Abstract
Melanoma is one of the most aggressive metastatic cancers with resistance to radiation and most chemotherapy agents. This study highlights an alternative treatment for melanoma based on nanosecond pulsed dielectric barrier discharge (nsP DBD). We show that a single nsP DBD treatment, directly applied to a 5 mm orthotopic mouse melanoma tumor, completely eradicates it 66% (n = 6; p ≤ 0.05) of the time. It was determined that reactive oxygen and nitrogen species produced by nsP DBD are the main cause of tumor eradication, while nsP electric field and heat generated by the discharge are not sufficient to kill the tumor. However, we do not discount that potential synergy between each plasma generated component (temperature, electric field and reactive species) can enhance the killing efficacy.
Keywords: atmospheric pressure plasma, cancer, dielectric barrier discharge, nanosecond pulsed electric field, reactive species
Graphical Abstract
1. Introduction
In this study, we show that nanosecond pulsed dielectric barrier discharge (nsP DBD) is an effective therapy for the eradication of B16F0 mouse orthotopic melanomas. Melanomas are highly aggressive cancers which are extremely resistant to most traditional forms of chemotherapy and radiotherapy.[1,2] Therefore, the current challenge is to discover and develop new therapies that can overcome this resistance and improve treatment efficacy. NsP DBD is different than other ablative therapies in that plasma generation combines ionized gases (plasma) containing ions, radicals and reactive oxygen and nitrogen species (ROS/RNS),[3–5] nanosecond pulsed electric fields (nsPEF), UV radiation and heat; each of which have individually shown efficacy in treating cancer and initiating tumor cell apoptosis.[6–16] Utilizing these modalities in combination promotes the unique effectiveness of nsP DBD. The aim of this study is then to determine if we could isolate the component of the nsP DBD that is primarily responsible for nsP DBD-induced tumor cell killing.
In our previous work, we investigated the use of microsecond pulsed (μsP) DBD for tissue regeneration, using either low dose, or short treatment times and showed plasma generated ROS facilitated cell differentiation, development, and regeneration by activating intracellular ROS-sensitive signaling pathways.[18] In the current study, we use nsP DBD with longer treatment times to induce cell death. Several studies have reported plasma induces apoptosis and DNA damage when used to treat cancer cells[19–44] including melanoma,[22–25,35,45,46] ovarian,[26,47] colorectal,[27] liver,[28] lung,[29,36] breast,[30,42] and brain[31] cancers. However, most of these studies used in vitro models to study the efficacy of plasma treatment on both malignant and normal cells and tissue.[26–28,30,35,38–40] Additionally, in these and other studies, a variety of plasma devices (mostly plasma jets) were utilized, as summarized by Hirst et al.[37] In another recent review,[48] a comprehensive table lists plasma treatment regimes used to treat cancer over the last decade. It is interesting, that so many different types of plasma devices and treatment protocols have all been used and proved effective as anti-cancer treatments.[48]
Even so, the mechanism of plasma action still remains unclear. While several studies suggest that plasma induces apoptosis and not necrosis, other studies report the opposite.[49–51] Still other studies have detailed plasma effects on the tumor cell cycle and the induction of DNA damage depending on treatment dose.[52] Even at low, non-ablative doses, plasma has been reported to induce senescence in melanoma cells.[25] However, the majority of studies have concluded that plasma-generated ROS is the mechanism predominantly responsible for the selective killing of cancer cells.[34,53] Preliminary μsP DBD treatment of glioblastoma showed that generation of a large amount of ROS resulted in the formation of DNA damage, multiphase cell cycle arrest (cellular senescence), and ultimately apoptosis.[7,50,54] The effective selectivity of the cancer cells to ROS-induced death has been attributed their higher metabolic rate which makes them significantly more susceptible to oxidative stress when compared to normal cells.[55] The effective killing of cancer cells using nsP electric field treatment has also been attributed to the stimulation of apoptosis, pyknosis, and DNA fragmentation both in vitro[56–66] and in vivo. [9,67,68] Studies report nsPEF treatment can be used to ablate melanoma,[59,62,66,69,70] with evidence of destruction of the tumor blood supply and complete remission after nsPEF treatment. In these studies, the nsPEF device generates a current flow through the tumor which causes ablation by heating (hyperthermia). Hyperthermia has been reported as an effective cancer therapy and could be produced by nsP DBD treatment.[71–75] Heating tumors is an established adjuvant cancer treatment technique that improves clinical outcomes of several superficial diseases, including superficial breast cancer and melanoma.[76–79] High temperatures result in coagulation necrosis and this effect is dependent on heating time. For instance, local heating at 50 °C for 6 min is sufficient for direct cell killing, and higher temperatures require less time.[80] Other possibile tissue reactions to plasma include tissue reoxygenation and an enhancement of the immune response.[81,82]
In this study, we show nsP DBD generated ROS/RNS and radicals create a reaction chemistry that is responsible for the elimination of subdermal B16 melanomas. We also show that neither the nsPEF, nor the heat associated with nsP DBD is sufficient to induce melanoma tumor remission without the presence of reactive species. However, we do not discount potential interactions between each of the component within nsP DBD to enhance the killing effect.
2. Materials and Methods
2.1. In vivo Subdermal B16 Melanoma Model
Male C57BL/6 mice (6–8 weeks old) were obtained from Charles Rivers Laboratories (Malvern, PA, USA) and maintained on a normal diet until use. The melanoma cells from B16-F10 mouse (CRL-6475) were purchased from American Type Culture Collection; their MAP (mitogen-activated protein) and mycoplasma were tested for purity and kept frozen at 80 °C under liquid nitrogen until resuscitated for use. For culture, cells were maintained with 90% DMEM (Dulbecco’s modified Eagle’s medium) and 10% heat inactivated FBS at 37 °C and 5% CO2. The cells were suspended at 2 × 106 cells ml−1 in 1× PBS (kept on ice) directly before injection. The mice were sedated with AErrane (isofluorane; Baxter) for the subcutaneous injection of B16 cells. Both right and left hind flanks were shaved before a subcutaneous injection of 50 μl of the cellular suspension on each side. Intradermal tumor development was monitored by digital caliper measurement daily for the duration of the experiments. The plasma treatment was applied to the tumor when the average tumor diameter reached approximately 5 mm, which occurred 7–9 days after B16 injection. We chose B16 cells because they induce tumors that aresimilar in gross and histological features.[83] All animal studies were conducted according to the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by IACUC of the Thomas Jefferson University.
2.2. Nanosecond-Pulsed Dielectric Barrier Discharge Plasma Treatment
The plasma was generated using a nanosecond-pulsed generator (FID technology, FPG20-1NM10) and we applied a positive polarity voltage in a 2 mm gap between the high-voltage electrode and the tumor. The mouse was placed on its side on a grounded metal plate with the tumor facing upward. The treatment conditions were: voltage of 33.6 kV, 20 ns pulse duration, 2 ns rise time, 3 ns fall time, and repetition rate of 236 Hz. The treatment time was set to 7 min, unless noted otherwise. The planar, high-voltage electrode included an inner copper core (1.03 cm diameter) surrounded by an outer insulating acrylic shell (1.25 cm diameter).[17,84] The surface electrode area was 0.83 cm2. Discharge waveforms were previously reported by Seepersad et al.[85]
2.3. Histology
Representative sets of mice were euthanized using a CO2 chamber. The entire tumor was excised, fixed overnight with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO), and embedded in paraffin wax. Serial 6 μm transverse sections from the middle of the tumor were stained according to the manufacturer instructions with hematoxylin (Thermo Fisher Scientific Inc., Waltham, MA) and eosin (Thermo Fisher Scientific Inc., Waltham, MA) (H&E), or Trichrome (Sigma-Aldrich, St. Louis, MO).
2.4. Survival Analysis
The health and behavior of the mice were assessed daily for the entire duration of the study. The tumors and extent of necrosis were also measured daily (length and width) using a digital caliper. Upon presentation of defined criteria associated with tumor burden and disease progression (abnormal feeding behavior, diminished response to stimuli, and failure to thrive or when one or more tumor reached diameter of 12 mm or more), mice were humanely euthanized according to approved IACUC guidelines. The survival time was recorded, tumors were excised and weighed. If only one of the tumors reached the critical diameter and the other tumor had not reoccurred, we implemented a histological analysis to confirm the absence of tumor and counted such studies as a survival statistic. The survival distribution presented in the study accounts for successful tumor eradication as the number of tumors was greater than the number of mice (two tumors/mouse). Statistical analysis between groups was performed using log-rank test to determine the p-value.
2.5. Using a Gel as an Adjuvant Treatment
The main hypothesis of this study is that the production of reactive species, radicals, and ions is responsible for the nsP DBD ability to eradicate the melanoma tumor. Thus, we prepared a gel composed of glycerol (C3H8O3) and sodium bicarbonate (NaHCO3) to buffer the pH and to prevent ROS/RNS and ions generated within the plasma from directly interacting with the skin surface. The sodium bicarbonate acts as a buffer because it is dissociated into sodium carbonate, carbon dioxide, and water. We applied 500 μl of gel on the tumor surface prior to the nsP DBD treatment and left it turned on for 10–15 min after treatment. A solution of hydrogen peroxide (H2O2) was added to the gel (gel/H2O2) at a final concentration of 3%. Each gel batch, with or without H2O2, was made freshly prior to treatment. In addition, we added 5 μl of 5-Fluorouracil (5FU, InvivoGen, San Diego, CA) at concentration of 10 mg ml−1 to 10 ml of the gel. 5FU with and without 3% H2O2 were applied daily to the tumor until the mice was sacrificed.
2.6. Temperature Measurement
The thermal component of the nsP DBD was evaluated in two animal models: the mouse B16 melanoma tumor and fresh porcine skin (approximately 10 × 10 × 2 cm) obtained from the butcher. Temperature was measured every 4 s from fiber optic sensors placed below the plasma generation site (Tskin), and at a control site approximately. 5 cm away (Tref) from the plasma to account for environmental temperature changes. The mouse tumor temperature was measured in situ and postmortem, using a fiber optic temperature sensor inserted in the center (approximately 3 mm below the skin surface) of a 6 mm tumor. The porcine skin temperature was measured at a depth of approximately 2 mm below the skin surface (interface between epidermal and dermal layers). The sensors are 0.56 mm diameter, have 0.1 °C accuracy (from 0 to 160 °C), and were connected to a 4-channel fluoroptic thermometer (Luxtron Model 3100) controlled with Labview software (National instruments, Austin, TX). The reference and skin temperatures were subtracted at each measurement time to obtain the temperature differential ΔT = Tskin − Tref. We performed three experiments for each model and determined the mean and standard deviation for ΔT.
2.7. Image Collection and Analysis
Photographs of the tumor were taken every weekday. The images were acquired with an iPhone next to a ruler to calibrate size and also measured with a digital caliper. Some images were enhanced to clearly show the tumor. Histology images were taken with Nikon Eclipse E800 and Evolution QEi camera for monochrome images and with an LCD filter to acquire color images (MediaCybernetics). All analysis was performed with Image Pro Plus Software (MediaCybernetics, Silver Spring, MD).
3. Results
3.1. Nanosecond-Pulsed Dielectric Barrier Discharge Treatment Effectively Increases the Survival of Mice with Subdermally Injected B16 Melanoma Tumors
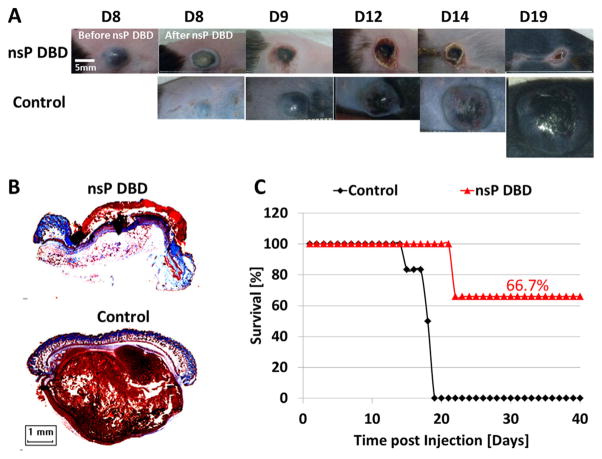
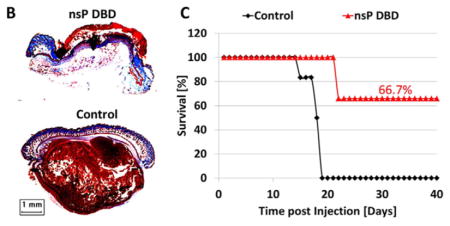
Prior to this study, we determined that a nsP DBD treatment of 7 min at 33.6 kV with a pulse repetition rate of 250 Hz resulted in complete eradication of greater than 60% of the subdermal B16 melanoma tumors (diameter approximately 5 mm when treated). This treatment was developed by trial and error testing of multiple, frequencies, voltages, and treatment times (data not shown). Figure 1A shows representative images of the tumor growth from Day (D) 8 to D19 post-injection (PI), comparing nsP DBD treatment with control (untreated) tumors. A significant decrease in tumor size was observed immediately after nsP DBD treatment. At 24 h (D9) after treatment a scab had formed on the skin where the tumor had been, while the control tumor continued to grow to 31.3 mm2 (Figure 1A). By D12 and D14, the scab on the nsP DBD treated mouse had begun to peel off and no reoccurrence of the tumor was visible. On D19, the control tumor had reached 12 mm in diameter and the mouse had to be sacrificed. Histological staining with trichrome at D22 PI confirmed the nsP DBD-treated tumor had been completely eradicated, with no signs of melanoma, while the control (D19 PI) showed a dense tumor mass (brown) below the epithelium (Figure 1B). A comparison of the survival distribution between control and nsP DBD treated tumors showed significantly increased survival after nsP DBD treatment (66.7%; n = 6/group; p <0.005) (Figure 1C). Starting at D15 PI, 83% of control tumors had grown above the allowable size and had to be sacrificed, with no control mouse surviving longer than D19 PI.
Figure 1.
The nsP DBD treatment eradicates the melanoma tumor. (A) B16 Melanoma cells were injected on the rear flank of C57BL/6 mice. After 8 d, the tumor was treated a single time for 7 min with nsP DBD at 236 Hz and 33.6 kV. The control tumor continued to grow up to day 19 (D19) post-injection until it was sacrificed. Mice treated with nsP DBD continued to heal with a scab up to 22 days and after were tumor free. (B) Trichrome staining of D22 nsP DBD treated tumor (top) and Control tumor (bottom) on D19 post injection. Histology of the nsP DBD treated tumor shows red skin staining confirming scab formation but no tumor below the epithelium is visible. (C) Survival for nsP DBD treated tumors (red triangle) and control untreated tumors (black diamond) as a function of time post-injection (n = 6 each). The nsP DBD treatment resulted in significant improvement of survival rate (66.7% post-treatment) compared to control (0%, p <0.005).
Treatment of the melanoma tumor with nsP DBD in the presence of glycerol/NaHC03 gel eliminates the ability of plasma to eliminate the tumor growth.
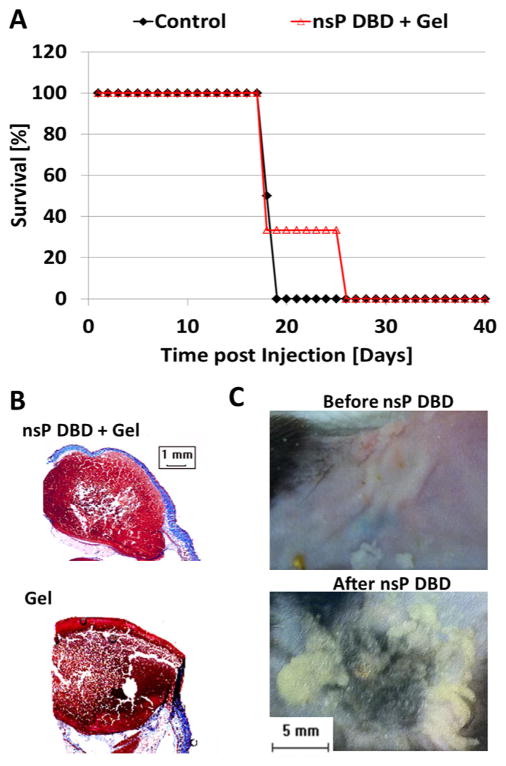
To test the hypothesis that the production of reactive species, radicals and ions was responsible for the ability of nsP DBD to eradicate the melanoma tumor, we prepared a gel composed of glycerol (C3H8O3) and sodium bicarbonate (NaHCO3) to buffer the pH and quench the ROS/RNS and radical generation. Immediately prior to nsP DBD treatment, 0.5 ml of the gel was applied to each tumor (nsP DBD and the untreated control). Some of the tumors treated with nsP DBD in the presence of the gel showed an initial reduction in size, but the nsP DBD no longer showed the ability to eliminate the tumor, which reoccurred in all mice. However, nsP DBD treatment with the gel did decrease the growth rate of the tumors resulting in an increased survival rate of 33.3% of the mice to D25 (n = 6/group; not significant), as compared to D19 in control (Figure 2A). The tumor treated with gel without plasma treatment had a survival curve identical to that observed in the untreated control (data not shown). No differences in tumor morphology between the two treatments were apparent after trichrome staining (Figure 2B).
Figure 2.
The nsP DBD treatment is ineffective in the presence of a gel composed of glycerol and NaHCO3. (A) Graph showing survival for nsP DBD treated mice with Gel (red empty triangle) and Control tumor (black diamond) as a function of time post-injection (n = 6 each). The nsP DBD treatment with gel did not significantly prolong mice survival, compared to Control: 33% (treated) versus 0% (control) at day 19 post-injection (D19) and by D25 survival was 0% for both cases (p >0.01). (B) Trichrome staining of nsP DBD treated tumor with gel (top) on D25 and control tumor with gel (bottom) on D19 post injection. Histological analysis shows no significant differences between the control and treated tumors. (C) Normal mouse skin before (top) and after (bottom) nsP DBD treatment with gel. The gel mixture was transformed to moist powder after nsP DBD treatment on mouse skin.
3.2. Nanosecond-Pulsed Dielectric Barrier Discharge Treatment Causes Absorption of the Glycerol into the Tissue
Of note, the nsP DBD treatment of the tumor with gel resulted in a noticeable reduction in the aqueous composition of the gel leaving only a residual moist powder at the end of the treatment. To determine if the gel evaporated during nsP DBD treatment the same volume of gel was placed on paper, weighed, and treated for 1 min with the same nsP DBD treatment condition (33.6 kV, 250 Hz). Under these conditions, no changes in the appearance, the weight, or the pH (steady at 7.2) of the gel were observed after treatment. This result indicates that treatment with nsP DBD does not cause evaporation of the gel and confirms the gel penetration into the skin. However, it should be noted that skin and paper have very different electrical conductive properties and this will have an effect on the DBD properties. To confirm that gel penetration was not specific to tumor physiology, 0.5 ml of the gel was placed on normal mouse skin (tumor-less) and treated with nsP DBD (33.6 kV, 250 Hz, 7 min). After nsP DBD treatment, the moist powder again appeared on the skin surface (Figure 2C).
3.3. Neither the nsPEF nor the Temperature Generated by Nanosecond-Pulsed Dielectric Barrier Discharge Treatment Significantly Contributes to the Elimination of the Melanoma Tumor in the Absence of Reactive Species
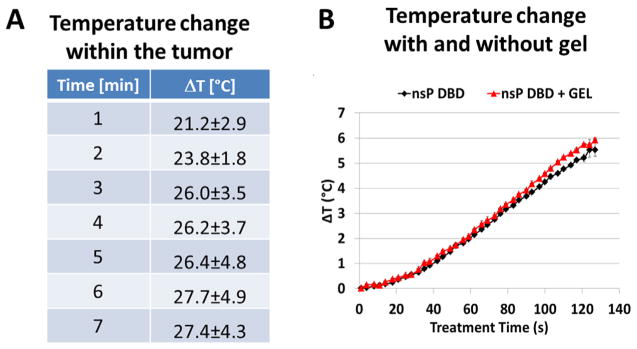
In addition to determining that reactive species, radicals and ions are responsible for the ability of nsP DBD to eradicate the melanoma, the results of the nsP DBD treatment with NaHC03 gel, addressed the tumor killing ability of the nsP electric field (EF) produced by nsP DBD. Treatment with the NaHC03 gel proved unsuccessful and as the application of the gel should have little or no effect on the nsPEF produced by nsP DBD, we can conclude that this component is notresponsible for eradicating the melanoma tumor. Additionally, as the temperature produced by nsP DBD also has the ability to contribute to melanoma cell death, we measured the temperature rise associated with nsP DBD treatment. Fiber optic temperature sensors were placed in the center of an approx. 6 mm melanoma tumor and the temperature rise over the 7 min treatment time is summarized in the table shown in Figure 3A. The temperature was increased over 27° after 7 min of treatment. This temperature increase was enough to significantly contribute to cell death associated with nsP DBD treatment. This experiment was repeated on fresh pig skin obtained from the butcher with and without the glycerol/NaHCO3 gel. The fiber optic sensor was placed directly under the epithelium at a depth of approx. 1 mm. The temperature was measured at 4 s intervals throughout the 2 min treatment and showed no significant difference between nsP DBD in the presence or absence of gel (Figure 3B). Of note, the differences in the ΔT between the tumor measured at 3 mm and the pig skin measured at 2 mm are most likely due to the vascular nature of the tumor, as compared to the solid thickness of the pig epithelium.
Figure 3.
Temperature increases associated with nsP DBD treatment are not affected by the presence of the gel. (A) Temperature measured in the center of a 6 mm diameter melanoma tumor in a postmortem mouse treated with nsP DBD. (B) Measurement of temperature 2 mm below the pig skin surface during nsP DBD treatment for 2 min, with or without gel, showed no significant differences (with gel – red triangles; without gel – black diamonds).
3.4. The Addition of Hydrogen Peroxide (H2O2) to the Glycerol/NaHC03 Gel Restores the Ability of the Nanosecond-Pulsed Dielectric Barrier Discharge Treatment to Eliminate the Melanoma Tumor
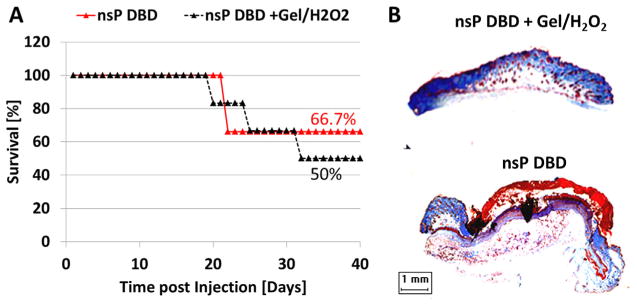
H2O2 was added into the glycerol/NaHC03 gel (gel/H2O2) to determine if the efficacy of nsP DBD treatment could be restored. NsP DBD treatment with gel/H2O2 was compared to nsP DBD treatment alone. The survival distributions showed mice treated with nsP DBD treatment with gel/H2O2 resulted in a survival rate of 50% as compared to the 66% survival of the nsP DBD treatment alone. However, the differences between the two distributions were statistically insignificant (p >0.1; Figure 4A). Trichrome staining showed that at D62 PI the gel/H2O2 coated tumor treated with nsP DBD was no longer present. A comparable stained section of the tumor treated with nsP DBD treatment (no gel) at D22 PI also showed the tumor had been eliminated (Figure 4B). Therefore, we concluded that the addition of H2O2 to the gel restores the efficacy of nsP DBD treatment.
Figure 4.
Tumor killing efficacy of nsP DBD is restored by adding H2O2 to the gel. (A) Mice survival for tumors treated with nsP DBD alone (red triangle) and tumors treated with nsP DBD and gel/H2O2 (black triangle) as a function of time (n = 6 each). The survival distribution are not significantly different (p >0.1). (B) Trichrome staining of nsP DBD treated tumor with gel/H2O2 (top, D62 post-injection) and tumor treated with nsP DBD alone (bottom, D22 post-injection). Histological analysis shows no recurrence of the tumor for either treatment.
3.5. H2O2, 5-Fluorouracil or a Combination of both in the Glycerol/NaHC03 Gel is not Sufficient to Eliminate the Melanoma Tumor
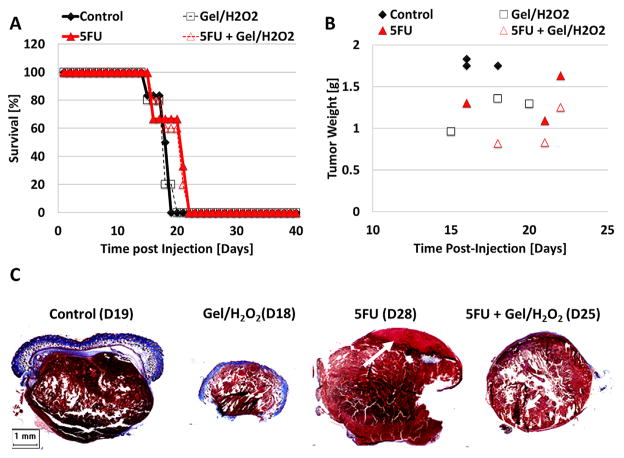
In order to determine if the gel/H2O2 without nsP DBD has any effect on melanoma tumor growth, the gel/H2O2 was applied daily to the tumors for the duration of experiment and compared to untreated control. By D19 PI, all control tumors had reached maximum size and the mice were sacrificed. However, the growth of tumors treated with gel/H2O2 was retarded and the last animal was not sacrificed until D25 (Figure 5A). Additionally, we assessed whether the common chemotherapy agent, 5-Fluorouracil (5FU) added to the gel either alone or in combination with H2O2 would have an effect on survival or size of the melanoma tumor. Both 5FU and 5FU/H2O2 were applied daily for the duration of experiment. The survival distributions comparing mice receiving the glycerol/NaHC03 gel (control), or the gel with H2O2, 5FU, or 5FU/H2O2 did not show significant differences (p > 0.1; Figure 5A). However, when comparing the tumor weight distribution, treatments with 5FU alone, 5FU/H2O2 and H2O2 all resulted in progressively decreased weights, respectively, as compared to control (Figure 5B) indicating a slowing of cell proliferation in the presence of these agents. Additionally, it should be noted that if the melanoma is not completely eradicated it will continue to grow. Therefore, the longer the mouse survives, the larger the size of the tumor. Trichrome staining of tumors extracted after each treatment is shown in Figure 5C. Only 5FU alone showed some necrosis of the upper tumor layer (white arrow, pink area on the top).
Figure 5.
Treatments of melanoma tumor with gel containing 5FU, H2O2, or a combination of both slow tumor growth, but do not eliminate the tumor. (A) Survival for standard topical melanoma treatment 5FU with (empty filled triangle, n = 3) and without H2O2 (red filled triangle, n = 5), with gel/H2O2 alone (black empty square, n = 5) and control (black diamond, n = 6) as a function of time post-injection. No significant differences were found between the treatments (p >0.1). B) Tumor weight upon extraction as a function of time post-injection is shown for all treatment options. All the treatments reduce tumor weight as compare to control. (C) Trichrome staining of tissue targets comparing all four treatments: (1) control, (2) gel/H2O2, (3) gel/5FU without H2O2, and (4) gel/5FU with H2O2.
4. Discussion
This study reports the ability for nsP DBD to successfully eradicate subdermal B16 melanoma tumors in the C57BL6 mouse. Additionally, we determined that by blocking the effects of nsP DBD generated ROS/RNS and radicals with glycerol/NaHC03 gel, the treatment was no longer effective at eliminating the melanoma. In addition to ROS/RNS and radicals, nsP DBD simultaneously produces a nsPEF, radiation, and heat. Use of the glycerol/NaHC03 gel should not significantly affect the penetration of the nsPEF or, as we also show (Figure 3), affect the temperature generation,[86–88] thereby strengthening the conclusion that the killing is primarily due to ROS/RNS and radicals. This conclusion does not refute other studies reporting nsPEF as a successful treatment for melanoma[59,62,66,69,70] but only that the nsPEF associated with the generation of nsP DBD is not sufficient. Interestingly, a recent study showed when a μsPEF was applied in addition to plasma treatment, an enhanced antibacterial effect was observed.[89] This may indicate the nsPEF produced by plasma is not strong enough to have effects on its own but may certainly enhance the effectiveness of other plasma components. Additionally, the ability of nsP DBD treatment to eliminate melanoma is restored when H2O2 is added to the glycerol/NaHC03 gel but the glycerol/NaHC03 gel with H2O2 or 5FU alone, or 5FU/H2O2 together does not eliminate the melanoma. While taken together the results of this study clearly implicate the reaction chemistry generated by nsP DBD treatment as the mechanism of action, but importantly the potential interactions between each component generated by nsP DBD is not eliminated as a means to enhance the killing effectiveness.
Multiple studies describing the effect of plasma treatment of cells and tissues have detailed the effects of ROS/RNS and radicals generated in the plasma to stimulate intracellular ROS. Depending on the treatment dose and time, intracellular ROS either initiates signaling pathways controlling migration, proliferation, or differentiation. Alternatively, and in response to higher plasma doses it causes DNA damage, multiphase cell cycle arrest (cellular senescence), and ultimately apoptosis.[7] From our previous studies using μsP DBD, we concluded lower doses enhanced cell differentiation in in vitro culture[18] and limb autopod survival, growth, and elongation in organ culture.[17]
The effect of higher dose plasma treatments have been shown to play a crucial role in killing cancer cells.[31,90,91] For example, in a successful treatment of glioblastoma using nsP DBD, the authors demonstrated that the increased intra-cellular ROS were responsible for the treatment effectiveness. In our study, we show blocking ROS with the glycerol/NaHC03, the effectiveness of the nsP DBD treatment was lost (Figure 4). These findings are consistent with our previous reports[17,86] as well as work by others[5,40,92–96] which identifies the ROS production by plasma as the main mechanism of interaction with cells and tissues. Thus, taken together with these and other studies, we conclude that he reaction chemistry generated by the ROS/RNS and radicals produced in the plasma in an interaction with the molecules present in cells and tissues result in the death of cancer cells and the elimination of melanoma, to create an effective cancer therapy.
The other two major components generated during nsP DBD treatment are nsPEF and heat. Both of these factors individually have had some success in killing cancer cells in vitro and tumors in vivo.[71–75] Several differences exist between the nsPEF generated within the plasma and the types of nsPEF reported as an effective cancer treatment. First, the nsPEF generated in these studies used a special apparatus with electrodes designed to promote the flow of electric current through the tumor tissue. The applied nsPEF associated with the generation of nsP DBD is created between the high voltage electrode and the tissue which serves as the second electrode. Additionally, filaments generated within the plasma generate a local EF at their tips. However, the magnitude of a current flow through the tumor tissue cannot be compared to that generated by the nsPEF. Finally, the characteristics of the “in vivo” second electrode, which may change with the addition of gel, the vascularity the tissue composition, etc., can alter the characteristics of the plasma and thereby may create some differences in the electric field, temperature, or species production.
Cancer treatment with hyperthermia has also had some success. Goldberg et al. found that coagulation necrosis of tissues is induced in vitro when the local temperature is maintained at 50 °C for 6 min or longer.[80] While DBD plasmas have been described as non-thermal from a physical perspective, nsP DBD treatment can significantly elevate tissue temperature and potentially cause thermal damage in a biological sense. After a 7 min treatment, the tissue temperature was increased 26 ± 2 °C at 1 mm below the skin surface (Figure 3). However, as we show the addition of gel does not affect the ability of nsP DBD to increase tissue temperature (Figure 3), the temperature increase alone must be insufficient to eradicate the melanoma. While we determined that neither the nsPEF nor the hyperthermia generated by nsP DBD are primarily responsible for the successful elimination of the melanoma tumor, the combinatory effect of all plasma components most certainly enhance treatment effectiveness.
The effectiveness of the nsP DBD treatment was restored by the addition of H2O2 to the glycerol/NaHCO3 gel mixture, suggesting that H2O2 has the ability to reestablish/enhance the reaction chemistry produced by nsP DBD treatment. Topical gel/H2O2 treatment without plasma reduced tumor growth, but did not eliminate the tumor, suggesting that treatment with nsP DBD may induce a deeper penetration of the H2O2 (and the associated reaction chemistry) into the tumor. The interaction of plasma with H2O2 has the potential to generate toxic products including hydroxyl radicals and anions.[97] However, in the tumor tissue environment, the Fenton reaction (Eq. 1) may be the major source of these toxic products, as high concentrations of Fe2+/Fe3+ are present due to hemorrhage and red blood cell lysis.[98] Thus, in the presence of H2O2 there would be an increase of reaction products as illustrated below.
4.1. Equation 1: Fenton Reaction
| (1) |
Therefore, nsP DBD treatment could induce high concentrations of hydroxyl anions and radicals to initiate massive amounts of cellular damage and the effective killing of melanoma cells. The addition of the glycerol/NaHCO3 gel without H2O2 could also effectively inhibit this reaction. Additional chemical products generated by plasma include superoxide anion, ozone, and reactive nitrogen species and these can be involved in the following chemical reactions:[3–5,97–99]
4.2. Equation 2: Nitric Oxide Reactivity
| (2) |
4.3. Equation 3: Carbon Dioxide Reactivity
| (3) |
4.4. Equation 4: Ozone Reactivity
| (4) |
5. Conclusion
In conclusion, this study determined that nsP DBD treatment can be used effectively against melanoma tumors in the B16 orthotopic melanoma model. Based on the lack of tumor suppression in the absence of ROS and the success of the treatment with addition of H2O2, we propose that nsP DBD production of reactive oxygen and nitrogen species play a role in tumor suppression. We also show that neither the nsPEF nor the heat associated with nsP DBD significantly contributes to remission of the melanoma tumor. Taken together, our findings suggest that nsP DBD can be developed as a new therapeutic tool for melanoma and other epithelial cancers. Further studies are necessary to investigate the molecular and cellular mechanisms underlying the cellular response to nsP DBD and the interaction with the immune system as well as which species are most responsible for the tumor eradication. Together with other studies, these findings indicate that nsP DBD treatment is an effective cancer therapy that deserves further investigation.
Acknowledgments
We would like to thank Carol Diallo for her assistance with plasma treatment of melanoma and work with mice. We would like to thank Drexel University Co-ops Yang Wan, Nicolette Pepe, and Stephen Jo for assistance with this work. Special thanks go to Drs. Marla Steinbeck, Gary Freidman, and Paul Stauffer for their valuable discussions and insights into electric field properties, reactive species interactions, and thermal effects, respectively. In addition, we thank Drs. Alexander Fridman, Danil Dobrynin, and Greg Fridman from Drexel Plasma Institute for their technical assistance and discussions. The authors also thank Sin Park, Jeff Bulson, and Marc Zemel of MOE Medical Device, Inc., for their assistance with designing and building the electrodes used in this study and their generous gift to the Jefferson Foundation which helped fund this study. Additional funding from NIH supported the study of plasma/tissue interactions R01EB013011 (Freeman).
Contributor Information
Dr. Natalie Chernets, Department of Orthopaedic Surgery, Thomas Jefferson, University, 1015 Walnut Street, Philadelphia, Pennsylvania 19107
Deepa S. Kurpad, Department of Orthopaedic Surgery, Thomas Jefferson, University, 1015 Walnut Street, Philadelphia, Pennsylvania 19107
Dr. Vitali Alexeev, Department of Dermatology and Cutaneous Biology, Thomas Jefferson University, Philadelphia, Pennsylvania 19107
Dr. Dario B. Rodrigues, Department of Radiation Oncology, Thomas Jefferson University, Philadelphia, Pennsylvania 19107
Dr. Theresa A. Freeman, Department of Orthopaedic Surgery, Thomas Jefferson, University, 1015 Walnut Street, Philadelphia, Pennsylvania 19107. Department of Dermatology and Cutaneous Biology, Thomas Jefferson University, Philadelphia, Pennsylvania 19107.
References
- 1.Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS, Testori A, Larue L, Goding CR. Genes Dev. 2006;20:3426. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoek KS, Goding CR. Pigment Cell Melanoma Res. 2010;23:746. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 3.Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Plasma Process Polym. 2008;5:503. [Google Scholar]
- 4.Li Y, Kojtari A, Friedman G, Brooks AD, Fridman A, Ji HF. J Phys Chem B. 2014;118:1612. doi: 10.1021/jp411440k. [DOI] [PubMed] [Google Scholar]
- 5.Graves DB. J Phys D: Appl Phys. 2012;45:263001. [Google Scholar]
- 6.Brulle L, Vandamme M, Ries D, Martel E, Robert E, Lerondel S, Trichet V, Richard S, Pouvesle JM, Le Pape A. PLoS One. 2012;7:e52653. doi: 10.1371/journal.pone.0052653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B, Pouvesle JM, Pape AL. Int J Cancer. 2012;130:2185. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- 8.Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Nature. 2001;413:271. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- 9.Nuccitelli R, Chen X, Pakhomov AG, Baldwin WH, Sheikh S, Pomicter JL, Ren W, Osgood C, Swanson RJ, Kolb JF, Beebe SJ, Schoenbach KH. Int J Cancer. 2009;125:438. doi: 10.1002/ijc.24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuccitelli R, Tran K, Sheikh S, Athos B, Kreis M, Nuccitelli P. Int J Cancer. 2010;127:1727. doi: 10.1002/ijc.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beebe SJ, Blackmore PF, White J, Joshi RP, Schoenbach KH. Physiol Meas. 2004;25:1077. doi: 10.1088/0967-3334/25/4/023. [DOI] [PubMed] [Google Scholar]
- 12.Krysko DV, D’Herde K, Vandenabeele P. Apoptosis. 2006;11:1709. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 13.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP, Greten TF. Int J Cancer. 2003;103:205. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 14.Nuccitelli R, Tran K, Lui K, Huynh J, Athos B, Kreis M, Nuccitelli P, De Fabo EC. Pigment Cell Melanoma Res. 2012;25:618. doi: 10.1111/j.1755-148X.2012.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiming X, Liu LZ, Loizidou M, Ahmed M, Charles IG. Cell Res. 2002;12:311. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 16.Choudhari SK, Chaudhary M, Bagde S, Gadbail AR, Joshi V. World J Surg Oncol. 2013;11:118. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernets N, Zhang J, Steinbeck MJ, Kurpad DS, Koyama E, Friedman G, Freeman TA. Tissue Eng Part A. 2015;21:300. doi: 10.1089/ten.tea.2014.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinbeck MJ, Chernets N, Zhang J, Kurpad DS, Fridman G, Fridman A, Freeman TA. PloS One. 2013;8:e82143. doi: 10.1371/journal.pone.0082143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sensenig R, Kalghatgi S, Cerchar E, Fridman G, Shereshevsky A, Torabi B, Arjunan KP, Podolsky E, Fridman A, Friedman G. Ann Biomed Eng. 2011;39:674. doi: 10.1007/s10439-010-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Kim G, Kim W, Kim K, Lee J. Appl Phys Lett. 2010;96:021502. [Google Scholar]
- 21.Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, Friedman G, Azizkhan-Clifford J. PloS One. 2011;6:e16270. doi: 10.1371/journal.pone.0016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, Vasilets V, Friedman G. Plasma Chem Plasma Process. 2007;27:163. [Google Scholar]
- 23.Lee H, Shon C, Kim Y, Kim S, Kim G, Kong MG. New J Phys. 2009;11:115026. [Google Scholar]
- 24.Shi XM, Chang ZS, Wu XL, Zhang GJ, Peng ZY, Dong ZY, Shao XJ. Plasma Process Polym. 2013;10:808. [Google Scholar]
- 25.Arndt S, Wacker E, Li YF, Shimizu T, Thomas HM, Morfill GE, Karrer S, Zimmermann JL, Bosserhoff AK. Exp Dermatol. 2013;22:284. doi: 10.1111/exd.12127. [DOI] [PubMed] [Google Scholar]
- 26.Iseki S, Nakamura K, Hayashi M, Tanaka H, Kondo H, Kajiyama H, Kano H, Kikkawa F, Hori M. Appl Phys Lett. 2012;100:113702. [Google Scholar]
- 27.Kim CH, Kwon S, Bahn JH, Lee K, Jun SI, Rack PD, Baek SJ. Appl Phys Lett. 2010;96:243701. doi: 10.1063/1.3449575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Li M, Zhou R, Feng K, Yang S. Appl Phys Lett. 2008;93:021502. [Google Scholar]
- 29.Kim JY, Ballato J, Foy P, Hawkins T, Wei Y, Li J, Kim SO. Biosens Bioelectron. 2011;28:333. doi: 10.1016/j.bios.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Holmes B, Cheng X, Zhu W, Keidar M, Zhang LG. PloS One. 2013;8:e73741. doi: 10.1371/journal.pone.0073741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaushik NK, Attri P, Kaushik N, Choi EH. Molecules. 2013;18:4917. doi: 10.3390/molecules18054917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratovitski EA, Cheng X, Yan D, Sherman JH, Canady J, Trink B, Keidar M. Plasma Process Polym. 2014;11:1128. [Google Scholar]
- 33.Thiyagarajan M, Anderson H, Gonzales XF. Biotechnol Bioeng. 2014;111:565. doi: 10.1002/bit.25114. [DOI] [PubMed] [Google Scholar]
- 34.Kaushik N, Uddin N, Sim GB, Hong YJ, Baik KY, Kim CH, Lee SJ, Kaushik NK, Choi EH. Scientific Rep. 2015:5. doi: 10.1038/srep08587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishaq M, Kumar S, Varinli H, Han ZJ, Rider AE, Evans MD, Murphy AB, Ostrikov K. Mol Biol Cell. 2014;25:1523. doi: 10.1091/mbc.E13-10-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panngom K, Baik K, Nam M, Han J, Rhim H, Choi E. Cell Death Dis. 2013;4:e642. doi: 10.1038/cddis.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst AM, Frame FM, Maitland NJ, Connell D. BioMed Res Int. 2014;2014:15. doi: 10.1155/2014/878319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang JW, Kang SU, Shin YS, Kim KI, Seo SJ, Yang SS, Lee JS, Moon E, Lee K, Kim CH. PloS One. 2014;9:e92198. doi: 10.1371/journal.pone.0092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ja Kim S, Min Joh H, Chung TH. Appl Phys Lett. 2013;103:153705. [Google Scholar]
- 40.Min Joh H, Ja Kim S, Chung TH, Leem SH. Appl Phys Lett. 2012;101:053703. [Google Scholar]
- 41.Siu A, Volotskova O, Cheng X, Khalsa SS, Bian K, Murad F, Keidar M, Sherman JH. PloS One. 2015;10:e0126313. doi: 10.1371/journal.pone.0126313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SB, Kim B, Bae H, Lee H, Lee S, Choi EH, Kim SJ. PloS One. 2015;10:e0129931. doi: 10.1371/journal.pone.0129931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babington P, Rajjoub K, Canady J, Siu A, Keidar M, Sherman JH. Biointerphases. 2015;10:029403. doi: 10.1116/1.4915264. [DOI] [PubMed] [Google Scholar]
- 44.Akhlaghi M, Rajayi H, Mashayekh AS, Khani M, Hassan ZM, Shokri B. Biointerphases. 2015;10:029510. doi: 10.1116/1.4918806. [DOI] [PubMed] [Google Scholar]
- 45.Omata Y, Iida M, Yajima I, Takeda K, Ohgami N, Hori M, Kato M. Environ Health Prevent Med. 2014;19:367. doi: 10.1007/s12199-014-0399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar N, Attri P, Yadav DK, Choi J, Choi EH, Uhm HS. Scientific Rep. 2014;4:7589. doi: 10.1038/srep07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Utsumi F, Kajiyama H, Nakamura K, Tanaka H, Hori M, Kikkawa F. SpringerPlus. 2014;3:1. doi: 10.1186/2193-1801-3-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlegel J, Köritzer J, Boxhammer V. Clin Plasma Med. 2013;1:2. [Google Scholar]
- 49.Adachi T, Tanaka H, Nonomura S, Hara H, Kondo S-i, Hori M. Free Radical Biol Med. 2015;79:28. doi: 10.1016/j.freeradbiomed.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Ma Y, Ha CS, Hwang SW, Lee HJ, Kim GC, Lee KW, Song K. PloS One. 2014;9:e91947. doi: 10.1371/journal.pone.0091947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirst A, Simms M, Mann V, Maitland N, O’Connell D, Frame F. Br J Cancer. 2015;112:1536. doi: 10.1038/bjc.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volotskova O, Hawley TS, Stepp MA, Keidar M. Scientific Rep. 2012;2:636. doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu D, Liu D, Wang B, Chen C, Chen Z, Li D, Yang Y, Chen H, Kong MG. PloS One. 2015;10:e0128205. doi: 10.1371/journal.pone.0128205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arjunan KP, Sharma VK, Ptasinska S. Int J Mol Sci. 2015;16:2971. doi: 10.3390/ijms16022971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.López-Lázaro M. Mol Nutr Food Res. 2008;52:S103. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 56.Beebe SJ, Fox PM, Rec LJ, Willis EL, Schoenbach KH. FASEB J. 2003;17:1493. doi: 10.1096/fj.02-0859fje. [DOI] [PubMed] [Google Scholar]
- 57.Ren W, Beebe SJ. Apoptosis. 2011;16:382. doi: 10.1007/s10495-010-0572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall EH, Schoenbach KH, Beebe SJ. Apoptosis. 2007;12:1721. doi: 10.1007/s10495-007-0083-7. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Yin S, Hu C, Chen X, Jiang K, Ye S, Feng X, Fan S, Xie H, Zhou L. PloS One. 2014;9:e86421. doi: 10.1371/journal.pone.0086421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semenov I, Xiao S, Pakhomov AG. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2013;1828:981. doi: 10.1016/j.bbamem.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuccitelli R, Tran K, Sheikh S, Athos B, Kreis M, Nuccitelli P. Int J Cancer. 2010;127:1727. doi: 10.1002/ijc.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nuccitelli R, Chen X, Pakhomov AG, Baldwin WH, Sheikh S, Pomicter JL, Ren W, Osgood C, Swanson RJ, Kolb JF. Int J Cancer. 2009;125:438. doi: 10.1002/ijc.24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beebe SJ, Fox PM, Rec LJ, Willis ELK, Schoenbach KH. FASEB J. 2003;17:1493. doi: 10.1096/fj.02-0859fje. [DOI] [PubMed] [Google Scholar]
- 64.Beebe SJ, Blackmore PF, White J, Joshi RP, Schoenbach KH. Physiol Measure. 2004;25:1077. doi: 10.1088/0967-3334/25/4/023. [DOI] [PubMed] [Google Scholar]
- 65.Buescher ES, Smith RR, Schoenbach KH. Plasma Sci, IEEE Transactions on. 2004;32:1563. [Google Scholar]
- 66.Beebe SJ, Fox PM, Rec LJ, Somers K, Stark RH, Schoenbach KH. Plasma Sci, IEEE Transactions on. 2002;30:286. [Google Scholar]
- 67.Chen X, James Swanson R, Kolb JF, Nuccitelli R, Schoenbach KH. Melanoma Res. 2009;19:361. doi: 10.1097/CMR.0b013e32832f1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nuccitelli R, Pliquett U, Chen X, Ford W, Swanson RJ, Beebe SJ, Kolb JF, Schoenbach KH. Biochem Biophys Res Commun. 2006;343:351. doi: 10.1016/j.bbrc.2006.02.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X, Swanson RJ, Kolb JF, Nuccitelli R, Schoenbach KH. Melanoma Res. 2009;19:361. doi: 10.1097/CMR.0b013e32832f1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vernier PT, Sun Y, Marcu L, Salemi S, Craft CM, Gundersen MA. Biochem Biophys Res Commun. 2003;310:286. doi: 10.1016/j.bbrc.2003.08.140. [DOI] [PubMed] [Google Scholar]
- 71.Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, Zee Jvd, van Putten WL, van Rhoon GC, van Dijk JD, González DG. Int J Radiat Oncol Biol Phys. 1996;35:731. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 72.Sterzer F, Paglione R, Mendecki J, Friedenthal E, Botstein C. Spectrum, IEEE. 1980;17:32. doi: 10.1016/0360-3016(80)90019-x. [DOI] [PubMed] [Google Scholar]
- 73.Ko SH, Ueno T, Yoshimoto Y, Yoo JS, Abdel-Wahab OI, Abdel-Wahab Z, Chu E, Pruitt SK, Friedman HS, Dewhirst MW. Clin Cancer Res. 2006;12:289. doi: 10.1158/1078-0432.CCR-05-0210. [DOI] [PubMed] [Google Scholar]
- 74.Stehlin J, Giovanella Bd, De Ipolyi P, Muenz L, Anderson R. Surg Gynecol Obs. 1975;140:339. [PubMed] [Google Scholar]
- 75.Bolfarini GC, Siqueira-Moura MP, Demets GJ, Morais PC, Tedesco AC. J Photochem Photobiol B: Biol. 2012;115:1. doi: 10.1016/j.jphotobiol.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Dahl O. Acta Oncologica. 1999;38:863. doi: 10.1080/028418699432554. [DOI] [PubMed] [Google Scholar]
- 77.Stauffer P, Goldberg S. Int J Hyper. 2004;20:671. doi: 10.1080/02656730400007220. [DOI] [PubMed] [Google Scholar]
- 78.Overgaard J, Bentzen S, Gonzalez DG, Hulshof M, Arcangeli G, Dahl O, Mella O. Lancet. 1995;345:540. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 79.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. J Clin Oncol. 2005;23:3079. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 80.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Acad Radiol. 1996;3:212. doi: 10.1016/s1076-6332(96)80443-0. [DOI] [PubMed] [Google Scholar]
- 81.Miller V, Lin A, Fridman G, Dobrynin D, Fridman A. Plasma Process Polym. 2014;11:1193. doi: 10.1002/ppap.201400232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collet G, Robert E, Lenoir A, Vandamme M, Darny T, Dozias S, Kieda C, Pouvesle JM. Plasma Sources Sci Technol. 2014;23:012005. [Google Scholar]
- 83.Wosko TJ, Ferrara DT, Sartori LS. Cancer Lett. 1984;24:57. doi: 10.1016/0304-3835(84)90080-6. [DOI] [PubMed] [Google Scholar]
- 84.Chernets N, Zhang J, Steinbeck M, Kurpad DS, Koyama E, Friedman G, Freeman T. Tissue Eng. 2014 doi: 10.1089/ten.tea.2014.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seepersad Y, Pekker M, Shneider MN, Fridman A, Dobrynin D. J Phys D: Appl Phys. 2013;46:355201. [Google Scholar]
- 86.Lin A, Chernets N, Han J, Alicea Y, Dobrynin D, Fridman G, Freeman TA, Fridman A, Miller V. Plasma Process Polym. 2015 doi: 10.1002/ppap.201400232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Babaeva NY, Tian W, Kushner MJ. J Phys D: Appl Phys. 2014;47:235201. [Google Scholar]
- 88.Liu C, Dobrynin D, Fridman A. J Phys D: Appl Phys. 2014;47:252003. doi: 10.1088/0022-3727/47/25/252003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Q, Zhuang J, von Woedtke T, Kolb JF, Zhang J, Fang J, Weltmann KD. Appl Phys Lett. 2014;105:104103. [Google Scholar]
- 90.Sensenig R, Kalghatgi S, Cerchar E, Fridman G, Shereshevsky A, Torabi B, Arjunan KP, Podolsky E, Fridman A, Friedman G, Azizkhan-Clifford J, Brooks AD. Ann Biomed Eng. 2011;39:674. doi: 10.1007/s10439-010-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Panngom K, Baik KY, Nam MK, Han JH, Rhim H, Choi EH. Cell Death Dis. 2013;4:e642. doi: 10.1038/cddis.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laroussi M, Leipold F. Int J Mass Spectrom. 2004;233:81. [Google Scholar]
- 93.Murakami T, Niemi K, Gans T, O’Connell D, Graham WG. Plasma Sources Sci Technol. 2013;22:015003. [Google Scholar]
- 94.Arjunan KP, Friedman G, Fridman A, Clyne AM. J R Soc Interf. 2012;9:147. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gaunt LF, Beggs CB, Georghiou GE. Plasma Sci, IEEE Transactions on. 2006;34:1257. [Google Scholar]
- 96.Vandamme M, Robert E, Pesnel S, Barbosa E, Dozias S, Sobilo J, Lerondel S, Le Pape A, Pouvesle JM. Plasma Process Polym. 2010;7:264. [Google Scholar]
- 97.Wende K, Williams P, Dalluge J, Van Gaens W, Aboubakr H, Bischof J, von Woedtke T, Goyal SM, Weltmann KD, Bogaerts A. Biointerphases. 2015;10:029518. doi: 10.1116/1.4919710. [DOI] [PubMed] [Google Scholar]
- 98.Winterbourn CC. Nat Chem Biol. 2008;4:278. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 99.Lymar SV, Hurst JK. J Am Chem Soc. 1995;117:8867. [Google Scholar]