Abstract
The proprioceptive neuromuscular facilitation (PNF) sets up a feature of treatment developed with the objective to facilitate and improve the motor performance. The aim of this study was to investigate in healthy female individuals the effects of electrophysiological of a diagonal of the PNF upper limb. The sample consisted of 30 female participants aged between 18 to 28 years, randomly divided into 3 groups (G1, G2, and G3). The three groups had 2 moments of electroencephalographic signal detection, before and after the task. The statistical neurophysiological design allowed the analysis of the relative power of alpha band in three leads (Fz, F7, and F8). Thus, a three-way mixed factorial analysis of variance (ANOVA) was performed to investigate the factor inter subjects (groups) and intrasubjects (areas and moments), a two-way ANOVA to investigate the interactions between the three factors, and a one-way ANOVA to analyze separately the factors time and area. A P≤0.05 was considered as significance level. The results showed significant increase of alpha band in the three groups analyzed, being more evident to the G2 group. Therefore, the PNF can be considered favorable also in relation to the cortical behavior, reinforcing its use in rehabilitation processes, especially in the clinical practice of physiotherapy.
Keywords: Electroencephalography, Alpha band, Proprioceptive neuromuscular facilitation, Rehabilitation, Physiotherapy
INTRODUCTION
The central nervous system (CNS) modifies or improves the motor responses through the motor learning (Alencar et al., 2011), and the latter can be acquired or restored through proprioceptive neuromuscular facilitation (PNF), which constitutes a treatment resource developed with the objective of facilitating and improving the individuals’ motor performance with impairment in voluntary motor function (Joshi and Akre, 2015; Lee, 2016; Ribeiro et al., 2014). The PNF movement patterns perform a biarticular muscles movement, therefore, it makes their movements similar to actions found in the people’s daily routine and even with the exercises in sports practice (Kofotolis et al., 2005; Mesquita et al., 2015). The analysis of the repercussions generated by the application of the maneuvers regarding the electro-neurophysiological behavior can be performed through electroencephalography (EEG), which according to the study by Hosokawa et al. (1985) the change in the level of cortical excitation due to postural changes and patterns of movements such as investments in PNF, could be detected through the tool.
EEG investigates a brain electrical activity in different areas of the scalp, describing it in wave form or band, and in turn, varies in frequency and amplitude (Lee et al., 2015). Frequently used for the diseases diagnosis and for the medical follow-up of people with dementia (Han, 2013), epilepsy, and other clinical conditions (Cunha et al., 2009). Regarding physiotherapy, it has been used to evaluate individuals’ cortical activity of during an application or simulation of motor and cognitive tasks (Machado et al., 2014).
The alpha band (8–13 Hz) constitutes one of the main components of the cortical electrical signal (Sanei and Chambers, 2007), since they are dominant in the occipital region during rest and surveillance states (Klimesch et al., 2011; Osipova et al., 2008) and it is still considered a relevant predator of the effective cortical information processing and inhibitor of conflicting processes cerebral cortex, for example, during cognitive and sensory-motor activities (Babiloni et al., 2010; Park et al., 2015). It should also be noted that alpha band activity is inversely proportional to cortical activation (Machado et al., 2007; Machado et al., 2014). Studies that associate investigation of cortical behavior during the PNF use are still scarce in the literature. Thus, the present study aimed to investigate in healthy female individuals the electroneurophysiological effects of an upper limb diagonal of PNF.
MATERIALS AND METHODS
Sample composed of 30 female participants with the age range from 18 to 28 years, weight between 50 and 80 kg (mean±standard deviation, 57.6±7.3), allowing a variation of ±2 kg (Moreira et al., 2017). This is a controlled cross-sectional study developed at the Cerebral Mapping and Functionality Laboratory from the Federal University of Piauí, Parnaíba - Piauí/Brazil, approved by the Research Ethics Committee (No. 1.087.478) and complying with the Criteria of Ethics in Research with Human Subjects included in the Declaration of Helsinki and Resolution (No. 466/2012) of the National Health Council.
In order to ensure better sample homogeneity in the muscle strength level, only female students were selected. The inclusion criteria adopted in the present study were: sedentary young women, age group between 18 and 28 years, body mass index (BMI) between 18.5 and 24.9 kg/m2 according to the WHO Expert Committee (1995) characterizes the range of normality for BMI, and, that they should not be familiar with the basic PNF principle. All these criteria were adopted with the purpose of making the sample more homogeneous. Young patients with musculoskeletal or joint pathologies in the right shoulder, cardiopulmonary or neurological pathologies with functional limitation to perform active-free and resisted movements, amputees and patients with sensory or cognitive deficits that made it impossible to perform the requested movement were excluded from the study. Regarding EEG, those who used psychotropic or psychoactive drugs and who had slept less than 8 hr the night before the experiment were excluded.
Procedures description
After signing the informed consent form, the participants were randomly assigned to three groups with specific tasks, namely: (a) control group (G1), the participants did not perform any type of movement throughout the experiment, remaining only eyes open; (b) PNF group (G2) performed the flexion-abduction-external rotation movement with extension of elbow, wrist and fingers and radial deviation of the right upper limb. The movement starting from the right shoulder in a position of slight internal rotation with elbow extension, flexion of the wrist and fingers and ulnar deviation, with the hand resting on the medial part of the contralateral thigh; (c) PNF load group (G3), performed the same movements of G2, however, with the addition of a load to the movement.
Throughout the procedure the volunteers remained comfortably seated in a chair with their feet resting on the floor, hip and knees in 90º flexion angle, and the electroencephalographic capture cap attached to the scalp and in front of them a monitor that emitted a visual stimulus. The three groups had 2 moments of EEG signal detection, before and after the task, and in G1 the rest corresponded to the sum-up of the task times of the experimental groups (G2 and G3), which totaled an average time of 40 min. The experimental groups performed the task 81 times (9 blocks with 9 repetitions) so that each block had duration of 1′ 30″ seg, with intervals of 3 min between each block, the movement being performed only with the right upper limb.
To determine the ideal G3 load and the number of suitable replicates applied during the experiment, pretests were performed with 60 randomized volunteers in 3 groups, whose load varied from 10%, 20%, and 30% of the weight of the upper limb. These are based on de Leva’s protocol (1996), in which the value of 0.0449×body weight of each volunteer is multiplied. In addition, the Borg Effort Perception Scale of 20 points was applied. Such a scale has a score ranging from 6 to 20, the intensities being considered, from “extremely easy” to “absolute maximum” (Borg, 1982), and it is commonly used to evaluate the effort subjective perception, that is, to monitor the exercise intensity and to quantify the dyspnea, being applied directly at the fatigue moment (Brandt et al., 2013; Cavallazzi et al., 2005).
When comparing the perceived exertion values of each participant, it was found that the 20% load did not generate a fatigue sensation above 15 points on the Borg scale, with mean values between 11–13 (“fairly light” to “a little Difficult”) recommendations for sedentary and untrained individuals (Scherr et al., 2013), not limiting the correct maneuver execution. The load variation was measured using a precision scale (MH-Series Pocket Scale 500 g/0.1 g). It should be emphasized, therefore, that the Borg scale was used only for the subjective determination of the sensation of fatigue and through this, to standardize the amount of repetition and also the load to be used for G3. To identify the dominance of manual laterality the Edinburgh inventory was used (Oldfield, 1971).
Based on the results of the pretests the G3 participants received a pair of gym gloves (Speedo, Nottingham, UK), which was positioned in the metacarpophalangeal joint with the adaptation of a load of 20% out of the mass of the upper limb. It is important to point out that it was placed in such a way that it allowed the range of movement of the interphalangeal and wrist joints, in order not to interfere in the correct maneuver execution.
Data processing
The EEG signal was captured with a BrainNet BNT 36 - EEG (EMSA-Medical Instruments, Rio de Janeiro, Brazil) using the international 10–20 system and 20 channels in a Braintech-3000 EEG System (EMSA-Medical Instruments). Twenty electrodes were placed in a nylon cap (EletroCap Inc., Fairfax, VA, USA), producing monopolar derivations with the lobes of the interconnected ear, and were used as reference points. The EEG impedance was maintained at 5–10 kΩ. The data were acquired using a total range of less than 100 V. The signal from the EEG was amplified with a gain of 22.000 Hz, and analogically filtered between 0.01 Hz (high-pass) and 60 Hz (low-pass), with 240 Hz. Data Acquisition (Delphi 5.0) software, developed at the Brain Mapping Laboratory Integration and a Sensory-Motor filter notch of 60 Hz with a high-pass of 0.001 Hz and a low-pass of 60 Hz was used. The capture room was acoustically isolated and had electrical grounding. The impedance of the skin-electrode interface was maintained between 5 and 10 kΩ. The data were later analyzed in the Matlab program and evaluated using the EEGlab tool consisting of an interactive useful Matlab tool for the continuous processing of EEG related events and other electrophysiological data, including independent component analyzes (ICA) (Delorme and Makeig, 2004).
Statistical analysis
The neurophysiological statistical design allowed the analysis of the relative power (RP) of the alpha band at the pre and posttask moments for the three groups (G1, G2, and G3). The derivations selected were Fz, F7, and F8 corresponding to areas of the prefrontal cortex (PFC). Thus, a three-way mixed factorial analysis of variance (ANOVA) was performed with factor between subjects, the “groups” (G1, G2, and G3) and intra subjects factor for “areas” (Fz, F7, and F8) and “moments” (pre- and posttask). For the three-way mixed factor ANOVA, the Mauchley’s test was used to evaluate the sphericity hypothesis and the Greenshouse-Geisser (G-Gɛ) procedure to correct the freedomdegrees. The analysis of the data normality and homoscedasticity were previously verified by the Levene and Shapiro–Wilk tests (P>0.05). Interactions between three factors were investigated using a two-way ANOVA for repeated measurements and a one-way ANOVA of repeated measures followed by the post hoc test performed with Bonferroni corrections. A one-way repeated measures ANOVA was analyzed separately for moment and area factors, and statistical significance was considered with an alpha-Bonferroni-adjusted alpha level of P<0.025.
The effect magnitude was interpreted using the recommendations suggested by Hopkins et al. (2009): 0.0=trivial; 0.2=small; 0.6=moderate; 1.2=great; 2.0=very large; 4.0=almost perfect. A 5% probability for type I errors was adopted in all analyzes (P=0.05). Thus, to detect if there was a real difference in the population, the statistical power was interpreted as 0.8 to 0.9, i.e., high power (Fayers and Machin, 1995). Analyses were conducted using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
RESULTS
According to the realization of a triple factorial mixed ANOVA an interaction between the factors group, area and moment was observed (F[4.1434]=31.583; P<0.001; η2p=0.08; power=1.00) for all groups. In the analysis of the interaction between the moment and area factors in all the groups interaction was observed for G1 (F[2.478]=138.414; P<0.001; η2p=0.36; power=1.00), G2 (F[2.478]=274.893; P<0.001; η2p=0.53; power=1.00), and G3 (F[2.478]=80.789; P<0.001; η2p=0.25; power=1.00).
In the comparison of the pre- and posttask moments for the Fz derivation, the post hoc test revealed that in G1 (F[1.239]=95.056; P<0.001; η2p=0.28; power=1.00) the RP of the alpha band was higher at 0.062 μV2 (95% confidence interval [CI], 0.049–0.074; P<0.001) at the posttask moment. In G2 (F[1.239]=26.446; P<0.001; η2p=0.10; power=1.00) the RP was higher at 0.036 μV2 (95% CI, 0.022–0.050; P<0.001) at the posttask moment and the G3 (F[1.239]=11.351; P<0.001; η2p=0.04; power=0.91) also presented higher RP at the posttask moment at 0.017 μV2 (95% CI, 0.007–0.027; P<0.001) as shown in Fig. 1.
Fig. 1.
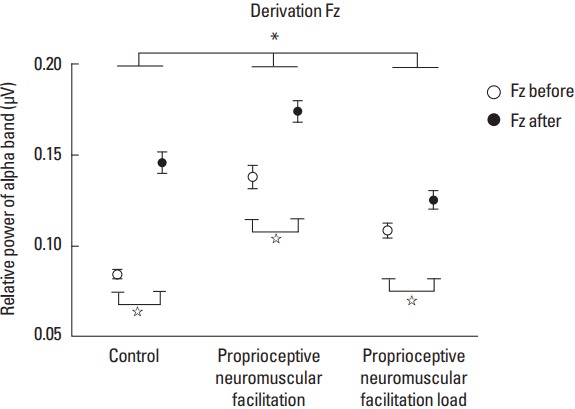
Derivation Fz. Statistically significant values between groups are represented by * and within the groups represented by ⋆.
For the F7 derivation in the comparison of the pre- and posttask moments the post hoc test revealed that for G1 (F[1.239]=38.596; P<0.001; η2p=0.13; power=1.00) there was an increase in RP by 0.021 μV2 (95% CI, 0.014–0.027; P<0.001) at the posttask moment. In G2 (F[1.239]=156.815; P<0.001; η2p=0.39; power=1.00) the power was higher at the posttask moment by 0.039 μV2 (95% CI, 0.033–0.046; P<0.001), and for G3 (F[1.239]= 43.151; P<0.001; η2p=0.15; power=1.00) the RP of the alpha band was also higher at the posttask moment at 0.027 μV2 (95% CI, 0.019–0.035; P<0.001) (Fig. 2).
Fig. 2.
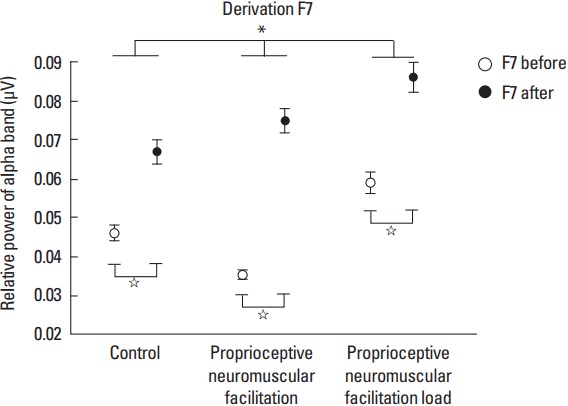
Derivation F7. Statistically significant values between groups are represented by * and within the groups represented by ⋆.
Regarding the F8 derivation for the pre- and posttask moment factor, the post hoc test demonstrated that G2 (F[1.239]=154.964; P<0.001; η2p=0.39; power=1.00) presented a RP increase of the alpha band at 0.040 μV2 (95% CI, 0.034–0.047; P<0.001) at the posttask moment, as well as (F[1.239]=19.880; P<0.001; η2p=0.07; power=0.99) presented in 0.015 μV2 (95% CI, 0.008–0.022; P<0.001) at the posttask moment as it can be seen in Fig. 3.
Fig. 3.
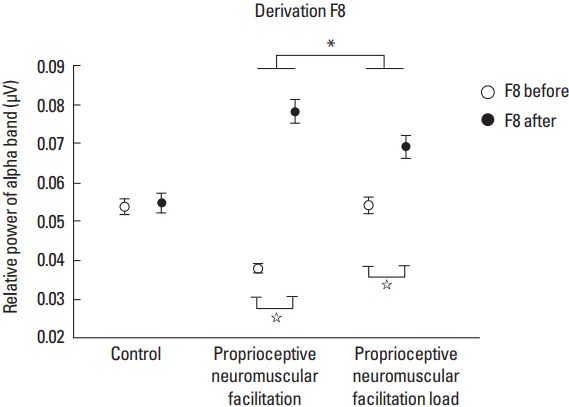
Derivation F8. Statistically significant values between groups are represented by * and within the groups represented by ⋆.
In the investigation of the pretask area factor for G1 (F[2.478]= 102.851; P<0.001; η2p=0.30; power=1.00), the post hoc test revealed that for the Fz derivation there was an increase in RP of the alpha band by 0.038 μV2 (95% CI, 0.031–0.045; P<0.001) compared to F7 derivation, and an increase in power of 0.031 μV2 (95% CI, 0.023–0.038; P<0.001) compared to F8 derivation. In the comparison of F7 and F8 derivations, there was an increase in RP by 0.008 μV2 (95% CI, 0.002–0.013; P<0.002) for the F8 derivation. In G2 (F[2.478]=220.779; P<0.001; η2p=0.48; power=1.00), the Fz derivation presented a RP increase in 0.102 μV2 (95% CI, 0.086–0.119; P<0.001) when compared to F7 derivation and increase of 0.100 μV2 (95% CI, 0.084–0.116; P< 0.001) compared to F8 derivation. For G3 (F[2.478]=128.885; P<0.001; η2p=0.35; power=1.00), the post hoc test showed that for the Fz derivation the power was greater in 0.050 μV2 (95% CI, 0.040–0.059; P<0.001) when compared to F7 derivation and greater in 0.054 μV2 (95% CI, 0.044–0.065; P<0.001) compared to F8 derivation.
In the investigation of the area factor after the task for G1 (F[2.478]=179.537; P<0.001; η2p=0.42; power=1.00), the Fz derivation presented an increase in RP by 0.079 μV2 (95% CI, 0.065–0.093; P<0.001) compared to F7 derivation and increase of 0.084 μV2 (95% CI, 0.071–0.098; P<0.001) compared to F8 derivation. For G2 (F[2.478]=205.274; P<0.001; η2p=0.46; power=1.00), the post hoc test revealed increased RP of the alpha band for Fz derivation at 0.099 μV2 (95% CI, 0.084–0.115; P<0.001) compared to F7 derivation and increase of 0.096 μV2 (95% CI, 0.080–0.112; P<0.001) when compared to the F8 derivation. In G3 (F[2.478]=88.521; P<0.001; η2p=0.27; power=1.00) in the area factor after the task, the Fz derivation presented a RP increase in 0.039 μV2 (95% CI, 0.028–0.050; P<0.001) compared to F7 derivation and increase of 0.056 μV2 (95% CI, 0.045–0.068; P<0.001) when compared to the F8 derivation. In the comparison of derivations F7 and F8, a power increase of 0.017 μV2 (95% CI, 0.009–0.025; P<0.001) was observed for the F7 derivation.
DISCUSSION
The objective of the present study was to investigate healthy females and the electroneurophysiological effects of an upper limb diagonal of the PNF, based on a RP analysis of the alpha band in the Fz, F7, and F8 leads. RP is understood to be a power ratio in a fixed frequency band analyzed (Fonseca et al., 2004), and its parameters in both the alpha band and in the others, have been used and documented and used in the clinical practice of EEG (Fonseca et al., 2003). In addition, the PFC represents an anterior region of the cerebral cortex, occupying about 1/4 of its total surface and comprising a non-motor area of the frontal lobe (Machado and Haertel, 2013).
The hypothesis for this study was that the diagonal of the upper limb (flexion, abduction, and external rotation of the shoulder) based on the PNF would produce an increase of the RP in the alpha band, that is, it would generate electroneurophysiological repercussions in cerebral cortex level, being that these repercussions would be verified with greater amplitude when an extra load was added to the member performing this task. Although there is no consensus on the actual role of PFC, the literature suggests its relation with attention maintenance, selection of actions and planning capacity, and these mechanisms are similar in performing a PNF maneuver (Kostopoulos and Petrides, 2016; Monk et al., 2006).
According to the results found, in the comparison of the pre- and posttask moments, it was possible to observe a statistically significant difference in the three derivations Fz, F7, and F8, and in almost all groups analyzed. The increase in RP found at the posttask moment for G1 in the analysis of the Fz and F7 derivations can be explained due to the fact that the participants did not perform any type of motor action during the whole experiment, remaining only with open eyes and attentive, besides that, that the resting time may have produced drowsiness in the participants, suggesting a low cortical activity. In this context, it should be remembered that the activity of the alpha band is inversely proportional to the cortical activity (Machado et al., 2007), being still related to the alertness and attention processes (Park et al., 2015).
The experimental groups, that is, G2 and G3 presented statistically significant results in the three derivations analyzed, being G2 the one with the highest RP. In both groups, the participants were previously educated about the positioning and correct execution of the PNF maneuver, starting only when they were able to do this task (Adler et al., 2007). Moraes et al. (2007) suggest that alpha band activity also increases after exercise, especially when compared to resting states. This result corroborates the fact that diagonal movement needs more attention to its execution (Adler et al., 2007).
In the investigation of the cortical area factor before and after the task, the results showed RP increase of the alpha band at both moments, being more evident in the Fz derivation in all the analyzed groups in relation to the other derivations, however, it should be emphasized that this increase was higher in G2, especially before the task. This may be explained due to fact that this region of the cortex, more precisely the dorsomedial PFC, is related to working memory (Raschle et al., 2015), which is understood as the individual’s cognitive ability to temporarily keep information about a certain action in the mind, as well as to serve as a guide for decision-making and future motor actions (Yang et al., 2014).
For the RP increase in the Fz derivation, especially in G2, it is assumed that this area of the cortex remained more active, since the participant already had a knowledge of the task that would be performed later, since it was previously trained, besides the Diagonal motion requires more attention because it is a movement that activates the CNS generally speaking (Rosário, 2011), acting on the three motion planes (frontal, sagittal, and transverse), and consequently promoting a greater neuromuscular recruitment (Pereira and Gonçalves, 2012; Rhyu et al., 2015). For this result one can also consider the fact that this derivation is located in a region of interconnection of the brain lateral areas, presumably assuming an increase in power concentration.
In the cortical area factor before the task only for the F7 and F8 derivations, the RP was higher in F8 derivation for the G1. In the other groups, both derivations showed no statistically significant difference. However, for such factor after the task, only G3 showed a significant difference between the aforementioned two derivations, the F7 derivation presented the highest increase in RP. The justification for this is that this derivation corresponds to the left ventrolateral prefrontal cortex (VLPFC), and this basically plays a crucial role in the action selection (Stern et al., 2000) and in the cognitive functions control such as memory, being more active during conditions that require the Semantic knowledge of specific objectives (Badre and Wagner, 2007). In addition, left VLPFC is located in the left hemisphere and the maneuver was performed only in the right upper limb, since the left hemisphere commands the tasks performed in the right hemisphere (Herrmann and Ribeiro, 2003; Pereira, 2004).
In conclusion, this study showed that both experimental groups presented significant results. However, regarding the group that performed the diagonal in an active-free way with the one that received an extra load, G1 demonstrated more positive repercussions in the cortical activity, suggesting then that not necessarily an additional load needs to be applied in motor activities so that the cerebral cortex responds more effectively. The influence of motor activities, such as the diagonal of the upper limb based on PNF on the cerebral cortex, are still far from being fully understood, however, the present study showed that such a concept of treatment can be considered favorable also in relation to the cortical behavior, since its muscular benefits are already well-disseminated in the literature, thus reinforcing its use and efficacy in the rehabilitation processes and treatments potentialization of patients, especially in the clinical physiotherapy practice.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Adler SS, Beckers D, Buck M. Um guia ilustrado. 2nd ed. Barueri: Manole; 2007. PNF: facilitação neuromuscular proprioceptiva. [Google Scholar]
- Alencar RF, Cordeiro TGF, Anjos PGS, Cavalcanti PL. Neuromuscular facilitation proprioceptive on the mat repurchase of functions in spinal cord injury. Rev Neurocienc. 2011;19:512–518. [Google Scholar]
- Babiloni C, Marzano N, Iacoboni M, Infarinato F, Aschieri P, Buffo P, Cibelli G, Soricelli A, Eusebi F, Del Percio C. Resting state cortical rhythms in athletes: a high-resolution EEG study. Brain Res Bull. 2010;81:149–156. doi: 10.1016/j.brainresbull.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Brandt M, Jakobsen MD, Thorborg K, Sundstrup E, Jay K, Andersen LL. Perceived loading and muscle activity during hip strengthening exercises: comparison of elastic resistance and machine exercises. Int J Sports Phys Ther. 2013;8:811–819. [PMC free article] [PubMed] [Google Scholar]
- Cavallazzi TG, Cavallazzi RS, Cavalcante TM, Bettencourt AR, Diccini S. Evaluation of the use of the modified scale of Borg in the asthmatic crisis. Acta Paul Enferm. 2005;18:39–45. [Google Scholar]
- Cunha M, Machado S, Miana LC, Machado D, Bastos VH, Velasques B, Cagy M, Basile LF, Piedade R, Ribeiro P. Effects of a cognitive modulator in the theta and alpha asymmetry during a typewriting task: a sensorimotor integration perspective. Arq Neuropsiquiatr. 2009;67(2A):214–218. doi: 10.1590/s0004-282x2009000200008. [DOI] [PubMed] [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J Biomech. 1996;29:1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Fayers PM, Machin D. Sample size: how many patients are necessary? Br J Cancer. 1995;72:1–9. doi: 10.1038/bjc.1995.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca LC, Tedrus GM, Chiodi MG, Cerqueira JN, Duran MH. Quantitative electroencephalography in children with benign childhood epilepsy with centrotemporal spikes: analysis of band power. Arq Neuropsiquiatr. 2004;62(2B):455–458. doi: 10.1590/s0004-282x2004000300014. [DOI] [PubMed] [Google Scholar]
- Fonseca LC, Tedrus GM, Martins SM, Gibert MA, de Antunes Td TA, Laloni DT. Quantitative electroencephalography in healthy school-age children: analysis of band power. Arq Neuropsiquiatr. 2003;61(3B):796–801. doi: 10.1590/s0004-282x2003000500018. [DOI] [PubMed] [Google Scholar]
- Han D. Development of a brain index for dementia diagnosis using quantitative EEG analysis. J Phys Ther Sci. 2013;25:497–500. [Google Scholar]
- Herrmann MA, Ribeiro AG. Correlation between the oral side preference during chewing and cerebral dominance. Rev CEFAC. 2003;5:49–53. [Google Scholar]
- Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41:3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Nakamura R, Kosaka K, Chida T. EEG activation induced by facilitating position. Tohoku J Exp Med. 1985;147:191–197. doi: 10.1620/tjem.147.191. [DOI] [PubMed] [Google Scholar]
- Joshi M, Akre A. Comparative effectiveness of static stretch and proprioceptive neuromuscular facilitation (PNF) stretch on hamstring flexibility in young adult females. Indian J Physiother Occup Ther. 2015;9:216–220. [Google Scholar]
- Klimesch W, Fellinger R, Freunberger R. Alpha oscillations and early stages of visual encoding. Front Psychol. 2011;2:118. doi: 10.3389/fpsyg.2011.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofotolis N, Vrabas IS, Vamvakoudis E, Papanikolaou A, Mandroukas K. Proprioceptive neuromuscular facilitation training induced alterations in muscle fibre type and cross sectional area. Br J Sports Med. 2005;39:e11. doi: 10.1136/bjsm.2004.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulos P, Petrides M. Selective memory retrieval of auditory what and auditory where involves the ventrolateral prefrontal cortex. Proc Natl Acad Sci U S A. 2016;113:1919–1924. doi: 10.1073/pnas.1520432113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK. Influence of the proprioceptive neuromuscular facilitation exercise programs on idiopathic scoliosis patient in the early 20s in terms of curves and balancing abilities: single case study. J Exerc Rehabil. 2016;12:567–574. doi: 10.12965/jer.1632796.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Lee SH, Jung BK. Analysis of cortical activation during three types of therapeutic activity. J Phys Ther Sci. 2015;27:1219–1222. doi: 10.1589/jpts.27.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Haertel LM. Neuroanatomia funcional. 3nd ed. São Paulo: Atheneu; 2013. [Google Scholar]
- de Machado DC, Lima GC, Souza Dos Santos R, Ramos AJ, Menezes de Sousa CC, Moreira Dos Santos RP, Coelho KK, Cagy M, Orsini M, Bastos VH. Comparative analysis electroencephalographic of alpha, Beta and gamma bands of a healthy individual and one with hemiparesis. J Phys Ther Sci. 2014;26:801–804. doi: 10.1589/jpts.26.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado S, Portella CE, Silva JG, Velasques B, Terra P, Vorkapic CF, Silva VF, Miana L, Basile L, Cagy M, Piedade R, Ribeiro P. Changes in quantitative EEG absolute power during the task of catching an object in free fall. Arq Neuropsiquiatr. 2007;65(3A):633–636. doi: 10.1590/s0004-282x2007000400017. [DOI] [PubMed] [Google Scholar]
- Mesquita LS, de Carvalho FT, Freire LS, Neto OP, Zângaro RA. Effects of two exercise protocols on postural balance of elderly women: a randomized controlled trial. BMC Geriatr. 2015;15:61. doi: 10.1186/s12877-015-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Moraes H, Ferreira C, Deslandes A, Cagy M, Pompeu F, Ribeiro P, Piedade R. Beta and alpha electroencephalographic activity changes after acute exercise. Arq Neuropsiquiatr. 2007;65(3A):637–641. doi: 10.1590/s0004-282x2007000400018. [DOI] [PubMed] [Google Scholar]
- Moreira R, Lial L, Teles Monteiro MG, Aragão A, Santos David L, Coertjens M, Silva-Júnior FL, Dias G, Velasques B, Ribeiro P, Teixeira SS, Bastos VH. Diagonal movement of the upper limb produces greater adaptive plasticity than sagittal plane flexion in the shoulder. Neurosci Lett. 2017;643:8–15. doi: 10.1016/j.neulet.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osipova D, Hermes D, Jensen O. Gamma power is phase-locked to posterior alpha activity. PLoS One. 2008;3:e3990. doi: 10.1371/journal.pone.0003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JL, Fairweather MM, Donaldson DI. Making the case for mobile cognition: EEG and sports performance. Neurosci Biobehav Rev. 2015;52:117–130. doi: 10.1016/j.neubiorev.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Gonçalves M. Proprioceptive neuromuscular facilitation improves balance and knee extensors strength of older fallers. ISRN Rehabil. 2012;2012 Article ID 402612. [Google Scholar]
- Pereira SA. The relationship between laterality and flexibility. R Min Educ Fís. 2004;12:101–112. [Google Scholar]
- Raschle NM, Menks WM, Fehlbaum LV, Tshomba E, Stadler C. Structural and functional alterations in right dorsomedial prefrontal and left insular cortex co-localize in adolescents with aggressive behaviour: An ALE meta-analysis. PLoS One. 2015;10:e0136553. doi: 10.1371/journal.pone.0136553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu HS, Kim SH, Park HS. The effects of band exercise using proprioceptive neuromuscular facilitation on muscular strength in lower extremity. J Exerc Rehabil. 2015;11:36–40. doi: 10.12965/jer.150189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro TS, de Sousa e Silva EM, Sousa Silva WH, de Alencar Caldas VV, Silva DL, Costa Cavalcanti FA, Lindquist AR. Effects of a training program based on the proprioceptive neuromuscular facilitation method on post-stroke motor recovery: a preliminary study. J Bodyw Mov Ther. 2014;18:526–532. doi: 10.1016/j.jbmt.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Rosário JL. Manual prático de facilitação neuromuscular proprioceptiva. São Paulo: Baraúna; 2011. [Google Scholar]
- Sanei S, Chambers JA. Eeg sinal processing. Nova Jersey: John Wiley & Sons; 2007. [Google Scholar]
- Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113:147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]
- Stern CE, Owen AM, Tracey I, Look RB, Rosen BR, Petrides M. Activity in ventrolateral and mid-dorsolateral prefrontal cortex during nonspatial visual working memory processing: evidence from functional magnetic resonance imaging. Neuroimage. 2000;11(5 Pt 1):392–399. doi: 10.1006/nimg.2000.0569. [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- Yang J, Yu Y, Kunita A, Huang Q, Wu J, Sawamoto N, Fukuyama H. Tactile priming modulates the activation of the fronto-parietal circuit during tactile angle match and non-match processing: an fMRI study. Front Hum Neurosci. 2014;8:926. doi: 10.3389/fnhum.2014.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]


