Abstract
Background and Purpose
Due to the limitation in treatment window of the rtPA (recombinant tissue plasminogen activator), the development of delayed treatment for stroke is needed. In this study, we examined the efficacy of delayed post-stroke treatment (post 3–8 days) of the sonic hedgehog pathway agonist (SAG) on functional recovery and the underlying mechanisms.
Methods
We evaluated functional recovery at 1 month after stroke using locomotion analysis and Barnes maze test for cognitive function. We utilized a genetically inducible NSC-specific reporter mouse line (nestin-CreERT2-R26R-YFP) to label and track their proliferation, survival and differentiation in ischemic brain. Brain tissue damage, angiogenesis and cerebral blood flow recovery was evaluated using MRI techniques and immunostaining.
Results
Our results show that delayed treatment of SAG in stroke mice results in enhanced functional recovery both in locomotor function and cognitive function at one month after stroke. Further, utilizing the nestincreERT2-YFP mice, we showed that post-stroke SAG treatment increased surviving newly born cells derived from both SVZ and SGZ neural stem cells (NSCs), total surviving DCX+ (Doublecortin) neuroblast cells and neurons (NeuN+/YFP+) in the ischemic brain. SAG treatment also improved the brain tissue repair in ischemic region supported by our T2 weighted MRI, Cerebral Blood Flow (CBF) map by Arterial-Spin-Labeling (ASL) and immunohistochemistry (alpha-smooth muscle actin and CD31 immunostaining).
Conclusions
These data confirm an important role for the hedgehog pathway in post-stroke brain repair and functional recovery, suggesting a prolonged treatment window for potential treatment strategy to modulate shh pathway after stroke.
Keywords: sonic hedgehog signaling, stroke, neurogenesis, angiogenesis
Subject Terms: Ischemic Stroke, Neurogenesis
Introduction
Due to the limitation in treatment window of the rtPA (recombinant tissue plasminogen activator), only a small percentage of patients are treated in the acute window (4.5 h) after stroke onset 1. Therefore, therapeutic strategies targeting the subacute and chronic stages of the disease are needed. Recent studies utilizing genetic ablation systems to specifically diminish neuroprogenitor cells or immature neuroblasts in stroke mice demonstrate the contribution of adult neural stem cells (NSCs) and their progeny in the functional recovery in stroke 2, 3. However, the spontaneous brain repair process is limited and does not lead to full recovery after stroke 4. One of the major challenges is the survival of newly generated cells after stroke. It has been estimated that 80% of newly born cells originating from the adult stem cell niche SVZ (subventricular zone) die during the first 2 weeks after their formation in stroke animals 5. Therefore, enhancing the survival of newly born cells is an important priority for enhancing neurogenesis after stroke. Potential therapeutic treatments such as stem cell transplantation have shown efficacy in the subacute and chronic stroke stages in animal models, potentially through facilitating endogenous repair mechanisms such as neurogenesis and angiogenesis 6, 7.
Sonic hedgehog (shh) signaling play important roles in the self-renewal, proliferation, and migration of NSCs 8, 9. We and others have shown the beneficial effects of shh activation in brain injury animal models, including stroke 10–12. In a previous study, we have shown that the shh gene is upregulated in multiple cell types after stroke and deletion of shh signaling in NSCs lead to compromised functional recovery in stroke mice. In the same study, we found that post-stroke shh agonist (SAG) treatment lead to enhanced functional recovery in the subacute phase of stroke (post-stroke day 7) in a distal middle cerebral artery occlusion (MCAo) model 12. However, whether the SAG molecule leads to long-term functional recovery and its effects on neurogenesis and the survival of newly generated cells in the transient filament MCAo model is unknown. In this study, we evaluated the efficacy of delayed post-stroke treatment (post-stroke day 3–8) of SAG in long-term functional recovery after MCAo and brain tissue repair and CBF (Cerebral Blood Flow) recovery using MRI technique. In addition, to specifically examine the effects and mechanisms of shh signaling activation on NSCs in the SVZ and SGZ (subgranular zone) in stroke mice, we utilized a genetically inducible NSC-specific reporter mouse line (nestin-CreERT2-R26R-YFP) to label and track their proliferation, differentiation and survival.
Materials and Methods
Animals
All animal protocols were conducted under National Institutes Health (NIH) Guidelines using the NIH handbook Animals in Research and were approved by the Institutional Animal Care and Use Committee (Case Western Reserve University). Male C57BL/6 mice (10–12 weeks old) were used in this study. To evaluate neurogenesis in stroke mice, the nestin-CreERT2-R26R-YFP strain was used in this study.
Chemical
SAG was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). SAG is dissolved in 0.5%Cellulose tween-80 solution at a final concentration of 1 mg/ml. Animals received SAG at the dosage of 10mg/kg/day through gavage feeding from post-stroke day 3–8. Activity of each batch of SAG was confirmed in lab by its effects on stimulating proliferation of in vitro cultured SVZ NSCs neurosphere culture.
Study design and timeline
A total of 138 mice were used in this study (102 C57BL/6 male mice and 36 nestin-CreERT2-R26R-YFP male mice). Male mice were used in this study to reduce experimental variability in stroke. The animals were subjected to the following experiments: 28 mice were subjected to MCAo and received either vehicle or SAG treatment from post-stroke day3–8. Mice were subject to locomotion behavioral functional test before MCAo and at 3, 7, 21, 28 days after MCAo. Additional 36 mice were subjected to MCAo and received either vehicle or SAG treatment (post-stroke day3–8) and were evaluated for Barnes maze test to evaluate memory and learning at 30 days post stroke. 18 mice from the Barnes maze test group were subjected to MRI study on post-stroke day2 and 30 to evaluate ischemic damage and CBF. To evaluate the effect of SAG treatment on Barnes maze performance on control mice, mice received SAG or vehicle treatment (10mg/kg for 6 days, n= 9 for each group) without surgery and were subjected to Barnes Maze test 21 days later. To evaluate the proliferation of SVZ and SGZ cells, Bromodeoxyuridine (BrdU) pulse labeling were carried out on post-stroke day 6 after either vehicle or SAG treatment and brain were harvested 24 hours later to quantify BrdU+, PCNA+ (proliferating cell nuclear antigen) or Ki67+ cells (20 mice). To evaluate neurogenesis and long-term survival of NSC progeny: 20 nestin-CreERT2-R26R-YFP mice were treated with tamoxifen (TAM) to induce yellow fluorescent protein (YFP) labeling of SVZ and SGZ NSCs and treated with either vehicle or SAG (post-stroke day 3–8). YFP+, DCX+ or NeuN+ cells were analyzed by unbiased stereological method at 30 days post stroke. Mice from the MRI study and the nestin-CreERT2-R26R-YFP were combined to analyze the DCX+ cells and angiogenesis as well. To examine gene expression in SVZ NSCs, additional 16 nestin-CreERT2-R26R-YFP mice were subjected to MCAo and SAG or vehicle treatment (post-stroke day 3–8) and SVZ tissue microdissected using methods described in 13 for quantitative RT-PCR. To evaluate angiogenesis by histochemistry, brain sections from post-stroke day 30 mice were subjected to immunostaining using two different markers, alpha-smooth muscle actin (α-SMA) and CD31 and quantitatively analyzed as described in detail in the supplemental information.
All the experimental groups are randomized and all outcome analysis was carried out by independent study team members blinded to the treatment condition. Details on experimental timeline, randomization and blinding of groups, animal numbers for each experiment, and survival rate/exclusions due to death are included in online supplemental data. Details on each assay are also included in online supplemental data.
Statistics
Results are expressed by mean±SEM of the indicated number of experiments. Statistical analysis was performed using Student’s t-test, and one- or two-way analysis of variance (ANOVA), as appropriate, with Student-Newman-Keuls post-hoc tests or Bonferroni post-hoc tests for repeated behavioral measurements. A p value equal to or less than 0.05 was considered significant.
Results
Post-stroke shh agonist (SAG) treatment (post-stroke day 3–8) after MCAo enhanced the locomotor behavioral recovery and cognitive function at 1 month after stroke
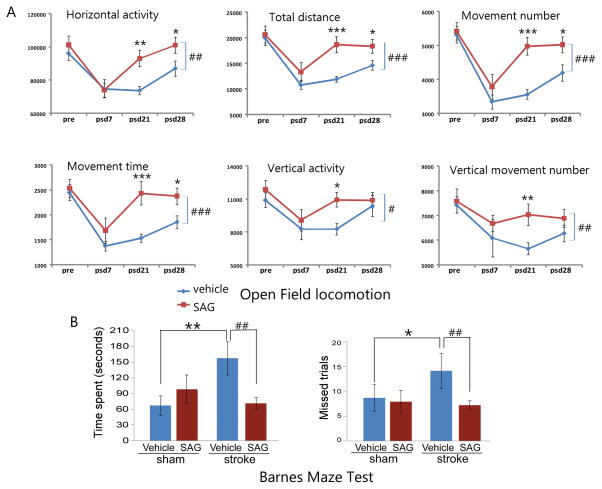
To examine whether post-stroke SAG treatment lead to long-term improvement in behavioral function in the tMCAo model, we tested the vehicle or SAG-treated mice with behavioral tests that evaluated either locomotor or cognitive function. There is no difference in any the parameters measured in the two groups before or at post-stroke day 3 after MCAo (Supplemental Table I). Mice that received post-stroke SAG treatment (n= 11) showed enhanced recovery in motor function compared to vehicle-treated mice (n=12), demonstrated by significant increases in multiple behavioral measurements including total horizontal activity (HACTV, Fig 1A, Two-way ANOVA for repeated measurements, SAG vs. vehicle: F1, 91 = 8.189, p=0.005), distance traveled (TOTDIST, Fig 1A, SAG vs vehicle: F1, 91 = 12.917, p<0.001), number of movements (MOVNO, Fig 1A, SAG vs vehicle: F1, 91 = 15.423, p<0.001,), movement time (MOVTIME, Fig 1A, SAG vs vehicle: F1, 91 = 13.868, p<0.001), vertical activity (VACTV, Fig 1A, SAG vs vehicle: F1, 91 = 4.402, p=0.039) and vertical movement number (VMOVNO, Fig 1A, SAG vs vehicle: F1, 91 = 7.343, p=0.008) compared to the control group at 3 and 4 weeks after MCAo surgery. (p values for post-hoc pairwise comparison of SAG vs vehicle groups at different time points in all parameters are listed in Supplemental Table II). SAG treatment administered on post-stroke day 3 for 6 days showed a trend of improvement on multiple parameters starting on post-stroke day 7 which became highly significant in all the parameters measured at 3 weeks post stroke but plateaued at 4 weeks after stroke, which still were significantly improved compared to vehicle-treated mice in multiple parameters including HACTV, TOTDIST, MOVNO, MOVTIME and RESTIME (Fig 1). At the last time point (post-stroke day 28), the vertical activities between the two groups were no longer statistically significant.
Figure 1.
Post-stroke SAG treatment enhanced functional recovery in both locomotor function and cognitive function. (A). locomotor function measured by automatic open field locomotion chambers at different time points in vehicle or SAG-treated (post-stroke day 3–8) mice. Multiple parameters are shown as labeled in panels. MEAN ± SEM. # or ## p<0.05 or p<0.01, two-way ANOVA vehicle vs SAG. * or **, p<0.05 or p<0.01, post-hoc Newman-Keuls test (B). Barnes maze test showing time spent and missed trials before mice found the escape hole in control or stroke mice that received vehicle or SAG treatment. *or **, p<0.05 or p<0.01, control vs stroke and ##, p<0.01, vehicle vs SAG.
To determine whether post-stroke SAG treatment affects stroke-induced functional deficits in learning and memory, we also evaluated cognitive function in control or post-stroke SAG-treated mice. To minimize the interference of other behavioral tests on cognitive function test, a separate group of mice was used in this test. Four groups of mice were tested at 21 days after SAG or vehicle treatment (control or stroke + vehicle or SAG). Two variables (surgery and treatment) were used to analyze changes in behaviors using a two-way ANOVA. Results show that stroke induced deficits in learning and memory in vehicle-treated mice (Fig 1B, p=0.008 for time spent and p=0.019 for error trials, ANOVA, post-hoc Student-Newman-Keuls test, n=9 for control+vehicle and n=12 for stroke+vehicle). In control mice, SAG or vehicle treatment shows no difference (n=9 for each group) suggesting SAG does not promote memory in naïve young animals. For stroke mice, MRI at 2 days after MCAo showed that there was no difference in infarct size between these two groups of mice (33.95 ± 5.79 % hemisphere for vehicle and 32.64+5.83 % hemisphere for SAG-treated mice) before the treatment started. However, at 30 days after stroke, stroke mice that received SAG treatment spent significantly less time to find the escape hole compared with vehicle-treated stroke mice (Fig 1B, p=0.006, ANOVA, post-hoc Student-Newman-Keuls test, n=12 for stroke+vehicle and n=14 for stroke+SAG) which is not significantly different from control mice (p= 0.416, control+SAG vs stroke+SAG). In addition, SAG-treated mice also took less error trials to find the escape hole (Fig 1B, p=0.003, stroke+vehicle vs stroke+SAG), which is not significantly different from control mice (p=0.830, control+SAG vs stroke+SAG). These data indicate that SAG treatment restored cognitive function in stroke mice.
Post-stroke shh agonist (SAG) treatment (post-stroke day 3–8) after MCAo increased the survival but not proliferation of SVZ and SGZ NSCs progeny and total neuroblast cells at 1 month after stroke
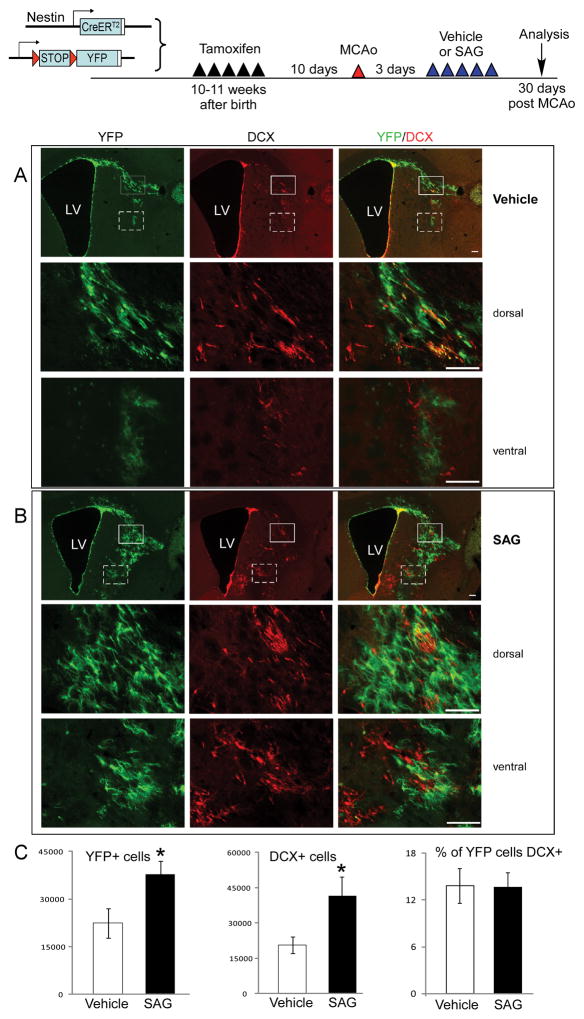
To examine whether SAG post-stroke treatment improved behavioral functions through modulation of proliferation or survival of NSCs and their progeny, we utilized a NSC-specific inducible cell labeling system as previously reported by us and others 12, 14, 15. Using this mouse line, we labeled NSCs before MCAo and tracked their survival and differentiation into neuronal phenotype at 30 days after MCAo in vehicle or SAG-treated mice using unbiased stereological counting of YFP and DCX positive cells. On contralateral side, there is no migration of YFP or DCX positive cells into the striatal or cortical region in vehicle or SAG-treated groups. On the ipsilateral side of the brain, there are significant expansion and migration of YFP+ cells into striatal and some cortical region from SVZ to the ischemic core (Fig 2A). Importantly, SAG treatment resulted in increased YFP+ cells in striatal and cortical area on ipsilateral side (Fig 2B–C, Student’s t-test, t= 2.469, p=0.03, n= 7 for each group). In addition, total DCX+ cells migrated into the striatal and cortical area were also increased in SAG-treated mice (Fig 2B–C, Student’s t-test, t= 2.368, p=0.026, n= 13 for each group). The percentage of YFP+ cells that were also DCX+ does not differ between vehicle and SAG-treated mice (Fig 2B–C, p=0.96, Student’s t-test), suggesting that post-stroke SAG treatment increased the total surviving YFP+/DCX+ cells at 30 days post stroke without affecting the neuronal differentiation percentage. Similarly, using unbiased stereological quantification, we also observed increase total number of YFP+/NeuN+ cells in striatal and cortical area of the ipsilateral side in SAG-treated mice compared to vehicle-treated mice (Supplementary Figure IA, p<0.05, Student’s t-test).
Fig 2.
Post-stroke SAG treatment promote the survival of SVZ NSCs migrated into ischemic region. YFP+ and DCX+ NSCs migrate into striatal and cortical area in ischemic hemisphere in vehicle (A) or SAG (B) treated mice at 30 days post stroke. (C) unbiased stereological quantification of YFP+, DCX+ cells and percentage of DCX+ cells in YFP+ cells. MEAN ± SEM. * p<0.05 compared to vehicle-treated group. n=7 for YFP+ cells quantification and n=13 for DCX+ cell quantification. Dorsal striatal (solid box) and ventral striatal (dashed box) are shown in higher magnifications in lower panels in (A) and (B). scale bar= 100 μm.
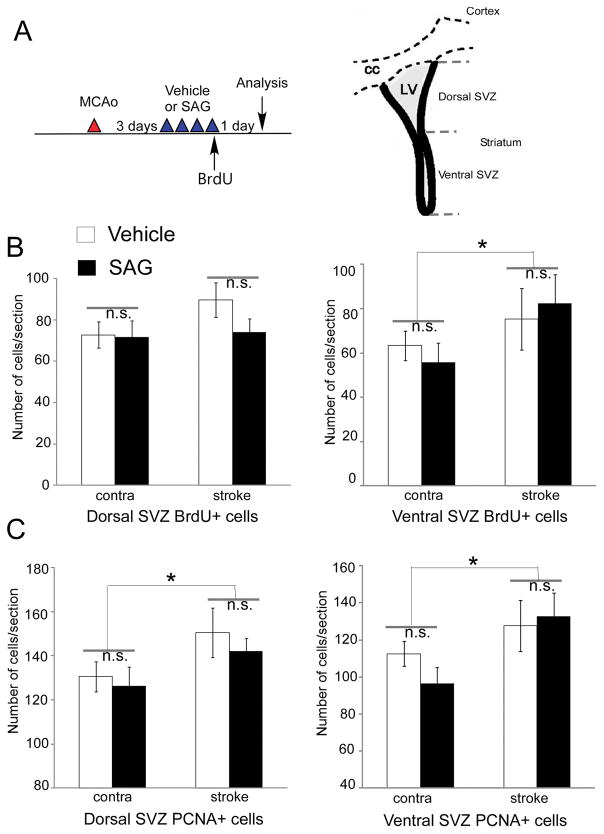
To determine whether post-stroke SAG increased the total number of YFP+ cells at 30 days by enhancing the proliferation of NSCs or the survival of newly born cells that are progeny of the SVZ NSCs, we treated mice with either vehicle or SAG (post-stroke day 3–7) and injected BrdU on post-stroke day 6 to evaluated proliferating cell numbers by both BrdU labeling and PCNA labeling. Ventral SVZ NSCs differ from dorsal SVZ NSCs in their expression of smoothened (Smo) receptor and might respond to SAG differentially16, we quantified proliferating cells in vehicle or SAG-treated mice in both dorsal and ventral SVZ. Our results indicate that ipsilateral side ventral SVZ showed increased proliferation compared to contralateral side SVZ in both vehicle and SAG-treated mice ( Fig 3, for BrdU+ cells, p=0.206 for dorsal SVZ and p=0.05 for ventral SVZ: contralateral side vs ipsilateral side; for PCNA+ cells, p=0.042 for dorsal SVZ and p=0.03 for ventral SVZ: contralateral side vs ipsilateral side, two-way ANOVA), however there is no difference between vehicle and SAG-treated mice measured by either BrdU + cells (Fig 3, p=0.271 for dorsal SVZ and p=0.758 for ventral SVZ: vehicle vs SAG) or PCNA + cells (p=0.457 for dorsal SVZ and p=0.499 for ventral SVZ: vehicle vs SAG, two-way ANOVA, n=7 each group). In general, dorsal or ventral SVZ NSCs showed similar trend. (Fig 3). To further confirm that this in vivo SAG treatment regimen indeed increase shh signaling in NSCs at the SVZ, we harvested SVZ tissue in nestin-CreERT2-R26R-YFP using technique described previously 13 and analyzed gene expression in both contralateral and ipsilateral SVZ in vehicle or SAG-treated stroke mice. Our results show no significant difference in two housekeeping genes (Hmbs and Hprt1) in any groups but a significant increase of smoothened (smo) gene expression in the ipsilateral side in both vehicle and SAG-treated mice (p<0.01, contralateral vs ipsilateral side, ANOVA n=5–7), suggesting ischemia induces smo gene expression in SVZ NSCs. More importantly, SAG treatment increased the shh signaling pathway target gene Gli1 expression in the ipsilateral side of the SVZ compared to the vehicle-treated mice, confirming activation of shh signaling pathway in NSCs by SAG in vivo treatment (supplementary Figure II, p<0.05, n=5–7).
Fig 3.
Post-stroke SAG (10mg/kg from post-stroke day 3–6) does not affect the proliferation of SVZ NSCs in stroke mice. (A) Experimental timeline and schematic diagram showing the dorsal and ventral SVZ regions. (B) Dorsal and ventral SVZ BrdU+ cells after vehicle or SAG treatment. (C) Dorsal and ventral SVZ PCNA+ cells after vehicle or SAG treatment. MEAN± SEM. *, p<0.05 contralateral side vs ipsilateral side, two-way ANOVA. No difference in vehicle vs SAG groups (n=7 each group).
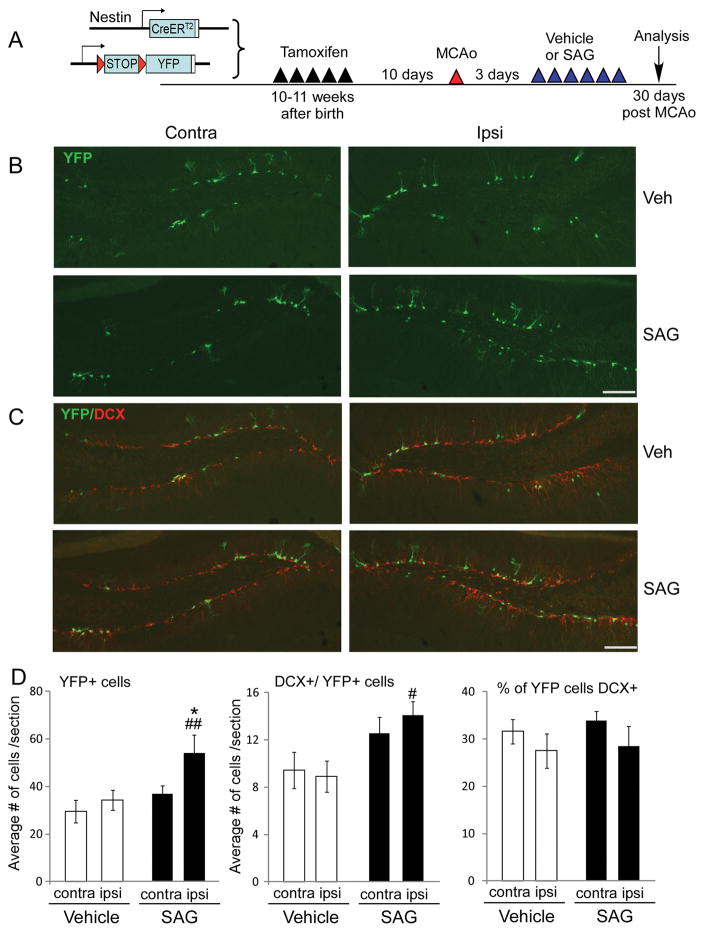
Since SAG-treated mice have enhanced cognitive function, we also examined the effects of SAG on the proliferation and survival of hippocampal SGZ NSCs. Similar to SVZ NSCs, BrdU positive cells in SGZ 7 days after MCAo showed that the ipsilateral side had significantly more cell proliferation demonstrated by increased BrdU+ cells in ipsilateral SGZ compared to the contralateral side in both vehicle and SAG-treated group (Supplementary Fig III, for BrdU+ cells, ANOVA, vehicle vs SAG: F1, 21 = 12.5, p=0.002, for Ki67+ cells, ANOVA, vehicle vs SAG: F1, 17 = 8.871, p=0.01, n=5–6). SAG treatment did not further enhance the increased proliferation in ischemic side (Supplementary Fig III, Two-way ANOVA, contralateral vs ipsilateral side: F1, 21 = 0.287, p=0.599, n=5–6). Ki67+ cells confirmed same trend for proliferating cells in hippocampus (Supplemental Fig III,). Consistent with the SVZ NSCs data, at 30 days we also observed increased number of total YFP+ cells, YFP+/DCX+ and YFP+/NeuN+ cells in the ipsilateral side of dentate gyrus (DG) in hippocampus of SAG-treated mice (Fig 4, p=0.008 for YFP+ cells and p=0.015 for DCX+/YFP+ cells, vehicle vs SAG, ANOVA, post-hoc t-test, n=7 each group. Supplementary Figure IB, p= 0.04 for YFP+/NeuN+ cells, vehicle vs SAG, ANOVA, post-hoc t-test), suggesting enhanced survival of newly generated neuroblasts and NeuN+ neurons by SAG treatment. There is no difference between the percentage of YFP+ cells that are DCX+ (Fig 4, p=0.573, n=7) or NeuN+ (p=0.552, n=7), indicating no effect of SAG on the differentiation of SGZ NSCs.
Fig 4.
Post-stroke SAG treatment promote the survival of SGZ NSCs in hippocampus. (A) experimental timeline to label SGZ NSCs. (B) YFP+ and (C) YFP+ and DCX+ NSCs and their progeny in contralateral or ipsilateral side of hippocampus in vehicle or SAG-treated mice at 30 days post stroke. (D) Quantification of YFP+, DCX+ cells and percentage of DCX+ cells in YFP+ cells in DG of hippocampus. MEAN± SEM. * p<0.05 compared to contralateral side. # or ##, p<0.05 or p<0.01 compared to vehicle-treated mice, ANOVA. n= 7 each group. scale bar= 100 μm.
Post-stroke shh agonist (SAG) treatment (post-stroke day 3–8) after MCAo decrease T2 value and increase CBF and angiogenesis in ischemic area at 1 month after stroke
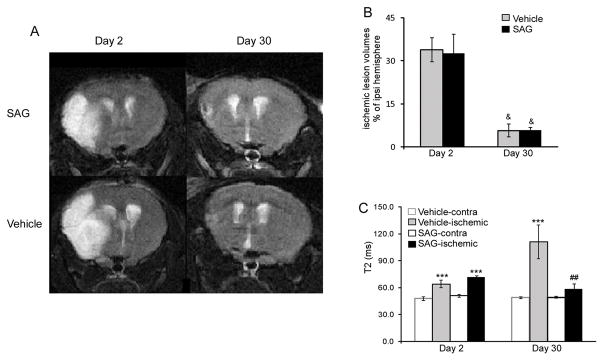
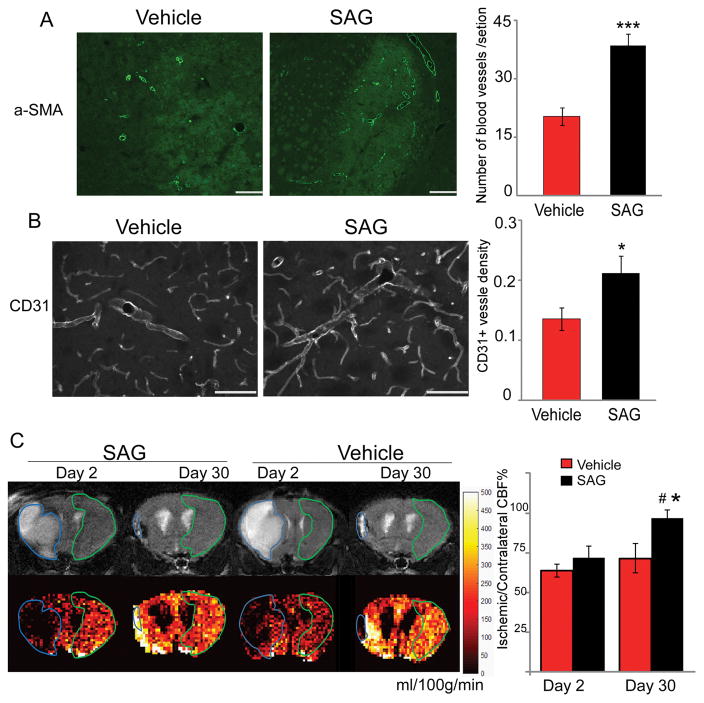
Multiple processes have been implicated to be important in the survival of adult born neuronal lineage cells, especially under pathological condition. Angiogenesis has been coupled with neurogenesis and regulate the proliferation, survival and migration of newly born cells17–20. Therefore, we examined the tissue damage and CBF at day 2 and day 30 after MCAo in vehicle and SAG-treated mice by MRI. T2-weighted images of SAG- and vehicle-treated mice on day 2 and day 30 showed that ischemic edema volume in SAG-treated mice was similar to vehicle-treated mice at both day 2 and day 30. At day 2, ischemic region in both groups showed significantly higher T2 signaling than the contralateral hemisphere and there was no difference in either edema volume or T2 relaxation time between the two groups (Fig 5, p>0.05, ANOVA). At day 30, the volume of T2 elevated areas had decreased in both groups and there was no significant difference in the T2 elevated volume between the two groups (Fig 5, p>0.05, ANOVA). Vehicle-treated mice showed further increase in T2 relaxation time in the ischemic region as compared to day 2, possibly implying irreversible neuronal damage 7. In contrast, SAG-treated mice showed decreased T2 in the ischemic area, reaching a similar level as the contralateral brain tissue (Fig 5, p=0.002, vehicle vs SAG at day 30, ANOVA, n=7). In addition, SAG-treated mice also showed increased CBF in the peri-lesion and lesion core areas (T2 elevated area) at day 30 compared to vehicle-treated mice (Fig 6, p=0.021, vehicle vs SAG at day 30, ANOVA, n=7), indicating enhanced angiogenesis and blood flow supply. Consistent with MRI and CBF imaging, we found that the number of α-SMA positive blood vessels was significantly enhanced by SAG treatment (Fig 6A, p<0.001, Student’s t-test, n=8–9) in the ischemic region. In addition, CD31 immunostaining also confirms higher density of CD31 positive blood vessels in the peri-infarct area of SAG-treated mice compared to vehicle-treated mice (Fig 6B, p=0.034, Student’s t-test, n=8). These data consistently suggest that post-stroke SAG treatment increased vascularization in the lesion side striatum and cortex, a mechanism that might contribute to the increased survival of NSC progeny.
Fig 5.
(A) Representative T2-weighted images of SAG-treated (top) and vehicle-treated (bottom) brains at day 2 (left) and day 30 (right). (B) Percentage ischemic volume at day 2 and day 30. Error bars indicate standard error. &<0.05, day 2 versus day 30. (C)Changes in T2 in ischemic region and contralateral brain region. MEAN± SEM. ***p<0.001, ischemic region versus nonischemic tissue, ##p<0.01, vehicle vs SAG group, n=7.
Fig 6.
Post-stroke SAG treatment enhances angiogenesis and cerebral reperfusion in ischemic region 30 days after stroke. (A) alpha-SMA positive blood vessels and (B) CD31 positive blood vessels in ischemic region after vehicle or SAG treatment and quantification of alpha-SMA or CD31 positive blood vessel density are shown in the right panels. (C). Panel shows the representative region of interest (ROI) for edema and contralateral brain definition. Top row shows the T2 weighted images of the brains and ROI for edema and contralateral brain are outlined by blue and green, respectively. Bottom row are the CBF maps (ml/100g/min) with the co-registered ROI. Contralateral-normalized CBF quantification in ischemic region is shown in right panel. MEAN± SEM. * or *** p<0.05 or p<0.001, SAG-treated versus vehicle-treated. #, p<0.05, day 2 vs day 30. n=7. scale bar= 100 μm.
Discussion
The sonic hedgehog signaling pathway has been reported to regulate SGZ proliferation after hippocampal ischemia because inhibition of shh pathway by antagonist Cyclopamine reduces ischemia-induced proliferation in the SGZ 21. However, it is unknown whether stimulation of shh signaling would further potentiate ischemia-induced proliferation in the SGZ. We and others have reported that shh pathway is upregulated in multiple cell types after stroke and we have shown that post-stroke treatment of a sonic hedgehog agonist (post-stroke day 3) in distal MCAo lead to functional improvement in the subacute phase after stroke12. An independent study also reported that treatment of a different sonic hedgehog pathway agonist (Purmorphamine) administered at early post-stroke stage (6 hours) lead to decreased neuronal damage, BBB integrity and reduced reactive astrogliosis11. However, since the 6-hour treatment after stroke does not distinguish neuroprotection from neuroregeneration mechanisms, it is not clear whether delayed activation of shh signaling would alone lead to enhanced brain repair.
Our data showed that delayed treatment of SAG (post-stroke day 3–8) significantly improved locomotion function in multiple parameters as well as cognitive function in memory and learning in Barnes maze test at one month after stroke. To specifically track and fate map SVZ and SGZ NSCs and quantify their differentiation and survival under SAG treatment, we utilized Nestincre-ERT2-YFP mice to induce the expression of YFP reporter gene in SVZ and SGZ NSCs in adult mice before MCAo onset. Although SVZ derived new born cells have been shown to differentiate into NeuN positive cells, only few new neurons survive in post ischemic brain5. Similarly, SGZ NSCs also respond to ischemia by increased proliferation, however, the survival rate of the newly born cells in ischemic animals is lower than that of non-stroke animal at 1 month later22. In this study, using the nestincreERT2-YFP mice in combination of analysis of proliferating cells in SVZ and SGZ by both BrdU labeling and endogenous proliferating markers PCNA or ki67, our data suggest that in vivo post-stroke SAG treatment at this dosage enhances the survival of newly born cells rather than further improving the proliferation. Whether there is a causal relationship between hippocampal neurogenesis and the recovery of poststroke cognitive function in this particular MCAo model warrants further investigation in the future, possibly using genetic tagging and ablation techniques that will specifically distinguish hippocampal neurogenesis.
In both the SVZ neurogenic niche and the ischemic area, angiogenesis and neurogenesis has been shown to be closely coupled. Using MRI imaging, we found that delayed SAG treatment does not affect the volume of T2 elevated area at 30 days post stroke. However, SAG-treated mice showed decreased T2 values in the ischemic area, reaching a similar level as the contralateral brain tissue while vehicle-treated mice showed further elevated T2, possibly implying long-term irreversible tissue damage23. Consistently, SAG treatment also lead to enhanced CBF reperfusion in the ischemic area, confirmed both by MRI and immunostaining, indicating enhanced angiogenesis in ischemic area and enhanced functional reperfusion. This could be one of the mechanisms for observed enhanced survival of newly born cells. Shh signaling has been reported to enhance angiogenesis, through regulation of VEGF, SDF-1 and angiopoietins24, 25.
In contrast to shh peptide, small molecule of shh pathway activator has the advantage of stability and ease of delivery. This SAG has been shown to readily cross BBB and induces shh pathway activation in vivo through regulation of smoothened function26–29. Recently this shh signaling pathway agonist (SAG) has also been reported to correct anatomical and behavioral deficits in a mouse Down syndrome model by a single injection at birth (20mg/kg dosage)30, also suggesting that temporary activation of shh pathway could lead to long term modulation brain development and function. In summary, our data show that delayed post-stroke treatment of systemic SAG administration lead to enhanced survival of adult NSCs in both SVZ and SGZ, enhanced angiogenesis and CBF reperfusion in ischemic brain and improved long-term functional recovery in stroke mice. These observations further indicate that modulating shh pathway might be a possible treatment strategy to improve functional recovery after stroke.
Supplementary Material
Acknowledgments
Sources of Funding: This study is funded by the AHA and NIH Grant (R01NS091213).
Footnotes
Disclosures: None.
References
- 1.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Sun C, Sun H, Wu S, Lee CC, Akamatsu Y, Wang RK, et al. Conditional ablation of neuroprogenitor cells in adult mice impedes recovery of poststroke cognitive function and reduces synaptic connectivity in the perforant pathway. J Neurosci. 2013;33:17314–17325. doi: 10.1523/JNEUROSCI.2129-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Mao X, Xie L, Sun F, Greenberg DA, Jin K. Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice. PloS one. 2012;7:e38932. doi: 10.1371/journal.pone.0038932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Zhang Z, Chopp M. Function of neural stem cells in ischemic brain repair processes. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:2034–2043. doi: 10.1177/0271678X16674487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 6.Napoli E, Borlongan CV. Recent advances in stem cell-based therapeutics for stroke. Translational stroke research. 2016;7:452–457. doi: 10.1007/s12975-016-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlongan CV. Age of pisces: Stem-cell clinical trials in stroke. Lancet. 2016;388:736–738. doi: 10.1016/S0140-6736(16)31259-4. [DOI] [PubMed] [Google Scholar]
- 8.Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult svz reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J Neurosci. 2007;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, Traiffort E. Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem cells. 2008;26:2311–2320. doi: 10.1634/stemcells.2008-0297. [DOI] [PubMed] [Google Scholar]
- 10.Bambakidis NC, Petrullis M, Kui X, Rothstein B, Karampelas I, Kuang Y, et al. Improvement of neurological recovery and stimulation of neural progenitor cell proliferation by intrathecal administration of sonic hedgehog. Journal of neurosurgery. 2012;116:1114–1120. doi: 10.3171/2012.1.JNS111285. [DOI] [PubMed] [Google Scholar]
- 11.Chechneva OV, Mayrhofer F, Daugherty DJ, Krishnamurty RG, Bannerman P, Pleasure DE, et al. A smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell death & disease. 2014;5:e1481. doi: 10.1038/cddis.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Y, Raviv N, Barnett A, Bambakidis NC, Filichia E, Luo Y. The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model. PloS one. 2015;10:e0124657. doi: 10.1371/journal.pone.0124657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W, Patzlaff NE, Jobe EM, Zhao X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nature protocols. 2012;7:2005–2012. doi: 10.1038/nprot.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrova R, Garcia AD, Joyner AL. Titration of gli3 repressor activity by sonic hedgehog signaling is critical for maintaining multiple adult neural stem cell and astrocyte functions. J Neurosci. 2013;33:17490–17505. doi: 10.1523/JNEUROSCI.2042-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: A dynamic duo during homeostasis, regeneration, and aging. Current opinion in neurobiology. 2013;23:935–942. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. The Lancet. Neurology. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Magueresse C, Alfonso J, Bark C, Eliava M, Khrulev S, Monyer H. Subventricular zone-derived neuroblasts use vasculature as a scaffold to migrate radially to the cortex in neonatal mice. Cerebral cortex. 2012;22:2285–2296. doi: 10.1093/cercor/bhr302. [DOI] [PubMed] [Google Scholar]
- 21.Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, et al. Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke. 2009;40:3618–3626. doi: 10.1161/STROKEAHA.109.561951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, et al. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 23.Lansberg MG, Thijs VN, O’Brien MW, Ali JO, de Crespigny AJ, Tong DC, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and t2-weighted signal intensity of acute stroke. AJNR. American journal of neuroradiology. 2001;22:637–644. [PMC free article] [PubMed] [Google Scholar]
- 24.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature medicine. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 25.Straface G, Aprahamian T, Flex A, Gaetani E, Biscetti F, Smith RC, et al. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. Journal of cellular and molecular medicine. 2009;13:2424–2435. doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roper RJ, Baxter LL, Saran NG, Klinedinst DK, Beachy PA, Reeves RH. Defective cerebellar response to mitogenic hedgehog signaling in down [corrected] syndrome mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1452–1456. doi: 10.1073/pnas.0510750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, et al. Small-molecule modulators of hedgehog signaling: Identification and characterization of smoothened agonists and antagonists. Journal of biology. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heine VM, Griveau A, Chapin C, Ballard PL, Chen JK, Rowitch DH. A small-molecule smoothened agonist prevents glucocorticoid-induced neonatal cerebellar injury. Science translational medicine. 2011;3:105ra104. doi: 10.1126/scitranslmed.3002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 30.Das I, Park JM, Shin JH, Jeon SK, Lorenzi H, Linden DJ, et al. Hedgehog agonist therapy corrects structural and cognitive deficits in a down syndrome mouse model. Science translational medicine. 2013;5:201ra120. doi: 10.1126/scitranslmed.3005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.