Abstract
Background
Understanding the precise anatomy in experimental animals is crucial to correct design of research projects. Rats are commonly used for scientific research in plastic surgery because of their availability in academic institutions, moderate cost and sizable vessels for microsurgical procedures. In past publications about rat anatomy, lymphatic mapping has been limited and incomplete. The aim of this study was to comprehensively map the superficial lymphatic system in the rat.
Methods
Twenty-seven Sprague–Dawley rats were used for this study. Indocyanine green (ICG) fluorescence lymphography was used to identify the lymphatic vessels and lymph nodes. Under general anaesthesia, ICG was injected intradermally at multiple spots along the dorsal and medial midlines, front and hind paws, and tail. The course of the lymphatic vessels was traced on the skin with marker pen and photographed. The superficial lymphatic vessels in each rat were sketched on a graphic template and all the templates were superimposed using graphic software to define the relationship between the lymphatic vessel and sentinel node.
Results
ICG fluorescence lymphography was able to demonstrate the superficial lymphatic vessels in the rat. Six groups of regional lymph node/s were identified and lymphatic pathways to those nodes delineated. Our lymphosome concept was successfully applied to the rat, with six lymphosomes identified.
Conclusions
We succeeded in performing superficial lymphatic mapping in the rat. Our anatomical findings can provide further information about the lymphatic system in the normal state and promote understanding of pathological changes generated by surgical manipulation for future studies.
Keywords: lymphatic system, lymphatic vessel, Rat, indocyanine green fluorescence lymphography, sentinel node
Introduction
Rats have been the workhorse for scientific research in plastic surgery for decades. Various skin flaps were designed using the rat model to investigate flap physiology and to test the effect of drugs on flap survival.1-3 In addition, the rat has arteries and veins of a good size that have enabled investigators to perform microsurgical procedures. Unlike larger experimental animals, rats are readily available in most of academic institutions and teaching hospitals and the material and housing costs they incur are moderate.
Understanding the precise anatomy in each species is crucially important to the design of experimental studies and collection of accurate data for testing hypotheses. There are published books available that describe the rat anatomy in detail and present the structures of muscles, bones, nerves and blood vessels similarly to an atlas of anatomy in humans.4,5 In these studies, vascular anatomy was mapped using a total-body injection technique with radiocontrast media, revealing that the skin could be demarcated into arterial territories, namely, angiosomes.6 However, there is very limited information available about the rat's lymphatic anatomy. Although some studies have focused on the anatomy of the lymphatic system in rats, they describe the location of the lymph nodes only, with description of the lymphatic pathways either fragmented or non-existent.7-10
We have also investigated the anatomy of the lymphatic system in human and animal cadaveric specimens using a radiographic microinjection technique developed by the author (HS).11-12 The technique is composed of two steps: identification of the lymphatic vessel with hydrogen peroxide followed by cannulation and injection of radiocontrast media directly into the vessel. We previously studied the lymphatic anatomy in rats using this microinjection technique, but this technique only worked for lymphatic vessels larger than 100 μm.13 We could identify the lymphatic vessels originating from the limbs and tail to the thoracic duct, but we were not able to do so in the head, neck and torso regions where the vessels were smaller.
Indocyanine green (ICG) fluorescence lymphography is an emerging imaging technology to demonstrate the lymphatic vessels in humans and animal experiments.14-17 We planned to apply this imaging technique to identify all the lymphatic vessels in a rat model for this study. Our aims were to comprehensively map the superficial lymphatic system in the rat using ICG lymphography and define the lymphatic territories (lymphosomes).
Materials and Methods
The animal protocol for this study was reviewed and approved by the University of Texas Institutional Animal Care and Use Committee (IACUC), which is accredited by the American Association for Laboratory Animal Science (AALAS). The anatomical terminology used for the rat lymph nodes followed the description in Tinley's article.10
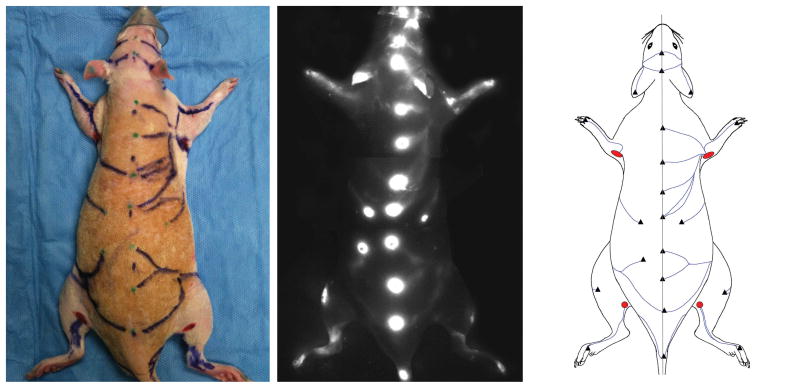
The study used twenty-seven Sprague–Dawley rats of mixed gender and weighing 300-400g. The rats were anesthetised with isoflurane gas and the body hair was shaved with electric clippers. Firstly, the rat was placed in the abdominal position. The dorsal skin was distributed evenly on both sides and the dorsal midline marked with a skin marker pen. A 0.01 ml solution of indocyanine green (IC Green, Akorn, Inc. IL, USA) comprising 0.25mg of ICG dye was injected intradermally along the dorsal midline at 1.5 cm intervals with a 30G needle and 1cc syringe. After the injection, the ICG fluorescence lymphography system (Photodynamic Eye, Hamamatsu K.K., Hamamatsu, Japan) was used to identify the lymphatic vessels. If the lymphatic vessel was not detected at the injection site after 10 min, an additional 0.01 ml of ICG solution was injected a few centimetres to the side of the initial injection site. Each lymphatic vessel was traced until it connected to a lymph node (Fig 1, left) and the course of the lymphatic vessel was traced on the skin with marker pen. Secondly, the body was flipped over and placed in the spinal position. The ventral midline was marked through the umbilicus and the ICG solution was injected in the same manner. The lymphatic vessels on the ventral side were also followed to their connecting lymph nodes and traced on the skin with marker pen. Additional ICG injections were given at the paws (n=9), ears (n=2) and nose (n=1). The lymphatic pathways in the dorsal and ventral views were photographed (Fig 1, middle) and the superficial lymphatic vessels in each rat were sketched on a graphic template (Fig 1, right). After sacrificing the rat, the skin at the lymph nodes was cut and an ICG lymphography scan was performed again to confirm the connections between the lymphatic vessel and the lymph node (Fig 2).
Fig. 1.
ICG fluorescence imaging in the rat (left). Bright spots are the ICG injection sites. Lymphatic vessel pathways are marked with marker (middle). The course of the lymphatic vessels and lymph nodes (red) is sketched on a graphic template (right).
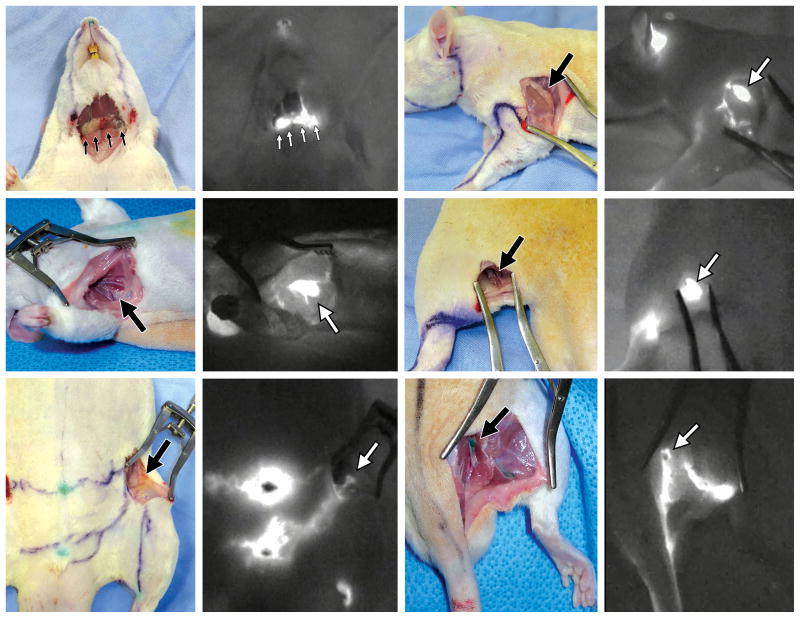
Fig. 2.
Six pairs of images show groups of sentinel node/s (arrow/s) with a photograph (left) and ICG lymphography image (right). Clockwise from top left: superficial cervical, brachial, popliteal, gluteal, inguinal, and axillary nodes.
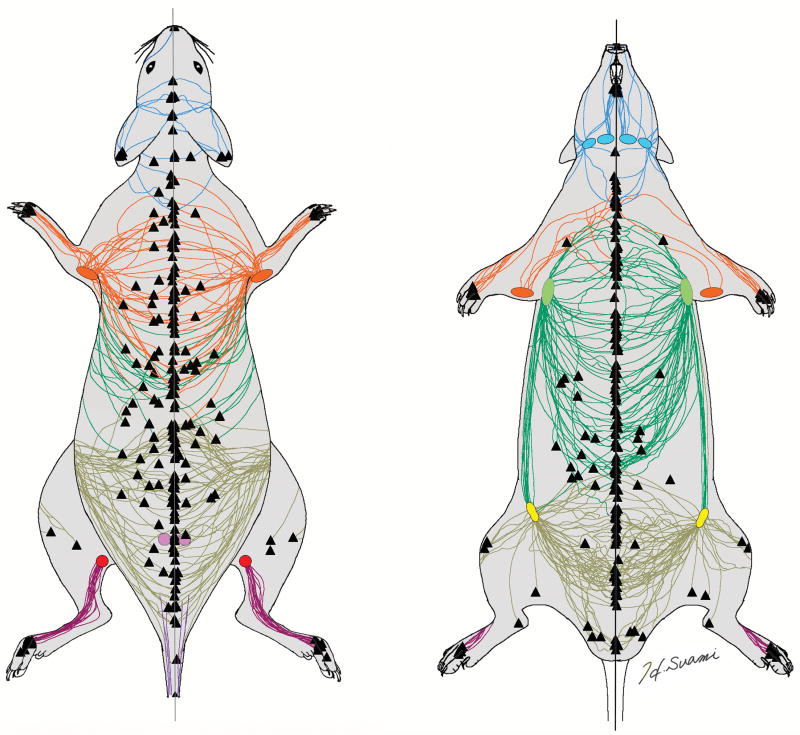
The lymphosome is our new anatomical concept by which the skin can be divided into lymphatic territories according to their corresponding sentinel node.15-17 We were also able to apply this concept to the subjects of this study. Each regional group of sentinel node/s was color-coded and the lymphatic vessels connecting to a particular node were color-coded with the same colour. Each graphic template was scanned and all the templates were then superimposed using graphic software (Adobe Photoshop CC, Adobe Systems Inc) (Fig 3). In this way, we could map the superficial lymphatic system in the rat to clarify the relationship between the skin region and the corresponding lymph node.
Fig. 3.
Superimposed diagrams of the superficial lymphatic vessels color-coded according to the lymphosome concept: superficial cervical (blue), brachial (orange), axillary (green), inguinal (yellow), popliteal (red) and gluteal (purple). Triangles are the ICG injection sites.
Results
We used ICG lymphography to identify the superficial lymphatic vessels in any region in the rat body. A few minutes after the ICG injection, the lymphatic vessels appeared near the injection spot. Once the ICG dye was taken up by the lymphatic vessel, we could facilitate its movement inside the lumen by massaging the skin with a finger and squeezing the vessel with the tip of a skin marker. The rat is a loose-skin animal and it was hard to accurately determine the dorsal and ventral midlines. These midlines worked as a lymph watershed between the two sides, so on some occasions we were only able to identify the lymphatic vessels on one side. However, we could identify additional lymphatic vessels in the blank area in 12 out of 27 rats (44.4 %) by doing extra ICG injections in the opposite side. When ICG was injected at the midline, the lymphatic vessels could be seen symmetrically on both sides. Midline crossing vessels never been observed on either the dorsal or ventral sides. These findings confirmed that the midline was one of the watersheds between lymphatic territories.
ICG fluorescence lymphography detected a sentinel node for each lymphatic vessel as a bright shiny spot. Unlike our previous studies in large animals, the lymph nodes in rats were located more superficially, less than 1cm below the surface of the skin.16,17 We could specify all the sentinel nodes once we cut the skin and used ICG lymphography to follow the course of each lymphatic vessel back to the node (Fig 2). The skin lymphatics connected to six groups of sentinel lymph nodes: superficial cervical, brachial, axillar, inguinal, gluteal and popliteal. Each sentinel node received lymphatic drainage from a different region, these being the head and neck, forelimb and dorsal upper torso, dorsal middle and ventral upper torso, dorsal lower, ventral lower torso and thigh, and lower leg respectively. We color-coded each lymph node group and then color-coded the corresponding lymphatic vessels to match. We were not able to demonstrate all the lymphatic vessels in each individual rat and thereby could not count the number of lymphatic vessels. However, the superimposed image reveals that the lymphatic drainage patterns were consistent for all the rats (Fig 3) and this enabled us to demarcate the skin into six lymphatic territories, namely, six lymphosomes.
The lymphatic system in rats demonstrated several specific characteristics that have never been found in our studies in other animals and humans.16-19 The brachial lymph node was located outside the axillary fossa, caudal from the triceps brachii and lateral from the lattismus dorsi. The lymphatic vessels in the forelimb and cranial part of the dorsal upper torso connected to this node. The efferent lymphatic vessels from the inguinal lymph node connected directly to the axillary node. In other animals and humans, a horizontal line at the umbilical level is the watershed between the thoracic and abdominal lymphatic territories. This unique pathway in the rat was consistently identified across the cohort.
Discussion
Rats are the most common animal model used in research and microsurgical training in plastic surgery and they have contributed to the development of new surgical procedures. In the field of lymphatic research, vascularised lymph node transfer (VLNT) was initially reported in the rat.20 The transferred lymph node retained functional lymphatic structure when examined through histology. This innovative finding led to the development of autologous lymph node transfer which has become one of the popular surgical options for the treatment of lymphoedema in the clinical setting.21 Several new VLNT donor sites were introduced in rats and functional mechanism was investigated.22-25 In addition, the patency of lymphovenous anastomosis was studied in rat models and lymphatic structural changes after surgical manipulation were investigated using ICG fluorescence lymphography.15,26-29 Different patterns of ICG fluorescence lymphography in lymphedema patients were investigated in relation to pathological changes in the lymphatic vessel.30 Future animal research must also focus more on the lymphatic vessels and therefore the baseline ICG image of the lymphatic system as discussed in this study would be expected to contribute to this aspect.
Several methods were developed to identify the lymphatic vessels in animals. Baum used dye injection to investigate the anatomy of the lymphatic system in different domestic animals.31,32 Dye injection is a simple and effective method in large animals, but the lymphatic vessels in small animals did not consistently take up the dye, and the injected dye contaminated the subcutaneous tissue.12 Another method is conventional lymphangiography which was also applied to rat studies and involved injecting mercury or radiocontrast media.8,9,12 ICG fluorescence lymphography was later introduced to describe the lymphatic vessels in the rat hind limb14. In this study, we found that ICG lymphography was useful in identifying the lymphatic vessels, not only in the rat hind limb, but also in other body regions.
The lymphosome concept is a new anatomical concept we have developed.16 -18 In the rat, a lymphatic vessel sometimes converged with other lymphatic vessels within a lymphosome, but connected to a single sentinel node only. This anatomical characteristic enabled us to delineate the borders of the lymphosomes (Fig 3 and 4). Tinley conducted a dye injection study to investigate the lymphatic drainage patterns in rats.10 His findings about skin lymphatic territories were consistent with our findings. However, our lymphatic mapping has the additional value of describing the pathway of the lymphatic vessel which could not be obtained through the dye injection method. Our detailed anatomical information about normal lymphatic pathways will be essential in evaluating lymph structural changes in post-surgical procedures.
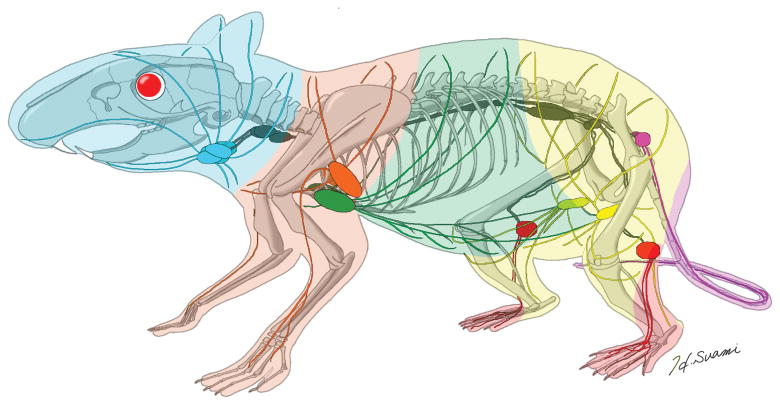
Fig. 4.
Three-dimensional diagram of the lymphatics and lymphosomes in the rat. Lymphosomes are indicated as: superficial cervical (blue), brachial (orange), axillary (green), inguinal (yellow), popliteal (red) and gluteal (purple). Deep lymphatics are shown in grey.
We combined our results for the superficial lymphatic system with our previous results for the deep lymphatic system to construct a summary three-dimensional diagram describing the lymphatic system in the rat (Fig 4).13 In both studies, we were able to determine the location of the sentinel lymph nodes, but in this current study we were able to use ICG to supplement our knowledge of the deep lymphatics with new information about the superficial lymphatic system. The direct connection of the lymphatic vessels between the inguinal and axillary lymph nodes is a unique anatomical structure in the rat. Understanding the anatomical differences between species is important for designing lymphatic research in experimental animals and translating new findings to the clinical setting.
Conclusions
We succeeded in mapping the superficial lymphatic system in the rat using ICG fluorescence lymphography, an outcome not possible with conventional injection methods. We discovered that the skin can be divided into six lymphosomes: cervical, brachial, axillar, inguinal, popliteal and gluteal territories. Our findings into precise lymphatic mapping in the rat will serve as a template for the normal rat anatomy and assist investigators to design new studies.
Acknowledgments
This research was supported by the University Cancer Foundation via the Institutional Research Grant Program of The University of Texas MD Anderson Cancer Center, the Kyte Plastic Surgery Research Fund, and the NIH/NCI under award number P30CA016672. We thank Philippa Sutton, Faculty of Health and Medical Sciences, Macquarie University for editing the manuscript.
Footnotes
Financial Disclosure: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Authors' Roles: Hiroo Suami: design of the study, performing animal procedures, collection of the data, imaging work, literature review, and writing of the manuscript.
Mario F. Scaglioni: performing animal procedures, collection of the data, and editing the manuscript.
References
- 1.Cormack GC, Lamberty BG. The microcirculation. In: Cormack GC, Lamberty BG, editors. The arterial anatomy of skin flaps. 2nd ed. London: Churchill Livingstone; 1994. pp. 15–69. [Google Scholar]
- 2.Lee S, editor. Experimental microsurgery. New York: Igaku-Shoin; 1987. [Google Scholar]
- 3.Siemionow MZ, editor. Plastic and reconstructive surgery; experimental models and research designs. London: Springer-Verlag; 2015. [Google Scholar]
- 4.Greene EC, editor. Anatomy of the rat. Philadelphia: Hunter Publishing Company; 1935. [Google Scholar]
- 5.Popsko P, Rajytova V, Horak J, editors. A colour atlas of the anatomy of small laboratory animals. Vol 2; Rat, mouse, golden hamster. London: Saunders; 1992. [Google Scholar]
- 6.Taylor GI, Minabe T. The angiosomes of the mammals and other vertebrates. Plast Reconstr Surg. 1992;89:181–215. doi: 10.1097/00006534-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Job TT. The adult anatomy of the lymphatic system in the common rat (epimys norvegicus) Anat Rec. 1915;9:447–455. [Google Scholar]
- 8.Engeset A. The route of peripheral lymph to the blood stream; an x-ray study of the barrier theory. J Anat. 1959;93:96–100. [PMC free article] [PubMed] [Google Scholar]
- 9.Engeset A, Tjotta E. Lymphatic pathways from the tail in rats and mice. Cancer Res. 1960;20:613–614. [Google Scholar]
- 10.Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109:369–383. [PMC free article] [PubMed] [Google Scholar]
- 11.Suami H, Taylor GI, Pan WR. A new radiographic cadaver injection technique for investigating the lymphatic system. Plast Reconstr Surg. 2005;115:2007–2013. doi: 10.1097/01.prs.0000163325.06437.b0. [DOI] [PubMed] [Google Scholar]
- 12.Suami H, Taylor GI, O'Neill J, Pan WR. Refinements of the radiographic cadaver injection technique for investigating minute lymphatic vessels. Plast Reconstr Surg. 2007;120:61–67. doi: 10.1097/01.prs.0000263321.64228.53. [DOI] [PubMed] [Google Scholar]
- 13.Suami H, Chang DW, Matsumoto K, Kimata Y. Demonstrating the lymphatic system in rats with microinjection. Anat Rec (Hoboken) 2011;294:1566–1573. doi: 10.1002/ar.21446. [DOI] [PubMed] [Google Scholar]
- 14.Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 15.Ogata F, Azuma R, Kikuchi M, et al. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg. 2007;58:652–655. doi: 10.1097/01.sap.0000250896.42800.a2. [DOI] [PubMed] [Google Scholar]
- 16.Suami H, Yamashita S, Soto-Miranda MA, Chang DW. Lymphatic territories (lymphosomes) in a canine: an animal model for investigation of postoperative lymphatic alterations. PLoS One. 2013;8:e69222. doi: 10.1371/journal.pone.0069222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito R, Suami H. Lymphatic Territories (Lymphosomes) in Swine: An Animal Model for Future Lymphatic Research. Plast Reconstr Surg. 2015;136:297–304. doi: 10.1097/PRS.0000000000001460. [DOI] [PubMed] [Google Scholar]
- 18.Suami H. Lymphosome concept: Anatomical study of the lymphatic system. J Surg Oncol. 2017;115:13–17. doi: 10.1002/jso.24332. [DOI] [PubMed] [Google Scholar]
- 19.Soto-Miranda MA, Suami H, Chang DW. Mapping superficial lymphatic territories in the rabbit. Anat Rec (Hoboken) 2013;296:965–970. doi: 10.1002/ar.22699. [DOI] [PubMed] [Google Scholar]
- 20.Shesol BF, Nakashima R, Alavi A, Hamilton RW. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast Reconstr Surg. 1979;63:817–823. [PubMed] [Google Scholar]
- 21.Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. doi: 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiecien GJ, Uygur S, Korn J, et al. Vascularized axillary lymph node transfer: A novel model in the rat. Microsurgery. 2015;35:662–667. doi: 10.1002/micr.22472. [DOI] [PubMed] [Google Scholar]
- 23.Visconti G, Constantinescu T, Chen PY, et al. The Venous Lymph Node Flap: Concepts, Experimental Evidence, and Potential Clinical Implications. J Reconstr Microsurg. 2016;32:625–631. doi: 10.1055/s-0036-1584527. [DOI] [PubMed] [Google Scholar]
- 24.Visconti G, Brunelli C, Mule A, et al. Septum-based cervical lymph-node free flap in rat: a new model. J Surg Res. 2016;201:1–12. doi: 10.1016/j.jss.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Ito R, Zelken J, Yang CY, et al. Proposed pathway and mechanism of vascularized lymph node flaps. Gynecol Oncol. 2016;141:182–188. doi: 10.1016/j.ygyno.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Fox U, Montorsi M, Romagnoli G. Experimental lymphatico-venous shunt in the rat: pressure controls and long-term patency. Lymphology. 1983;16:164–167. [PubMed] [Google Scholar]
- 27.Onoda S, Kimata Y, Matsumoto K, et al. Histologic Evaluation of Lymphaticovenular Anastomosis Outcomes in the Rat Experimental Model: Comparison of Cases with Patency and Obstruction. Plast Reconstr Surg. 2016;137:83e–91e. doi: 10.1097/PRS.0000000000001884. [DOI] [PubMed] [Google Scholar]
- 28.Takeno Y, Fujimoto E. Alterations of lymph flow after lymphadenectomy in rats revealed by real time fluorescence imaging system. Lymphology. 2013;46:12–19. [PubMed] [Google Scholar]
- 29.Buretta KJ, Brat GA, Christensen JM, et al. Near-infrared lymphography as a minimally invasive modality for imaging lymphatic reconstitution in a rat orthotopic hind limb transplantation model. Transpl Int. 2013;26:928–937. doi: 10.1111/tri.12150. [DOI] [PubMed] [Google Scholar]
- 30.Hara H, Mihara M, Seki Y, et al. Comparison of indocyanine green lymphographic findings ith the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast Reconstr Surg. 2013;132:1612–1618. doi: 10.1097/PRS.0b013e3182a97edc. [DOI] [PubMed] [Google Scholar]
- 31.Baum H. Das lymphgefasssystem den rindes. Berlin: Verlag von August Hirschwald; 1912. [Google Scholar]
- 32.Baum H. Das lymphgefasssystem den hundes. Berlin: Verlag von August Hirschwald; 1918. [Google Scholar]