Abstract
Background
Changes in the epidemiology of end stage liver disease may lead to increased risk of dropout from the liver transplant waitlist. Anticipating the future of liver transplant waitlist characteristics is vital when considering organ allocation policy.
Methods
We performed a discrete event simulation to forecast patient characteristics and rate of waitlist dropout. Estimates were simulated from 2015–2025. The model was informed by data from the Organ Procurement and Transplant Network, 2003–2014. National data are estimated along with forecasts for 2 regions.
Results
NASH will increase from 18% of waitlist additions to 22% by 2025. Hepatitis C will fall from 30% to 21%. Listings over age 60 will increase from 36% to 48%. The hazard of dropout will increase from 41% to 46% nationally. Wait times for transplant for patients listed with a MELD between 22 and 27 will double. Region 5, which transplants at relatively higher MELD scores, will experience an increase from 53% to 64% waitlist dropout. Region 11, which transplants at lower MELD scores, will have an increase in waitlist dropout from 30% to 44%.
Conclusions
The liver transplant waitlist size will remain static over the next decade due to patient dropout. Liver transplant candidates will be older, more likely to have NASH and will wait for transplantation longer even when listed at a competitive MELD score. There will continue to be significant heterogeneity among transplant regions where some patients will be more likely to drop out of the waitlist than receive a transplant.
Introduction
With the advent of highly effective medications for hepatitis C (HCV) and the ongoing epidemic of nonalcoholic fatty liver disease (NAFLD), the main etiology of end stage liver disease (ESLD) and liver transplantation (LT) will change over the next 2 decades.(1, 2) Implementation of birth-cohort screening for HCV and new medications with sustained virologic response rates >90% may render HCV an uncommon disease in the future.(1) Continued increases in diabetes and obesity will increase the incidence of NAFLD, and as the NAFLD population ages, many will develop cirrhosis and ESLD. The changing etiology of ESLD may impact the utility of listing patients for LT as older patients with multiple comorbidities may be more likely to die or be removed from the wait list than receive a transplant.(3, 4)
Already, the transplant community has seen stagnation if not slight decline in the numbers of deceased donor liver transplants performed nationally since 2006. Much of this decline is due to donor graft quality and donation after cardiac death (DCD) procurement strategies.(5) Transplant centers are less likely to use livers from donors with significant steatosis, advancing age, obesity, and DCD, even when they can transplant the donor’s other organs.(5) The recent decline in LT may be a harbinger of further reductions in LT in the coming years.(6) Such a decline in transplant availability would place a greater burden on centers managing ESLD patients on the waitlist.
Patient characteristics on the liver transplant waitlist have changed over the past decade and these attributes vary considerably by region.(4) In order to anticipate potential changes to waitlist attributes, we aimed to determine future demographic characteristics of the LT waiting list and determine the rate of waitlist removal and receipt of liver transplantation through discrete event simulation (DES). We performed our analyses using both national United Network of Organ Sharing (UNOS) data and data from 2 disparate regions to demonstrate the heterogeneity among UNOS regions.
Materials and Methods
Overview
We used the Standard Transplant Analysis and Research (STAR) dataset files from the Organ Procurement and Transplant Network (OPTN), a.k.a. the ‘UNOS database’ to create a DES model to predict patient characteristics and waitlist times on the liver transplant waitlist on a national level and for 2 selected UNOS regions. The DES model was informed by data from 2003–2012.(4) Data from 2013–2014 were withheld for validation of the model. We previously used this database to study the clinical and demographic attributes of the patient population on the waiting list (ie, describe demographic trends (gender, age, race) and other clinical attribute trends (model for end stage liver disease-MELD score, disease type, etc.)).(4) The current study focused on using a DES model to estimate waitlist times dynamically into the future while considering the changes in demographic and clinical attributes over time as well as liver availability. Each patient’s length of stay on the waitlist is controlled by his/her assigned “waiting time”, which is estimated by competing risk analysis. This is also used to determine their outcome ie, transplant versus drop out. Drop out is defined as removal from the list for any reason except transplant. While most dropouts occur because patients die or become too sick to transplant, a few (3.58% of waitlist additions nationally from 2003–2014)(4) occur due to ‘condition improved’. In this analysis, we include these patients because (i) 1 of our goals is to estimate waitlist size and these patients contribute to that; (ii) the number of these “good” dropouts is small in comparison and has been decreasing over time(4) and is expected to continue to do so.
Inclusion/Exclusion criteria
Inclusion and exclusion criteria for the simulation model are similar to the previously reported retrospective analysis.(4) Briefly, data from patients who received living donors and partial liver transplant were excluded. Pediatric patients under age 18 years of age were excluded. Acute liver failure (Status 1) patients were excluded. Data prior to the MELD era (February 2002) were excluded. We judged each of these groups to be systematically different than the clear majority of adults with ESLD awaiting an orthotopic liver transplant.
Setting
We studied the waitlist at the national level and specifically looked at region 5 and region 11. Region 5 includes Arizona, California, Nevada, New Mexico and Utah. It is 1 of the geographically largest and most populous transplant regions in US and transplants at very high MELD scores; we believe these data are representative of the high MELD regions. Region 11 includes North Carolina, Kentucky, South Carolina, Tennessee and Virginia. In contrast to region 5, region 11 is representative of smaller regions that can transplant at relatively lower MELD scores.
Variables
Initial MELD score
Initial MELD score is the first MELD score recorded when a patient joins the list. The score ranges from 6 to 40, and was categorized in quartiles based on clinical factors (6–15, 16–21, 22–27, 28–40). 22 and 28 were common levels for hepatocellular carcinoma exception points until October 2015 (patients now get 28 points after a 6-month waiting period).
Disease etiology
In the UNOS database, there are 2 variables for diagnosis: “primary diagnosis” and “secondary diagnosis”. The 2 variables have 79 different descriptions for the disease. We grouped them into 6 categories: hepatitis B (HBV), hepatitis C (HCV), nonalcoholic steatohepatitis (NASH), Alcoholic Cirrhosis, hepatocellular carcinoma (HCC) and other. The HCV group is inclusive of HCV plus alcohol. In the analysis, HCC became the primary diagnosis if it was listed as first or second due to the MELD exception point advantage it confers.
Construction of the Simulation Model
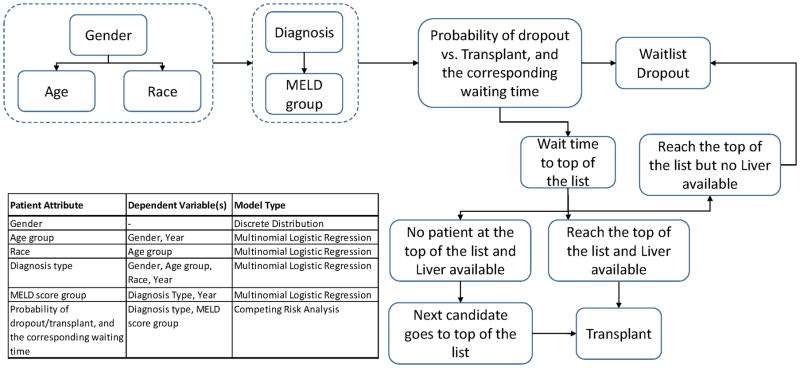
Hypothetical patient demographic (gender, age, race) and clinical characteristics (ESLD etiology and MELD score at list entry) are assigned probabilistically, per the flow chart in Figure 1. Statistical models, based on retrospective UNOS data analysis(4), predict whether individual patients with certain characteristics are likely to drop off the waitlist or receive transplant. A corresponding waiting time is assigned to the patients based on probabilistic analysis of historic wait times for patients with similar attributes. Once a patient has waited in the queue until his or her preassigned transplant time (ie, he/she is the next to receive transplant offer), the simulation determines the probability of liver availability based on historic trends. If a liver is available, the patient receives a transplant. If a liver is not available, the simulation proceeds until the next available liver appears. During this time, competing risk analysis determined if the listed patent can wait for the next available graft, or if the patient drops out from the waitlist. For example, a patient joins the list on day 1. The patient is probabilistically determined to move towards the top of the waitlist, and may be assigned a waiting time of 100 days. If no liver is available on the 100th day, he waits until the preassigned drop out time: hypothetically, the 120th day. During those 20 days, patient has priority to receive any new available liver. If no new liver is available, the patient drops out. The model was implemented using the software Arena version 15 (Rockwell Automation). The simulation model runs retrospectively from 2003–2014 and prospectively from 2015–2025 and is performed by running 5 replications; this number of replications allowed a 95% confidence interval half-width of 1% or less around the mean.
Figure 1.
Waitlist additions arrival and liver graft availability
For the waitlist additions and liver graft availability from 2003–2014, we used historical values. We proposed several possible trends for patient arrival and liver availability after 2014 which are based on a range of possible scenarios (see below). In the simulation model, patients are listed per a constant daily rate which is calculated by dividing the historical number of patient arrivals in that year by 365. Like patient arrivals, the total available livers in each year are distributed daily in the simulation system ie, livers arrive to the system per a constant daily rate which may vary in different years.
Model Validation
The simulation model was constructed using data from 2003–2012. Data from 2013–14 were withheld for validation. Actual and simulated national and regional values from 2014 are shown in Table 1. Retrospective model outputs are also compared to historical data in Figure 2
Table 1.
DES Model validation: actual and predicted national waitlist characteristics-2014.
| National | Region 5 | Region 11 | ||||
|---|---|---|---|---|---|---|
| Characteristic | 2014 actual value* (%) | 2014 simulated value (mean%, (95% CI**)) | 2014 actual value*(%) | 2014 simulated value (mean%, (95%CI**)) | 2014 actual value* (%) | 2014 simulated value (mean%, (95% CI**)) |
| Age, years | ||||||
| 18–39 | 8.79 | 7.3 (6.5–8.1) | 8.5 | 6.5 (5.7–7.3) | 9.5 | 6.9 (6.0–7.8) |
| 40–49 | 16.7 | 13.9 (12.3–15.5) | 16.8 | 15.0 (12.6–17.4) | 17.5 | 14.1 (11.0–17.2) |
| 50–59 | 41.8 | 44.8 (42.6–46.0) | 41.5 | 43.1 (40.1–46.1) | 42.7 | 47.0 (41.6–52.1) |
| 60+ | 32.8 | 34.1 (32.9–35.3) | 33.2 | 35.4 (31.1–39.7) | 30.3 | 32.0 (27.1–36.9) |
| Gender | ||||||
| Male | 62.9 | 64.7 (63.3–66.1) | 62.2 | 63.8 (59.5–58.1) | 63.7 | 66.8 (61.7–71.9) |
| Female | 37.1 | 35.1 (34.7–36.5) | 37.8 | 36.2 (32.7–39.7) | 36.3 | 33.2 (27.2–39.2) |
| Race/ethnicity | ||||||
| White | 70.0 | 72.8 (71.3–74.3) | 50.9 | 56.2 (55.4–57.0) | 84.9 | 82.5 (72.0–93.0) |
| Black | 7.3 | 8.3 (7.5–9.1) | 3.3 | 3.6 (2.0–5.2) | 9.5 | 12.6 (9.3–15.9) |
| Hispanic | 16.8 | 14.4 (13.5–15.3) | 33.8 | 29.9 (27.9–31.9) | 2.2 | 3.3 (0.1–6.5) |
| Other | 5.87 | 4.6 (4.4–4.8) | 12.1 | 10.4 (8.3–12.5) | 3.4 | 1.6 (0.4–2.8) |
| Disease etiology | ||||||
| HCV | 27.6 | 30.5 (29.5–31.5) | 39.9 | 33.2 (30.0–36.4) | 27.9 | 31.2 (24.3–38.1) |
| HBV | 2.1 | 1.4 (1.0–1.8) | 3.1 | 1.4 (0.7–2.1) | 1.2 | 1.2 (0.2–2.2) |
| NASH | 16.0 | 17.6 (16.3–18.9) | 11.5 | 13.9 (12.0–15.8) | 25.2 | 22.5 (16.5–28.5) |
| Alcohol | 19.7 | 17.7 (17.2–18.2) | 17.2 | 17.7 (14.6–20.8) | 16.1 | 15.3 (9.4–21.2) |
| HCC | 19.5 | 17.9 (17.0–18.8) | 17.5 | 14.0 (12.0–16.0) | 21.8 | 21.6 (13.9–29.3) |
| other | 15.1 | 15.0 (13.7–16.3) | 20.8 | 19.9 (18.8–21.0) | 7.8 | 8.2 (5.5–10.9) |
| Initial MELD score | ||||||
| 6–15 | 73.4 | 74.6 (73.2–76.0) | 74.6 | 76.5 (71.1–81.9) | 70.8 | 74.0 (64.3–83.7) |
| 15–21 | 21 | 19.7 (18.8–20.6) | 19.4 | 17.1 (14.5–19.7) | 25.5 | 21.6 (15.3–27.9) |
| 22–27 | 4.4 | 4.3 (3.9–4.7) | 4.8 | 4.4 (2.0–6.8) | 3.1 | 3.1 (0.9–5.3) |
| >28 | 1.2 | 1.4 (0.9–1.9) | 1.3 | 2.0 (1.2–2.8) | 0.7 | 1.3 (0.0–2.9) |
NOTE: All data are given as percentages. The DES model was created by UNOS data from 2003 to 2012. Available data from 2013–2014 were withheld from model construction and used for validation.
95% Confidence Interval.
Figure 2.
Scenario analyses
We considered 3 simulation scenarios acknowledging that there may be unanticipated events that impact the available numbers of grafts for liver transplantation, the volume of new arrivals on the transplant waitlist and the health and demographic characteristics of patients on the waitlist. Several scenarios were defined and analyzed (Table 2). Each scenario is composed of 3 parts: A: liver graft availability after 2014; B: Waitlist additions after 2014; C: Patients’ demographic and clinical trends after 2014. For each part, subscenarios are designed based on plausible trends (5–7). Liver graft availability may potentially increase as an unexpected consequence of the opioid epidemic impacting parts of the United States. Waitlist additions may change pending the success (or failure) of pharmacotherapy for NAFLD. Finally, patient clinical attributes may change via UNOS policy (HCC exception points), shifts in disease epidemiology (NAFLD replacing HCV) or patient demographics. By combining different possibilities from each of the 3 pieces, 3 scenarios are constructed: baseline, optimistic, and pessimistic scenarios.
Table 2.
Scenario descriptions.
| A. Liver Availability | B. Patient Arrival | C. Patient Attributes | |
|---|---|---|---|
| Baseline Scenario | Stagnant after 2014 | Increase 3% after 2014 | Stagnant after 2020 |
| Optimistic Scenario | Increase 2% after 2014 | Stagnant after 2014 | Stagnant after 2014 |
| Pessimistic Scenario | Decrease 2% after 2014 | Increase 5% after 2014 | Stagnant after 2020 |
Results
National waitlist
In our baseline scenario, the size of the national liver transplant waitlist will change from 13 950 in 2015 to 17 355 in 2025, a 24% increase. Nationally, the rate of wait list drop out will increase from 41.3% to 45.8% (Table 3). Patients on the waitlist will be older; the proportion older than 60 will increase from 35.9% to 47.1%. HCC will be the leading indication for liver transplantation, increasing from 16.5% to 28.5% and NASH will overtake HCV as the leading etiology of liver disease for new arrivals on the waitlist, increasing from 18.2% to 21.9%. We estimate that a patient listed in 2015 with a MELD score between 22 and 27 waited on average 82 days for a liver transplant. This time will increase to 123 days in 2020 and to 182 days in 2025.
Table 3.
Selected waitlist attributes 2015, 2020, and 2025, national data.
| Year | Male (%) | Age>=60 (%) | HCV (%) | NASH (%) | HCC (%) | Expected wait time for MELD 22–27, days | Waitlist dropout (%) | |
|---|---|---|---|---|---|---|---|---|
| Baselinescenario | 2015 | 64.7 | 35.9 | 29.6 | 18.2 | 16.5 | 82 | 41.3 |
| 2020 | 64.5 | 44.9 | 22.9 | 21.1 | 25.5 | 123 | 44.6 | |
| 2025 | 64.5 | 47.1 | 21.0 | 21.9 | 28.5 | 182 | 45.8 | |
| Optimisticscenario | 2015 | 64.7 | 35.3 | 30.2 | 18.0 | 15.4 | 88 | 39.3 |
| 2020 | 64.3 | 36.5 | 30.2 | 19.5 | 13.0 | 70 | 33.4 | |
| 2025 | 63.3 | 36.8 | 29.5 | 20.4 | 12.0 | 62 | 28.7 | |
| Pessimisticscenario | 2015 | 64.7 | 36.0 | 29.5 | 18.1 | 16.7 | 96 | 44.9 |
| 2020 | 64.7 | 45.3 | 22.2 | 20.5 | 27.7 | 205 | 59.8 | |
| 2025 | 64.9 | 47.7 | 19.6 | 20.7 | 32.4 | 368 | 69.9 |
NOTE: All data are given as percentages unless otherwise labeled
Under optimistic conditions (an increase in liver availability with unchanged waitlist additions and patient characteristics), the waitlist drop out will decrease from 39.3% to 28.7% by 2025. Accordingly, expected wait time for a patient entering the waitlist with a MELD score between 22 and 27 would fall from 88 days to 62 days.
Under pessimistic conditions (a decrease in liver graft availability, an increase in patients added to the waitlist and further changes in patient attributes), the dropout rate would increase from 44.9% to 69.9 % by 2025. Wait times for a patient entering the waitlist with a MELD score between 22 and 27 would increase from 96 days to 368 days by 2025. Selected characteristics of the national waitlist for the baseline, optimistic and pessimistic scenarios are shown in Table 3 and Figure 2a.
Regions 5 and 11
Regional waitlists will have considerable variability. In the baseline scenario, the region 5 waitlist size will change from 2681 in 2015 to 3107 in 2025, a 15.9% increase. The rate of drop out will change from 52.9% to 63.7%. Patients listed at a MELD score between 22 and 27 will have an increase in wait time from 110 days to 191 days by 2025. In comparison, the region 11 waitlist will change from 706 patients in 2015 to 863 patients in 2025, a 22.2% increase. The rate of dropout will change from 30.3% to 43.9%. Wait times in region 11 will increase from 48 days to 82 days by 2025. The region 5 waitlist will have a greater proportion of HCV and HCC while NASH will be more common in region 11. Patients in region 5 will be older at the time of listing with a higher proportion over age 60 compared to region 11. Selected characteristics of the region 5 and 11 waitlists for the neutral, optimistic and pessimistic scenarios are shown in Table 4 and Figure 2b.
Table 4.
Selected waitlist attributes 2015, 2020, and 2025, regions 5 and 11.
| Year | Male | Age>=60 (%) | HCV | NASH | HCC | Wait time for MELD 22–27, days | Waitlist dropout (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 11 | 5 | 11 | 5 | 11 | 5 | 11 | 5 | 11 | 5 | 11 | 5 | 11 | ||
| Baselinescenario | 2015 | 63.6 | 65.9 | 38.5 | 33.5 | 31.8 | 29.6 | 14.1 | 21.8 | 22.0 | 11.0 | 110 | 48 | 52.9 | 30.3 |
| 2020 | 63.9 | 65.6 | 50.3 | 45.1 | 20.9 | 21.8 | 14.9 | 26.0 | 36.3 | 19.5 | 144 | 59 | 57.7 | 38.4 | |
| 2025 | 64.2 | 68.5 | 54.0 | 49.7 | 20.0 | 19.7 | 13.7 | 25.3 | 39.8 | 29.4 | 191 | 82 | 63.7 | 43.9 | |
| Optimisticscenario | 2015 | 63.2 | 66.2 | 36.4 | 32.9 | 33.5 | 29.2 | 14.4 | 22.3 | 19.6 | 9.8 | 93 | 40 | 50.2 | 24.2 |
| 2020 | 62.7 | 64.6 | 38.1 | 35.2 | 31.2 | 28.6 | 15.4 | 23.6 | 17.0 | 9.8 | 52 | 38 | 46.3 | 14.3 | |
| 2025 | 62.1 | 66.2 | 38.3 | 35.1 | 31.0 | 30.4 | 15.6 | 23.3 | 16.1 | 10.3 | 50 | 30 | 41.1 | 7.1 | |
| Pessimisticscenario | 2015 | 63.8 | 65.8 | 38.6 | 33.7 | 31.4 | 28.8 | 13.9 | 22.2 | 22.6 | 11.2 | 118 | 46 | 54.5 | 30.6 |
| 2020 | 63.9 | 65.6 | 51.0 | 45.2 | 20.5 | 21.8 | 14.5 | 25.0 | 37.6 | 19.5 | 224 | 95 | 67.2 | 49.8 | |
| 2025 | 64.0 | 67.3 | 54.0 | 47.9 | 18.7 | 18.8 | 13.4 | 23.9 | 42.3 | 26.3 | 389 | 202 | 75.2 | 62.2 | |
NOTE: All data are given as percentages unless otherwise labeled
Discussion
This is the first detailed simulation that seeks to estimate future demographic and clinical attributes of patients on the liver transplant waitlist. Additionally, we estimate waitlist times and probability of waitlist drop out. Such modeling endeavors are critically important as we anticipate potential changes in UNOS region realignment and organ allocation as well as adjust to the reality of changing epidemiology of cirrhosis and end stage liver disease. We anticipate that there will be continued upward pressure on MELD scores for liver transplant listing, resulting in longer waitlist times and greater risk of waitlist dropout. These events may have a multifaceted impact on the transplant community.
Our simulation showed that the liver transplant waitlist size will remain relatively static on a national level over the next decade even as waitlist additions increase by 24%. This balance will not be achieved by additional transplants, but by increased waitlist drop out instead. The probability of drop out will exceed that of transplant under some scenarios and in regions that transplant at higher MELD scores. HCC will be the leading indication for liver transplant and NASH will overtake HCV as the leading etiology of cirrhosis. Liver transplant candidates will be older, with a growing proportion over the age of 60. Patients listed with MELD scores in the 22–27 range will wait for transplantation longer; some for greater than a year. There will continue to be significant heterogeneity among transplant regions where some patients will be more likely to drop out from the waitlist than receive a transplant. This phenomenon, already occurring in some regions, will become more common over time.
Quantification of these future trends will be important in informing public health policy. Important decisions looming for the transplant community include redrawing UNOS regions to lessen the effect of geographic location on access to organs. Accurate forecasting of future waitlist times and the economic impact of rezoning UNOS regions and donor service areas will be vital to crafting new policy. While rezoning the UNOS regions will not change the net number of livers available for transplant, waitlist times may change, thus anticipating future waitlist times will be integral. Such rezoning is controversial and focused primarily on equitable allocation of organs in the near (<5 years) term. There has been less emphasis on long term (~10 years) forecasting. To create an equitable strategy for organ allocation long term, multiple complex interactions must be considered with accurate forecasts of the changing transplant candidate and organ donor demographics.
Worsening organ shortages have already reignited a push for uniformly higher donation rates across the U.S. As patients get sicker on the waitlist, risk tolerance for marginal liver grafts may change. Communicating these risks to patients, payers, and the public will be vital to avoid unintended side effects of policy changes such as those made by CMS in 2007. Outcomes-based auditing likely altered waitlist management and clinical decision making leading to delisting of many patients who may have otherwise benefitted from transplant.(8) A future where patients are older and sicker on the transplant waitlist might exacerbate transplant centers’ perceptions of risk.
As patients wait longer for transplant, expensive complications will become more common. These issues have been raised previously,(6) but deserve updated emphasis as healthcare costs continue to spiral upwards. For example, complicated variceal bleeding may increase, which would lead to high hospitalization costs of $28 513 per patient in 2015 US dollars (adjusted for inflation from 2008 US dollars using the Consumer Price Index Medical Care component for all US cities).(6, 9, 10) From 2005 to 2009, inpatient charges for hepatic encephalopathy rose from $4.7 billion to $7.2 billion.(6, 11) Additionally, hospital readmission is often part of the dying process for a patient with ESLD, ultimately destined to drop off the list. In a single center study of 402 patients discharged after a complication of cirrhosis, 69% had at least 1 nonelective readmission. Additional costs for those readmissions ranged from $20 581 to $29 898 per patient.(12)
The increased cost and risk of worse outcome due to these trends in listed patients will also adversely affect posttransplant outcomes. Increased age at transplant is associated with worst posttransplant survival.(3) Such posttransplant effects will be compounded by decline in graft quality.(6) Sicker transplant recipients combined with marginal donors increase transplant related costs.(13) Donors in the highest risk quartile of the Donor Risk Index add $14 750 to the cost of transplant and another $27 000 to post transplant hospitalization, relative to low risk donors, pushing overall 1 year costs to approximately $250 000 in inflation adjusted 2015 U.S. dollars. DCD donors increased costs by $26 000 over standard donation after brain death (DBD) donors in adjusted 2015 dollars.(6, 10, 13) These costs are due to longer hospitalizations associated with higher risk donors. (6, 14)
Any forecasting model is subject to certain limitations. The accuracy and the validity of any modeling are dependent upon the data used to inform the model. In this simulation, we utilize over a decade of the best available patient data from the UNOS database. Interdependencies among health and demographic variables are calculated from 10 000s of patients. Moreover, discrete event stimulation offers unique strengths over Markov and other modeling methods, such as allowing dynamic transitions and making outcomes dependent on resource availability. We can also estimate person-specific heterogeneity in risks and outcomes.(15) A discrete event simulation can run retrospectively as well. We used this strategy to validate our model by comparing withheld data from 2014 and demonstrating that our model predicted most variables to within 1–2% of the actual values. Although some estimations do fall outside the 95% confidence interval, the absolute differences are small.
Additionally, we utilized alternate scenario analyses to account for potential changes in the waitlist. Unknowns such as donor availability and transplant volume could shift in an ‘optimistic’ direction due to an influx of victims of the opioid epidemic, or trend ‘pessimistically’ if clinical practices such as poor DCD utilization go unchanged.(6) New waitlist additions will be influences by widespread application of HCV therapy and the success (or failure) of pharmacologic interventions for NAFLD. Finally, patient level characteristics, like disease etiology, MELD score, or age and ethnicity of listed patients were altered to give a plausible range of optimistic and pessimistic outcomes.
In sum, based upon best available national data and well-established simulation modeling techniques, patients on the liver transplant waitlist will become older, wait longer, receive a transplant at a higher MELD score, and have a greater risk for waitlist drop out. Different regions will experience these changes to different degrees, which may lead to increased regional and demographic disparities in access to transplant and outcomes posttransplant. These epidemiologic changes in listed patients, their risk of drop out, increased overall cost, and risk of poorer posttransplant outcome must be considered when determining organ allocation in the decade to come. Our discrete events simulation modeling offers a clearer more accurate characterization of these changes in the long term.
Acknowledgments
Grant Support: Supported in part by the National Science Foundation (CMMI-1150732) and National Institutes of Health, KL2TR001106 and K23 DK109202.
Abbreviations
- UNOS
United Network of Organ Sharing
- LT
Liver Transplantation
- NAFLD
Non Alcoholic Fatty Liver Disease
- NASH
Nonalcoholic Steatohepatitis
- DCD
Donation after Cardiac Death
- DBD
Donation after Brain Death
- DES
Discrete Event Simulation
- HCV
Hepatitis C
- ESLD
end stage liver disease
- MELD
Model for End Stage Liver Disease
- STAR
Standard Transplant Analysis and Research
- OPTN
Organ Procurement and Transplant Network
- HCC
Hepatocellular Carcinoma
- HBV
Hepatitis B
Footnotes
Author Contributions: ZY: study concept and design, data analysis and interpretation, manuscript drafting, critical revision; MEM: study concept and design, data analysis and interpretation, manuscript drafting, critical revision; SBW: study concept and design, data analysis and interpretation, critical revision; PHH: study concept and design, data analysis and interpretation, critical revision; ESO: study concept and design, data acquisition, data analysis and interpretation, manuscript drafting, critical revision; ASB: study concept and design, data analysis and interpretation, manuscript drafting, critical revision, supervision.
Disclosures: The authors have no relevant conflicts of interest.
References
- 1.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161(3):170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton M. Cirrhosis and liver failure in nonalcoholic fatty liver disease: molehill or mountain? Hepatology. 2008;47(5):1431–1433. doi: 10.1002/hep.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su F, Yu L, Berry K, et al. Aging of liver transplant registrants and recipients: trends and impact on waitlist outcomes, post-transplantation outcomes, and transplant-related survival benefit. Gastroenterology. 2016;150(2):441–453. e446. doi: 10.1053/j.gastro.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Yi Z, Mayorga M, Wheeler SB, Hayashi PH, Barritt AS, Orman ES. The numbers of new waitlist registrants and removals are outpacing the number of liver transplants: national trends and regional variation from 2003 through 2014. Abstract presented at: AASLD Liver Meeting; November 13, 2016.2016. [Google Scholar]
- 5.Orman ES, Barritt ASt, Wheeler SB, Hayashi PH. Declining liver utilization for transplant in the United States and the impact of donation after cardiac death. Liver Transpl. 2013;19(1):59–68. doi: 10.1002/lt.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015;21(8):1040–1050. doi: 10.1002/lt.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toro-Diaz H, Mayorga ME, Barritt AS, Orman ES, Wheeler SB. Predicting liver transplant capacity using discrete event simulation. Med Decis Making. 2015;35(6):784–796. doi: 10.1177/0272989X14559055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolgin NH, Movahedi B, Martins PNA, et al. Decade-long trends in liver transplant waitlist removal due to illness severity: the impact of centers for medicare and medicaid policy. J Am Coll Surg. 2016;222(6):1054–65. doi: 10.1016/j.jamcollsurg.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Viviane A, Alan BN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11(1):1–3. doi: 10.1111/j.1524-4733.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- 10.Bureu of Labor Statistics. Consumer price index for all urban consumers: US city average, by commodity and service group. https://www.bls.gov/cpi/cpid10av.pdf. Published 2015.
- 11.Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034–1041. e1. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvalaggio PR, Dzebisashvili N, MacLeod KE, et al. The interaction among donor characteristics, severity of liver disease, and the cost of liver transplantation. Liver Transpl. 2011;17(3):233–242. doi: 10.1002/lt.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant. 2007;7(4):990–997. doi: 10.1111/j.1600-6143.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making. 2012;32(5):678–689. doi: 10.1177/0272989X12454941. [DOI] [PubMed] [Google Scholar]