Abstract
Recent advances have led to a greater appreciation of how mitochondrial dysfunction contributes to diverse acute and chronic pathologies. Indeed, mitochondria have received increasing attention as a therapeutic target in a variety of diseases since they serve as key regulatory hubs uniquely situated at crossroads between multiple cellular processes. This review provides an overview of the role of mitochondrial dysfunction in chronic kidney disease (CKD) with special emphasis on its role in the development of diabetic nephropathy (DN). We will examine the current understanding on the molecular mechanisms that cause mitochondrial dysfunction in the kidney and describe the impact of mitochondrial damage on kidney function. The new concept that mitochondrial shape and structure is intimately linked with its function in the kidneys is discussed. Furthermore, the mechanisms that translate cellular cues and demands into mitochondrial remodeling and cellular damage, including the role of microRNAs and lncRNAs, are examined with the final goal of identifying mitochondrial targets to improve treatment of patients with chronic kidney diseases.
Keywords: acute kidney injury, chronic kidney disease, diabetes, diabetic nephropathy, mitochondria, oxidative stress, Reactive Oxygen Species, Mitochondrial dysfunction
INTRODUCTION
Long recognized for their canonical roles in cellular respiration and oxidative phosphorylation, mitochondria have emerged as key participants in sensing and integrating cues from the environment to trigger adaptive and compensatory responses in cells. The spectrum of the regulatory responsibilities of mitochondria in the cell also includes central roles in the biosynthesis of macromolecules, regulation of cellular redox state, calcium homeostasis, inflammation, and cell death pathway. As a result, mitochondria are poised to play pivotal roles in the kidney’s function, an organ with high-energy demand and rich in mitochondria. In fact, the kidneys are second only to the heart in oxygen consumption and mitochondrial abundance. Interestingly, there is a significant variation along the nephron in energy generation, with proximal tubules generating ATP mainly via mitochondrial oxidative phosphorylation whereas podocytes, endothelial, and mesangial cells exhibit more flexibility in their glycolytic capacity to generate energy (1). This heterogeneity in generating ATP and the use of oxidative phosphorylation in different segments of the kidney could play an important role in determining the impact of mitochondrial dysfunction in the kidneys and progression to CKD.
The relationship between mitochondrial dysfunction and CKD has been long suspected, but dissecting the mechanistic nature of mitochondrial dysfunction in the development of CKD remains elusive. Several theories have attempted to establish a link between mitochondrial dysfunction and CKD progression. Establishing a valid relationship between mitochondrial dysfunction and CKD progression, however, has been elusive since this interaction seems to vary depending the definition of mitochondrial dysfunction, specific conditions, and the population studied or the model examined. Despite all these differences, there are some hallmark features of mitochondrial dysfunction that are commonly described in the context of CKD which include, changes in mitochondrial morphology and mitochondrial remodeling, enhanced mitochondrial oxidative stress, and a significant decrease in mitochondrial biogenesis and in ATP production (2).
Among several theories linking mitochondrial dysfunction with CKD, the mitochondrial dysfunction theory of diabetic complications also known as Unifying hypothesis deserves special attention since it has provided significant insights into the potential role of mitochondrial oxidative stress on mitochondrial dysfunction and CKD development in patients with diabetes (3). The Unifying hypothesis is based on the notion that mitochondrial dysfunction, mediated by the overproduction of ROS (reactive oxygen species), is a common pathway in the pathogenesis of microvascular complications of diabetes, including diabetic nephropathy and progression to CKD. However, several aspects of the Unifying theory have been recently challenged and many questions regarding the validity of the Unifying hypothesis remain to be carefully addressed.
The present review will provide an update on the recent advances on the causes of acquired mitochondrial dysfunction in the context of CKD with special emphasis on the recent progress and new insights into the role of mitochondrial remodeling in progression of CKD. A comprehensive characterization of mitochondrial dysfunction and its underlying molecular mechanism could offer insights and possible therapeutic options that could significantly impact our current management of CKD progression.
Mitochondrial Dynamics
Mitochondria are highly plastic organelles that constantly change their shape and size through fusion and fission processes, collectively known as mitochondrial dynamics, in response to metabolic and signaling cues in the cell environment (4). These opposing processes work in concert to maintain the overall morphology of mitochondria that range from spherical morphologies to hyperfused reticular networks.
During mitochondrial fission, a mitochondrion divides into two daughter organelles. Mitochondrial fusion is the opposite process where two mitochondria merge their outer and inner membranes resulting in a larger mitochondrion. An excess of mitochondrial fission results in mitochondrial fragmentation whereas enhanced mitochondrial fusion leads to mitochondrial hypertubulation. A precise balance between fission and fusion events is required for optimal mitochondrial fitness since unopposed mitochondrial dynamics could ultimately result in mitochondrial dysfunction and cell damage.
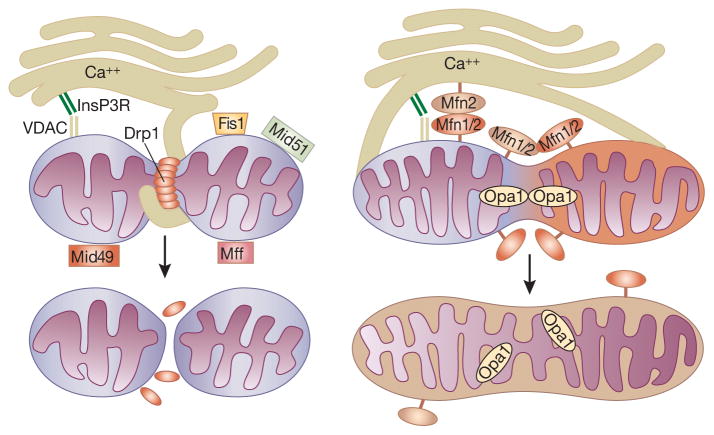
The molecular machinery involved in mitochondrial fission and fusion was first described in yeast, with homologous machinery found later in mammalian cells. Mitochondria fission is mainly mediated by Drp1 (dynamin related protein 1), a large dynamin-related GTPase. During mitochondrial fission, Drp1 is recruited to the outer mitochondrial membrane, forming large ring-like oligomers around the membrane to be partitioned. Using GTP hydrolysis for energy, Drp1 will then constrict the membrane until full fission has occurred (Figure-1). Binding of soluble Drp1 to the outer mitochondrial membrane is facilitated by several Drp1 receptors, including FIS1 (mitochondrial fission 1 protein), MFF (mitochondrial fission factor), and MiD49/MiD51 (mitochondrial dynamics proteins of 49 and 51 kDa). Drp1 activity is regulated by post-translational modifications including phosphorylation, ubiquitination, sumoylation, and S-nitrosylation. Defective regulation of Drp1 leads to enhanced mitochondrial fission and mitochondrial release of cytochrome c which is pathologically implicated in end organ damage. In contrast, outer mitochondrial fusion is mediated by two dynamin-related GTPases, mitofusins 1 and mitofusin 2 (Mfn1/2), which can interact both homo- and hetero-typically to mediate mitochondrial fusion. Mfn2 has also been recently demonstrated to serve an additional role of mitochondrial tethering to the endoplasmic reticulum, facilitating interorganelle cross talk and calcium signaling (5). Inner membrane fusion and cristae stabilization, on the other hand, involves another dynamin-related GTPase, Opa1 (optic atrophy 1). The membrane potential across the mitochondrial inner membrane also plays an important role in mitochondrial fusion by regulating post-translational changes in OPA1 (6).
Figure 1. Mitochondrial dynamics.
Left panel: Mitochondrial Fission. Drp1 oligomerizes and forms a ring around the mitochondrion in order to constrict and partition the mitochondrion using GTPase activity. Right Panel: Mitochondrial Fusion: MFN1 and MFN2 are found on the mitochondrial outer membrane and can interact as homo or heterodimers. OPA1 facilitates the fusing of the inner membrane. Fis1=Mitochondrial fission protein 1, Mff=Mitochondrial fission protein, MiD49 and MiD51=Mitochondrial dynamics proteins of 49 and 51 kDa, Mfn1/2=Mitofusion proteins 1 or 2, VDAC=Voltage-dependent anion channel, InsP3R=Inositol 1,4,5-trisphosphate receptor.
Defective mitochondrial dynamics have been implicated in a wide array of diseases including cancer, cardiovascular diseases, neurodegenerative diseases and diabetes. Specifically, increased mitochondrial fragmentation has been associated with kidney injury and CKD (7). Two recent studies have described enhanced fragmented mitochondria in kidney tubules in CKD models (8, 9). Our own laboratory has reported increased mitochondrial fission in the podocytes of experimental models of diabetes (10, 11). We generated a mouse model that specifically deleted Drp1 in podocytes (10). This mouse, when crossed to the db/db diabetic model, conferred protection against key features of diabetic nephropathy with reduced albuminuria, mesangial matrix expansion, and improved podocytes foot processes effacement compared to Drp1 wild-type diabetic mice. As expected, cultured podocytes from Drp1 deficient mice exhibited elongated mitochondria in their podocytes and oxygen consumption rate (OCR) was restored to control levels regardless of glucose concentration. Consistent with these findings, pharmacological inhibition of Drp1 using Mdivi-1 (mitochondrial division inhibitor 1) also yielded podocytes with fit mitochondria and protected against progression of diabetic nephropathy. The findings suggest that Drp1 and mitochondrial fission are critical in maintaining mitochondrial fitness with optimal energy production capacity in the kidneys.
The regulation of Drp1 by post-translational modifications is important for Drp1 translocation to mitochondria. A series of kinases can influence subcellular localization of Drp1 by phosphorylating two main conserved serine residues of phosphorylation of Drp1. Importantly, we have recently shown that Drp1 phosphorylation at Ser637/656 (human/rat) (corresponding to Ser600 in mouse Drp1 isoform b), promotes mitochondrial fission in response to high glucose conditions in podocytes (10). However, whereas another report, consistent with our findings, demonstrated that phosphorylation of Drp1 at the same conserved serine residue by the Ca2+/calmodulin-dependent protein kinase Iα (CaMKIα) enhanced Drp1 recruitment to the mitochondria (12), several other reports suggest that PKA phosphorylation of Drp1 at Ser600 decreases Drp1 GTPase activity (13, 14). These contradictory observations may indicate that the effects of phosphorylation at this residue are likely cell-context and stimulus dependent.
Overall, these findings suggest that targeting mitochondrial dynamics may lead to therapies that could improve mitochondrial morphology and fitness in the context of CKD. We can expect the emergence of new targets and drugs against different components of mitochondrial dynamics in CKD and other common diseases in which mitochondrial dysfunction is implicated. However, for mitochondrial dynamics to reach its full potential as a target for CKD treatment, much work still remains to be developed.
Mitochondrial Biogenesis
Since mitochondria are involved in a variety of key cellular processes, the abundance and functional properties of mitochondria are finely tuned to meet specific metabolic and energetic demands of the cell. The fine-tuning of mitochondrial biogenesis is achieved largely through an interconnected set of transcription factors that link environmental cues to cellular energy status and adaptive responses in the cell.
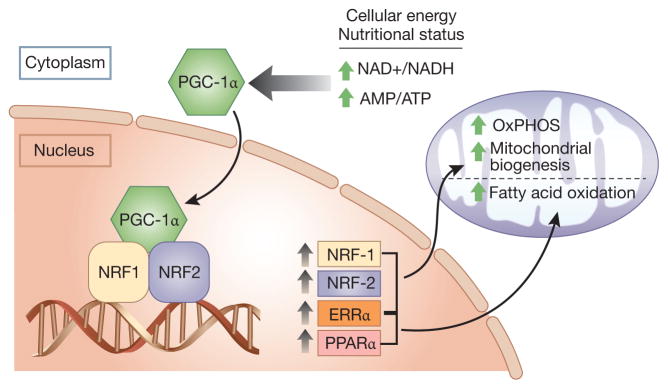
The peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1 family of transcriptional coactivators (PGC-1α, PGC-1β and PRC) are master regulators of mitochondrial biogenesis and energy metabolism. PGC-1α was initially identified by Spiegelman et al. as a protein interacting with PPARγ (15), later known to be highly expressed in tissues with high energy demands, such as the heart and kidneys. PGC-1α does not bind to DNA directly, but it docks on transcription factors bound at their respective response elements and coactivates them to exert their regulatory effects on mitochondrial function (Figure-2). Transcription factors such as nuclear respiratory factor-1 (NRF-1), NRF-2 and the estrogen-related receptors (ERR) are among multiple key transcription factors coactivated by PGC-1α (16, 17). The interaction between PGC-1α and these DNA-binding transcriptional factors will then allow for the concerted control of multiple classes of mitochondrial genes leading to regulation of mitochondrial biogenesis, respiration, fatty acid β-oxidation, Krebs cycle and oxidative phosphorylation. The relative contributions of these binding partners is cell type dependent and explains, at least in part, the different metabolic programs elicited in different cell types.
Figure 2. PGC-1α and mitochondrial biogenesis.
The master regulator, PGC-1α, drives mitochondrial biogenesis by co-activating transcription factors whose target genes increase biogenesis, oxidative phosphorylation, and fatty acid oxidation. ERRα=Estrogen Related Receptor Alpha, PPARα=Peroxisome Proliferator Activated Receptor Alpha, NRF1 and NRF2=Nuclear Respiratory Factors 1 and 2, AMP=Adenosine monophosphate, ATP=Adenosine triphosphate, NAD+=nicotinamide adenine dinucleotide, NADH=dihydronicotinamide adenine dinucleotide, OxPHOS=oxidative phosphorylation.
PGC-1α is highly responsive to cues from the environment and plays a critical role in relaying energetic or metabolic stimuli to changes in mitochondrial gene expression in a tissue specific manner. Recent gene knockout and silencing studies of many members of the PGC-1 network have revealed the central role of PGC-1 family in integrating mitochondrial biogenesis and energy production. As an example, transgenic expression of PGC-1α leads to an increase in mitochondrial content and increased expression of mitochondrial genes. Conversely, loss of PGC-1α results in viable mice with modest decreases in expression of mitochondrial genes, and phenotypes of moderate mitochondrial dysfunction.
The reduced efficiency of mitochondrial biogenesis and downregulation of PGC-1α have been frequently reported in multiple etiologies of CKD, including diabetic kidney disease. Indeed, the development of nephropathy in diabetes has been associated with PGC-1α signaling pathway (6, 18–21). PGC-1α is significantly downregulated in streptozotocin-induced diabetic rat renal tubules. Several experimental models of diabetic kidney disease, including OVE26 and Akt2, as well as db/db mice exhibit decreased PGC-1α levels. Recently, in an elegant study by Tran et al. the authors highlighted the interaction between mitochondrial dysfunction and kidney injury following ischemia–reperfusion injury through accumulation of fatty acids in kidneys, whereas restoring cellular NAD+ concentrations, a downstream metabolite of PGC-1α activation, improved mitochondrial dysfunction and protected against worsening kidney injury (22). Other reports, including another recent publication by Kang et al. have demonstrated the benefits of PGC-1α activation in CKD, where transgenic expression of PGC-1α in renal tubular cells improved kidney histology in mouse models of CKD by improving defective fatty acid oxidation and ATP depletion in the kidney (23). However, the broad nature of PGC-1α overexpression opens the possibility that fatty acid oxidation improvement could have only been partially responsible for the reported improvement in CKD phenotype as effects on other mitochondrial functions would also be anticipated by PGC-1α expression.
The family of NAD+-dependent deacetylases known as Sirtuins (SIRT1-7) regulate several aspects of mitochondrial biogenesis and could play important roles in kidney diseases (24–26). For instance, Yuan et al. reported that the SIRT1-mediated deacetylation of PGC-1α ameliorates aldosterone-induced podocyte injury and Resveratrol, an activator of SIRT1, protected mitochondrial function against podocyte injury (25). Importantly, proximal tubular specific overexpression of SIRT1 prevented histological changes in diabetic glomeruli, while knockout of SIRT1 resulted in albuminuria in non-diabetic mice and exacerbated diabetic changes in the glomeruli of both streptozotocin and db/db diabetic mouse models (20). Of note, PGC-1α can also increase levels of mitochondrial SIRT3 which has also been implicated in progression of CKD (24, 26).
Considering the broad involvement of PGC-1α as an important component of many disease states, it is not surprising that PGC-1α is actively considered as a novel pharmacological target in several metabolic diseases such as obesity and diabetes (27). However, an important limitation of using PGC-1α as a therapeutic target is that its functions are highly cell-specific and thus, novel targeted and biologic agents are under evaluation in targeting mitochondrial biogenesis.
PGC-1α is not the only suspect leading to defective bioenergetics in CKD, and several additional factors have also been proposed to contribute to altered bioenergetics in CKD, which among others include, deletions and mutations in mitochondrial DNA, changes in the lipid composition of mitochondrial membranes, and defective quality control by mitophagy (28). Of note, although altered mitochondrial biogenesis has an important effect on the development and progression of CKD, it is less clear whether restoring mitochondrial biogenesis could ameliorate progression of CKD. Further studies are clearly needed to assess the role of restoring mitochondrial function on CKD progression.
Non-Coding RNAs & Mitochondrial Remodeling
Non-coding RNAs (ncRNAs) are RNA molecules that do not translate into proteins, but are actively transcribed from the mammalian genome and exert important gene regulatory effects. ncRNAs can be classified by their size as short ncRNAs (19–31 nucleotides), midsize ncRNAs (~20–200 nucleotides), and long noncoding RNAs (>200 nucleotides). Short microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), the most extensively studied ncRNAs in CKD, have been extensively implicated in CKD pathogenesis in both protective and pathogenic roles (29).
miRNAs are highly conserved ncRNAs of ~22 nucleotides (nt) in length, acting as post-transcriptional gene regulators that canonically target 3′-UTR of many mRNAs through translational repression and/or mRNA degradation. We and others have shown a modulatory role of miRNAs in CKD pathogenesis. An example is miR-93, a signature miRNA in the diabetic environment, that have been shown to be downregulated in the diabetic milieu, and its conditional forced expression in podocytes prevented progression of diabetic nephropathy via a Msk2 (mitogen and stress-activated kinase-2)-mediated epigenetic modulation (30, 31). Others have more specifically shown a modulatory role of miRNAs on progression of CKD through their regulatory effects on the mitochondria. For instance, miR21, one of the first mammalian miRNAs identified, has been reported to be significantly upregulated in patients with a variety of kidney diseases and in animal models of CKD (32). Importantly, mice lacking miR-21 were protected against kidney fibrosis in part through improving mitochondrial fatty acid oxidation and targeting of peroxisome proliferator activated receptor-α (PPAR), a direct target of miR21 (33).
lncRNAs, in contrast to miRNAs, tend to be much less conserved among different species, and their regulatory effects on gene expression are much more diverse and less known than that of miRNA-mediated post-transcriptional mRNA gene regulation. A regulatory role for lncRNAs on mitochondrial function in the context of CKD has recently been suggested. A recent report by our group suggest that taurine-upregulated 1 (Tug1), a well-known lncRNA, contributes to diabetic nephropathy progression (34). We performed an unbiased RNA-Seq profiling of kidney glomeruli from db/db mice, an established model of Type 2 diabetes, and identified Tug1 as a differentially expressed lncRNA in the diabetic milieu. Podocyte-specific overexpression of Tug1 in diabetic mice improved biochemical and histological features associated with diabetic kidney disease. Mechanistically, we showed that overexpression of Tug1 rescues the expression of PGC-1α and its transcriptional targets. Unexpectedly, we found that Tug1 regulates mitochondrial bioenergetics in podocytes by targeting the expression of PGC-1α (the peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1). We further showed that by binding to Tug1-binding element (TBE) upstream of the PGC-1α promoter and transcription factor PGC-1α, Tug1 acts as scaffold molecule, to enhance transcription of PGC-1α mRNA.
The prevalence of ncRNAs targeting mitochondria will likely increase as many ongoing studies are specifically addressing the impact of ncRNAs on mitochondrial dysfunction. What is well-known is that while a few ncRNAs are generated within the mitochondria genome, many more are imported into the mitochondria from the cytosol (35). The presence of ncRNAs in the mitochondria coupled with their ability to regulate a broad set of nuclear genes encoding mitochondrial proteins including PGC-1α and HIF1 (hypoxia inducible factor 1) will likely have an important impact on how mitochondrial function is dynamically regulated in response to a wide range of pathological cues from their environment in the kidneys. With a growing number of ncRNAs identified in a variety of kidney diseases, there is no doubt that we are just starting to understand their significance in CKD progression. Much of the literature has not fully addressed or investigated ncRNA effects on mitochondria and CKD progression, and considering the recent advances in next-generation sequencing, we expect to gain a more comprehensive understanding of the diverse biological functions and molecular mechanisms of lncRNAs on mitochondrial function and progression of CKD in a near future. Indeed, a number of clinical trials are underway to assess the role of targeting ncRNA in several pathologies (36).
Mitochondrial Oxidative Stress
Reactive oxygen species (ROS) are molecules derived from oxygen that can readily oxidize other molecules. ROS have a physiological function at low levels and participate in eliciting proliferation and survival in response to stress conditions. However, at higher levels, mitochondrial ROS could be pathogenic and causing cellular damage (Figure-3). The observed dichotomy in mitochondrial ROS function could be explained if ROS is regarded as a stress-induced survival signal that primarily serve to activate compensatory survival mechanisms. However, when the levels of stress increase beyond a threshold or the cell stress becomes chronic, mitochondrial ROS production will no longer be protective contributing to further mitochondrial dysfunction and cellular damage.
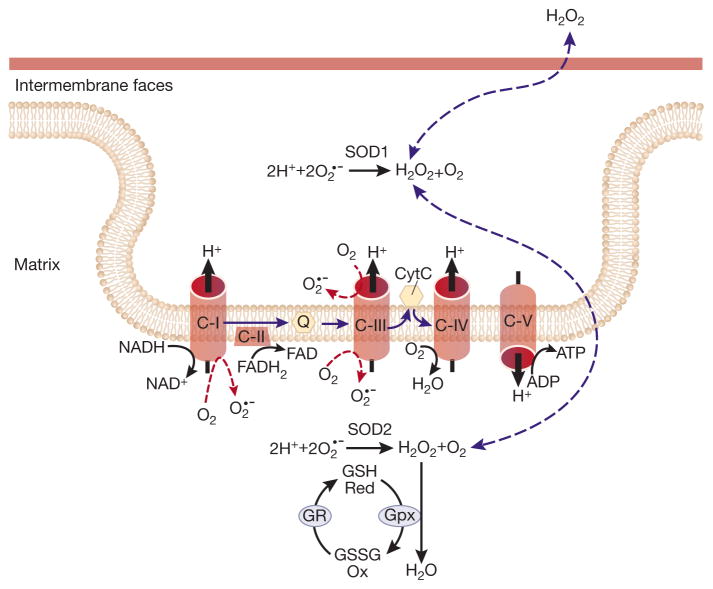
Figure 3. Mitochondrial ROS generation.
Electron escape during mitochondrial electron transport at complexes I and III generates superoxide anions both in the intermembrane space and matrix. CI, CII, CIII, CIV, CV=Complexes I–V, cytC=cytochrome C, Q=ubiquitone, O2′−=superoxide, GR=Glutathione reductase, Gpx=Glutatione peroxidase, SOD=Superoxide Dismutase, GSH=glutathione, GSSG=glutathione disulfide, FAD= flavin adenine dinucleotide, FADH2=Dihydroflavine adenine dinucleotide, H2O2=hydrogen peroxide, NAD+=nicotinamide adenine dinucleotide, NADH=dihydronicotinamide adenine dinucleotide.
Enhanced mitochondrial ROS has been implicated in numerous processes ranging from aging to cancer, and it has maintained center stage as a key mediator of kidney damage for decades. A large body of evidence based on studies not only in experimental models of kidney injury but also in patients have suggested that the generation of ROS is significantly increased in failing kidneys. Of particular interest is the free radical theory of diabetic microvascular complications also known as the Unifying hypothesis that inked mitochondrial ROS to mitochondrial dysfunction and end-organ damage in kidneys. The Unifying hypothesis proposes that overproduction mitochondrial ROS is associated with mitochondrial dysfunction which ultimately causes cellular damage and progression of kidney disease. We and others have identified enhanced mitochondrial ROS in vitro and in kidneys of diabetic mice in vivo (3, 11, 37). One such study suggested that glucose-induced ROS production contributes to podocyte apoptosis and podocyte depletion both in vitro and in vivo (37). Furthermore, we have recently utilized a redox sensitive GFP based biosensor that is specifically localized to mitochondrial matrix to demonstrate enhanced oxidative stress generated specifically in the mitochondria in live diabetic db/db mice (Galvan, D.L. et al., unpublished data). Consistent with these findings, several other reports suggest that the administration of mitoTEMPO, a mitochondrial targeted antioxidant, can alleviate key features of diabetic nephropathy (38, 39). These observations are in contrast to recent findings utilizing dihydroethidium to assess mitochondrial ROS in diabetic mice in vivo that found reduced mitochondrial ROS generation in diabetic environment (18). The use of DHE to assess mitochondrial ROS as well as a crosstalk linking cytosolic and mitochondrial oxidative stress in kidney diseases could be possible explanations for these seemingly contrasting findings.
Despite all controversies, it is well-established that the overproduction of mitochondrial ROS is harmful to the cells in multiple ways, including by inducing nuclear and mitochondrial DNA damage and promoting cell apoptosis (40). However, the traditional view that only overproduction of mitochondrial ROS is harmful has been recently challenged and it seems that both “too much” or “too little” of mitochondrial ROS could be detrimental for cell survival and as a result, the health of kidney cell could depend on the right amount of ROS generation since very low levels of mitochondrial ROS could also dysregulate multiple signaling pathways and interfere with the proper function and activity of a number of redox-dependent proteins.
Future Directions
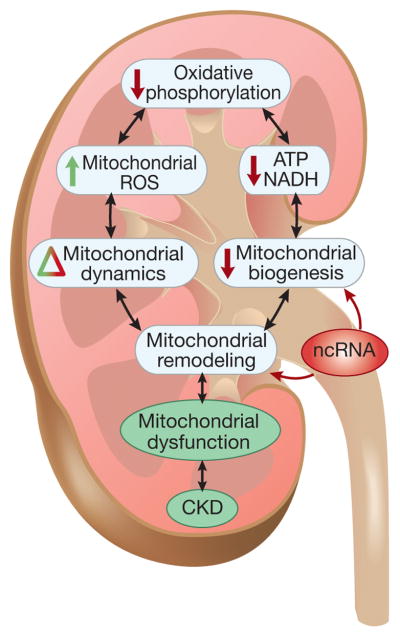
While it has become increasingly clear that mitochondrial dysfunction is involved in the development and progression of a variety of kidney diseases leading to CKD (Figure-4), our understanding of the impact of mitochondrial dysfunction in patients with CKD remains limited. There is reason for optimism, however, as additional studies using state-of-the-art strategies are underway to more comprehensively address the role of mitochondrial dysfunction in CKD progression. A global assessment of mitochondrial function should help to delineate the value as well as the limitations of therapeutic strategies for restoring mitochondrial function in patients with CKD.
Figure 4. Hallmarks of mitochondrial dysfunction in CKD.
The interrelated hallmarks provide a framework for further understanding the diverse aspects of mitochondrial dysfunction in CKD.
Acknowledgments
This work was supported by NIH grants R01DK091310 and R01DK078900 to FD.
Footnotes
Disclosure.
All authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wirthensohn G, Guder WG. Renal substrate metabolism. Physiol Rev. 1986;66:469–497. doi: 10.1152/physrev.1986.66.2.469. [DOI] [PubMed] [Google Scholar]
- 2.Zschiedrich S, Bork T, Liang W, et al. Targeting mTOR signaling can prevent the progression of FSGS. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 4.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tubbs E, Rieusset J. Metabolic signaling functions of ER-mitochondria contact sites: role in metabolic diseases. J Mol Endocrinol. 2017;58:R87–R106. doi: 10.1530/JME-16-0189. [DOI] [PubMed] [Google Scholar]
- 6.Morigi M, Perico L, Rota C, et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015;125:715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks C, Wei Q, Cho SG, et al. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galloway CA, Lee H, Nejjar S, et al. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes. 2012;61:2093–2104. doi: 10.2337/db11-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan M, Usman IM, Sun L, et al. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol. 2015;26:1304–1321. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayanga BA, Badal SS, Wang Y, et al. Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J Am Soc Nephrol. 2016;27:2733–2747. doi: 10.1681/ASN.2015101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Wang Y, Long J, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han XJ, Lu YF, Li SA, et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 14.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puigserver P, Wu Z, Park CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 16.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 17.Baldelli S, Aquilano K, Ciriolo MR. Punctum on two different transcription factors regulated by PGC-1alpha: nuclear factor erythroid-derived 2-like 2 and nuclear respiratory factor 2. Biochim Biophys Acta. 2013;1830:4137–4146. doi: 10.1016/j.bbagen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Dugan LL, You YH, Ali SS, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakatani Y, Inagi R. Epigenetic regulation through SIRT1 in podocytes. Curr Hypertens Rev. 2016;12:89–94. doi: 10.2174/1573402112666160302102515. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa K, Wakino S, Simic P, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19:1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platt C, Coward RJ. Peroxisome proliferator activating receptor-gamma and the podocyte. Nephrol Dial Transplant. 2017;32:423–433. doi: 10.1093/ndt/gfw320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran MT, Zsengeller ZK, Berg AH, et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang HM, Ahn SH, Choi P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershberger KA, Martin AS, Hirschey MD. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol. 2017;13:213–225. doi: 10.1038/nrneph.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Y, Huang S, Wang W, et al. Activation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 2012;82:771–789. doi: 10.1038/ki.2012.188. [DOI] [PubMed] [Google Scholar]
- 26.Perico L, Morigi M, Benigni A. Mitochondrial Sirtuin 3 and renal diseases. Nephron. 2016;134:14–19. doi: 10.1159/000444370. [DOI] [PubMed] [Google Scholar]
- 27.Sharabi K, Lin H, Tavares CD, et al. Selective chemical inhibition of PGC-1alpha gluconeogenic activity ameliorates type 2 diabetes. Cell. 2017;169:148–160. e115. doi: 10.1016/j.cell.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartleben B, Godel M, Meyer-Schwesinger C, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badal SS, Danesh FR. MicroRNAs and their applications in kidney diseases. Pediatr Nephrol. 2015;30:727–740. doi: 10.1007/s00467-014-2867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long J, Wang Y, Wang W, et al. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. 2010;285:23457–23465. doi: 10.1074/jbc.M110.136168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badal SS, Wang Y, Long J, et al. miR-93 regulates Msk2-mediated chromatin remodelling in diabetic nephropathy. Nat Commun. 2016;7:12076. doi: 10.1038/ncomms12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClelland AD, Herman-Edelstein M, Komers R, et al. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 2015;129:1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 33.Zhong X, Chung AC, Chen HY, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–674. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 34.Long J, Badal SS, Ye Z, et al. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vendramin R, Marine JC, Leucci E. Non-coding RNAs: the dark side of nuclear-mitochondrial communication. EMBO J. 2017;36:1123–1133. doi: 10.15252/embj.201695546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13:622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 37.Dieter BP, Alicic RZ, Meek RL, et al. Novel therapies for diabetic kidney disease: Storied past and forward paths. Diabetes Spectr. 2015;28:167–174. doi: 10.2337/diaspect.28.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Chen JK, Harris RC. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol. 2015;26:1115–1125. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sims CR, MacMillan-Crow LA, Mayeux PR. Targeting mitochondrial oxidants may facilitate recovery of renal function during infant sepsis. Clin Pharmacol Ther. 2014;96:662–664. doi: 10.1038/clpt.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishi Y, Satoh M, Nagasu H, et al. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int. 2013;83:662–673. doi: 10.1038/ki.2012.475. [DOI] [PubMed] [Google Scholar]