lonnroth K. Choosing algorithms for TB screening: a modelling study to compare yield, predictive value and diagnostic burden. BMC Infect Dis. 2014;14:532. doi: 10.1186/1471-2334-14-532. https://doi.org/10.1186/1471-2334-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon JA, Jenkins HE, Liu L, Cohen T, Black RE, Vos T, Becerra MC, Graham SM, Sismanidis C, Dodd PJ. Counting children with tuberculosis: why numbers matter. Int J Tuberc Lung Dis. 2015;19(Suppl 1):9–16. doi: 10.5588/ijtld.15.0471. https://doi.org/10.5588/ijtld.15.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starke JR. Improving tuberculosis care for children in high-burden settings. Pediatrics. 2014;134(4):655–7. doi: 10.1542/peds.2014-1652. https://doi.org/10.1542/peds.2014-1652. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Roadmap for childhood tuberculosis: towards zero deaths. 2013. [Last accessed on June 14th 2017]. http://apps.who.int/iris/bitstream/10665/89506/1/9789241506137_eng.pdf?ua=1&ua=1.
- 13.Central Intelligence Agency. The World Factbook Guinea Bissau. 2016. [Last accessed on June 14th 2017]. https://www.cia.gov/library/publications/the-world-factbook/geos/pu.html.
- 14.Lemvik G, Rudolf F, Vieira F, Sodemann M, Østergaard L, Rodrigues A, Gomes V, Aaby P, Wejse C. Decline in overall, smear-negative and HIV-positive TB incidence while smear-positive incidence stays stable in Guinea-Bissau 2004–2011. Trop Med Int Health. 2014;19(11):1367–76. doi: 10.1111/tmi.12378. https://doi.org/10.1111/tmi.12378. [DOI] [PubMed] [Google Scholar]
- 15.Gomes VF, Andersen A, Wejse C, Oliveira I, Vieira FJ, Joaquim LC, Vieira CS, Aaby P, Gustafson P. Impact of tuberculosis exposure at home on mortality in children under 5 years of age in Guinea-Bissau. Thorax. 2011;66(2):163–7. doi: 10.1136/thx.2010.141309. https://doi.org/10.1136/thx.2010.141309. [DOI] [PubMed] [Google Scholar]
- 16.Colombatti R, Penazzato M, Bassani F, Vieira CS, Lourenço AA, Vieira F, Teso S, Giaquinto C, Riccardi F. Malaria prevention reduces in-hospital mortality among severely ill tuberculosis patients: a three-step intervention in Bissau, Guinea-Bissau. BMC Infect Dis. 2011;11:57. doi: 10.1186/1471-2334-11-57. https://doi.org/10.1186/1471-2334-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombatti R, Vieira CS, Bassani F, Cristofoli R, Coin A, Bertinato L, Riccardi F. Contamination of drinking water sources during the rainy season in an urban post-conflict community in Guinea Bissau: implications for sanitation priority. Afr J Med Med Sci. 2009;38(2):155–61 P. Mid:20175419. [PubMed] [Google Scholar]
- 18.Colombatti R, Coin A, Bestagini P, Vieira CS, Schiavon L, Ambrosini V, Bertinato L, Zancan L, Riccardi F. A short-term intervention for the treatment of severe malnutrition in a post-conflict country: results of a survey in Guinea Bissau. Public Health Nutr. 2008;11(12):1357–64. doi: 10.1017/S1368980008003297. https://doi.org/10.1017/S1368980008003297. [DOI] [PubMed] [Google Scholar]
- 19.Vieira F, Sanha MS, Riccardi F, Colombatti R. Short term advantages of a public-private partnership for tuberculosis in Guinea Bissau: reduction of mortality and increased diagnostic capacity. Mediterr J Hematol Infect Dis. 2014;6(1):e2014049. doi: 10.4084/MJHID.2014.049. https://doi.org/10.4084/mjhid.2014.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministério da Saúde Pública Programa Nacional de Luta contra a Tuberculose. Manual de Tratamento. Bissau, Guinea Bissau: 2010. [Last Accessed on June 14th 2017]. http://www.nationalplanningcycles.org/sites/default/files/country_docs/Guinea-Bissau/pndsii_2008-2017_gb.pdf. [Google Scholar]
- 21.Blok L, Sahu S, Creswell J, Alba S, Stevens R, Bakker MI. Comparative meta-analysis of tuberculosis contact investigation interventions in eleven high burden countries. PLoS One. 2015 Mar 26;10(3):e0119822. doi: 10.1371/journal.pone.0119822. https://doi.org/10.1371/journal.pone.0119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lala SG, Little KM, Tshabangu N, Moore DP, Msandiwa R, van der Watt M, Chaisson RE, Martinson NA. Integrated Source Case Investigation for Tuberculosis (TB) and HIV in the Caregivers and Household Contacts of Hospitalised Young Children Diagnosed with TB in South Africa: An Observational Study. PLoS One. 2015;10(9):e0137518. doi: 10.1371/journal.pone.0137518. https://doi.org/10.1371/journal.pone.0137518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puryear S, Seropola G, Ho-Foster A, Arscott-Mills T, Mazhani L, Firth J, Goldfarb DM, Ncube R, Bisson GP, Steenhoff AP. Yield of contact tracing from pediatric tuberculosis index cases in Gaborone, Botswana. Int J Tuberc Lung Dis. 2013;17(8):1049–55. doi: 10.5588/ijtld.12.0933. https://doi.org/10.5588/ijtld.12.0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patsche CB, Rudolf F, Mogensen SW, Sifna A, Gomes VF, Byberg S, Wejse C. Low prevalence of malnourishment among household contacts of patients with tuberculosis in Guinea-Bissau. Int J Tuberc Lung Dis. 2017;21(6):664–669. doi: 10.5588/ijtld.16.0673. https://doi.org/10.5588/ijtld.16.0673. [DOI] [PubMed] [Google Scholar]
- 25.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24(2):351–76. doi: 10.1128/CMR.00042-10. https://doi.org/10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafson P, Gomes VF, Vieira CS, Rabna P, Seng R, Johansson P, Sandström A, Norberg R, Lisse I, Samb B, Aaby P, Nauclér A. Tuberculosis in Bissau: incidence and risk factors in an urban community in sub-Saharan Africa. Int J Epidemiol. 2004;33(1):163–72. doi: 10.1093/ije/dyh026. https://doi.org/10.1093/ije/dyh026. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson P, Gomes VF, Vieira CS, Samb B, Nauclér A, Aaby P, Lisse I. Clinical predictors for death in HIV-positive and HIV-negative tuberculosis patients in Guinea-Bissau. Infection. 2007;35(2):69–80. doi: 10.1007/s15010-007-6090-3. https://doi.org/10.1007/s15010-007-6090-3. [DOI] [PubMed] [Google Scholar]
- 28.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(6):359–68. doi: 10.1016/S1473-3099(08)70071-9. https://doi.org/10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 29.Karki B, Kittel G, Bolokon I, Jr, Duke T. Active Community-Based Case Finding for Tuberculosis With Limited Resources. Asia Pac J Public Health. 2017;29(1):17–27. doi: 10.1177/1010539516683497. https://doi.org/10.1177/1010539516683497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes VF, Wejse C, Oliveira I, Andersen A, Vieira FJ, Carlos LJ, Vieira CS, Aaby P, Gustafson P. Adherence to isoniazid preventive therapy in children exposed to tuberculosis: a prospective study from Guinea-Bissau. Int J Tuberc Lung Dis. 2011;15(12):1637–43. doi: 10.5588/ijtld.10.0558. https://doi.org/10.5588/ijtld.10.0558. [DOI] [PubMed] [Google Scholar]