Abstract
Objective
To discuss standard-of-care and emerging imaging techniques employed for screening and detection, diagnosis and staging, monitoring response to therapy, and guiding cancer treatments.
Data Sources
Published journal articles indexed in the National Library of Medicine database and relevant websites.
Conclusion
Imaging plays a fundamental role in the care of cancer patients and specifically, breast cancer patients in the neoadjuvant setting, providing an excellent opportunity for interprofessional collaboration between oncologists, researchers, radiologists and oncology nurses. Quantitative imaging strategies to assess cellular, molecular, and vascular characteristics within the tumor is needed to better evaluate initial diagnosis and treatment response.
Implications for nursing practice
Nurses caring for patients in all settings must continue to seek education on emerging imaging techniques. Oncology nurses provide education about the test, ensure the patient has appropriate pre testing instructions, and manage patient expectations about timing of results availability.
Keywords: Quantitative imaging, MRI, PET, ultrasound, optical imaging, interprofessional collaborations
Interprofessional collaboration is based on the concept that when providers consider the perspective of their colleagues, as well as that of the patient, they can deliver better care. Indeed, harmonious interactions between radiation, medical, surgical and research oncologists, nurses, pathologists, and radiologists are the foundation to optimizing patient care in oncology. This multidisciplinary approach is especially important in the current landscape where standard-of-care approaches to cancer treatment are evolving towards highly targeted and image directed oncologic and surgical treatment plans.
Imaging plays an integral role in the care of cancer patients, providing a noninvasive evaluation of tumor status throughout all stages of cancer care, including screening and detection, diagnosis and staging, biopsy guidance, monitoring response to treatment, guiding cancer treatments, and assessment of recurrence. Emerging and novel approaches to imaging will play a central role in the future to assist in the long-term care of cancer patients. Additionally, quantitative imaging is needed to fully assess the responses induced by cytotoxic and targeted agents, and may play a significant role in other treatments, such as immunotherapy and ablation therapy. This provides an opportunity for interprofessional care between medical oncologists, surgical oncologists, radiation oncologists, oncology researchers, nurses, pathologists, and radiologists.
In particular, neoadjuvant care of breast cancer presents an important opportunity for interprofessional collaboration. This contribution will explore imaging approaches currently available to clinicians for the care of breast cancer in the neoadjuvant setting, as well as future possibilities. Current standard-of-care imaging techniques such as mammography, ultrasound, and magnetic resonance imaging (MRI) are employed in the neoadjuvant setting for breast cancer, primarily for disease detection and biopsy guidance. However, advanced imaging techniques using modalities such as ultrasound, MRI, optical imaging, single photon emission computed tomography (SPECT), positron emission tomography (PET), and multimodality imaging approaches, are being explored in clinical research to assess cellular, molecular and vascular characteristics within the tumor to better assess initial diagnosis and treatment response.
Neoadjuvant therapy (NAT) is the administration of therapy prior to definitive surgical resection of disease and is becoming the standard-of-care for patients with locally advanced breast cancer.1,2 As NAT options for breast cancer continue to expand, it is critical to ensure that imaging can effectively evaluate and direct available treatments. The purpose of NAT is to remove as much of the primary lesion and distant micrometastases as possible in order to improve disease-free and overall survival, while decreasing the initial tumor burden to enable a more limited, breast-conserving, surgery.2–7 Additionally, NAT allows for earlier initiation of systemic therapy and prevention of tumor regrowth following surgical resection, and provides the opportunity to deliver cytotoxic agents through an intact native vasculature, as well as to assess the in vivo response of the tumor to specific cytotoxic agents.2 There are many current standard-of-care techniques that can be improved and novel methods explored in which imaging can play a central role in the care of women facing NAT.
Clinical considerations when determining which imaging modality is most appropriate for detection and monitoring of treatment response are carefully determined to ensure high sensitivity, the true positive rate, while specificity, the true negative rate, is a secondary metric of concern. Currently, the National Comprehensive Cancer Network (NCCN) guidelines8,9 recommend clinical assessment of locoregional breast cancer disease with careful physical examination and the use of mammography and ultrasound with the decision to pursue MRI based on patient’s specific clinical situation and needs, especially if breast conservation surgery is desired. MRI is especially useful when local disease extent is unclear by physical exam, mammography, or ultrasound, such as patients with lobular carcinoma where conventional imaging is less sensitive.10 MRI can be essential in identifying the primary cancer in women with axillary nodal adenocarcinoma or with Paget’s disease of the nipple where the primary tumor is not identified on mammography, ultrasound, or physical examination.11,12 If patients being considered for NAT have clinical or breast imaging results notable for lymphadenopathy, a dedicated axillary ultrasound is recommended for further evaluation. Fine needle aspiration (FNA) or core needle biopsy, guided by ultrasound, should be performed on any suspicious appearing lymph nodes, although core needle biopsy is preferred when feasible due to greater accuracy.13
Determining an accurate, repeatable, and objective assessment of response of a primary tumor and any metastatic lesion is necessary to measure therapeutic effect. The evaluation of the residual tumor after NAT is extremely difficult by physical examination, mammography, or ultrasound because these methods are not able to differentiate between neoplastic tissue and chemotherapy-induced fibrosis.14 Conversely, MRI has the advantage of better delineating the extent of disease due to the evaluation of tissue vascularization and differentiating between vital tumor and fibrotic tissue.14,15 Studies also demonstrate that MRI is superior to physical exam, mammography, and ultrasound in assessing response to NAT and should be considered the gold standard, especially in patients where multifocal or multicentric disease is suspected.14,16 However, even in the absence of residual disease on breast MRI, definitive surgical resection is required to document pathologic response to therapy. Imaging within standard-of-care is typically completed after a few cycles of therapy or following the completion of NAT to evaluate extent of residual disease, unless physical or patient exams suggest intermediary imaging tests. Current standard-of-care assessment of treatment response in solid tumors during clinical trials is based on the Response Evaluation Criteria in Solid Tumors (RECIST), which evaluates the changes in the longest dimension of a tumor with MRI.17 However, biological, molecular, and vascular alterations that occur within the tumor prior to downstream changes in tumor size can be described by advanced imaging modalities and early imaging biomarkers of response to treatment.
As the number and type of neoadjuvant regimens continue to increase, medical imaging will grow in importance as an integral component of breast cancer care. Novel approaches in breast cancer imaging in the neoadjuvant setting may play a future role in the detection and management of acute toxicities and long-term effects of cancer treatment. Quantitative imaging is needed to fully assess the responses induced by both cytotoxic therapies (chemotherapy, radiation therapy) and targeted agents. Additionally, quantitative imaging strategies may play a significant role in evaluating other expanding therapies, such as immunotherapy, ablation, or embolization treatments. This review will explore imaging approaches currently available to clinicians and look to the future possibilities. The first discussion is about current standard-of-care imaging modalities, mammography, ultrasound, and MRI, and their use in detection, diagnosis and staging, monitoring response to treatment, and guiding cancer therapy. The second discussion presents motivation for improving and further integrating imaging into standard-of-care with advanced, emerging imaging techniques such as contrast-enhanced ultrasound imaging, dynamic contrast enhanced (DCE)- MRI, diffusion-weighted (DW)- MRI, diffuse optical imaging, 18F-fluorodeoxyglucose ( 18F-FDG)-PET, 18F-fluoromisonidazole ( 18F-FMISO)-PET, and multimodality imaging.
Current standard-of-care imaging modalities
Current standard-of-care imaging modalities contribute to breast cancer care in the neoadjuvant setting. Each of these modalities, mammography, ultrasound, and MRI, play an integral role in detection and staging to determine eligibility for NAT, as well as monitoring treatment response. The strengths and weaknesses of each modality are discussed in the following sections.
Mammography
Mammography is the most common imaging modality used for screening of primary breast disease. Mammography can also be used for stereotactic biopsies to assess pathological evaluation of disease and placement of core biopsy clips. Limitations of stereotactic biopsies include underestimation of the extent of disease, difficulty with not well-defined mass lesions, and accessibility of lesions close to the chest wall.18,19 Breast tomosynthesis, or 3D mammography, is an advanced method that is becoming more popular as it acquires multiple images of the breast for a more detailed exam, however it is not yet available in all imaging facilities.
Ultrasound
Ultrasound is a secondary imaging modality that is commonly used for detection and screening of primary breast disease, most typically ordered when palpation of a mass has occurred. Ultrasound is typically preferred to be used in place of mammography for focal clinical concerns if patients are less than 30 years of age. Furthermore, it can distinguish conditions such as a plugged milk duct, fluid-filled cyst, or fat lobule, that are otherwise unable to be distinguished with mammography. As ultrasound is portable, inexpensive (relative to other imaging modalities), and there is no ionizing radiation involved, it is typically utilized as a first pass through for assessing atypical lesions. Ultrasound is also a common method for biopsy guidance, with its main limitations including difficulty identifying a cluster or group of clustered calcifications and accurately targeting very small lesions. Ultrasound can be used to distinguish fluid-filled cysts from solid mass-like lesions.18,20 Furthermore, ultrasound is used to diagnose locally advanced breast cancer to determine which patients are candidates for NAT.
Magnetic Resonance Imaging (MRI)
MRI has a significant role in detection, monitoring, and guiding treatments in breast cancer. If detection of disease is unclear through ultrasound or mammography, or if a patient is at high-risk for disease, MRI will be utilized for diagnosis and/or initial screening. Suspicious findings on MRI can be biopsied using MRI guidance, however if the area of concern can by identified on ultrasound or mammography, ultrasound or stereotactic guidance is more commonly used. MRI, along with ultrasound, frequently aids in the diagnosis of locally advanced breast cancer in order to determine a patient’s eligibility for NAT. MRI is also useful for evaluating residual disease for the subset of tumors that cannot be assessed using ultrasound or mammography and has been reported to be equal or superior to ultrasound and mammography in the evaluation of tumor size after NAT when compared with pathologic tumor size.14,16 During NAT, contrast-enhanced MRI is commonly completed prior to initiation of treatment to access the extent of the disease and axillary involvement. Although not adopted as standard-of-care, contrast-enhanced MRI prior to and following NAT, is generally accepted as the optimal approach for evaluating extent of disease and monitoring treatment response. By demonstrating extent of disease, MRI also plays a significant role in surgical and radiation therapy planning.
Interprofessional Collaborations
Current standard-of-care imaging of cancer is not typically executed in a collaborative fashion, as the referring oncologist or advanced practice nurse (APN) usually orders a particular imaging procedure based on patient need, preference, and economics. Many breast health centers employ nurse navigators whose role is to coordinate testing during the diagnostic period to ensure efficiency and expediency. Additionally, patient navigators may be available as a healthcare extender in order to provide a patient and family with comfort and guide patients around barriers in complex healthcare systems. However, when inconclusive results emerge from an initial imaging procedure, radiologists, oncologists, APNs, and nurse navigators will collaborate to determine the best actions for an individual patient. One example of inconclusive results that would require collaboration to design the best diagnostic strategy is when there is no apparent change in tumor size on clinical exam or conventional imaging such as mammography and/or ultrasound, however there is a change in tumor appearance and kinetics as seen on contrast-enhanced MRI. These are cases whereby an interprofessional approach is used to determine the extent of response until technologies allow for improved evaluation through quantification of such cases. These situations lead to the need for development of novel emerging strategies for assessing response in NAT.
Emerging strategies
Motivation for advancements in imaging
NAT is recommended for locally advanced breast cancer patients, Stage I-Stage III, to reduce the tumor burden for surgical resection and to treat micrometastases.21,22 Pathological complete response (defined as pCR) following NAT has been shown to be highly correlated with overall survival.21,22 Patients whose primary breast tumor responds and achieves pCR in the neoadjuvant setting have increased rates of survival; however, patients with residual disease at the conclusion of treatment have an increased risk of early recurrence and death.3–7 Approximately 30% of human epidermal growth factor receptor 2 positive (HER2+) and triple-negative breast cancer patients achieve a pCR following NAT.5,7 Targeted biological agents such as anti-HER2 and endocrine therapies have significantly improved NAT strategies and overall response rates. Novel targeted agents for breast cancer subtypes are also emerging, and this increase in therapeutic options creates an environment where imaging is a necessity to optimally select and guide therapy for the individual patient.23–223–25 Imaging can also play a pivotal role in predicting pathological response, prognosis, and separating patients that will respond to therapy from the non-responders. Early predictions of response will allow the ability to determine, early in the course of treatment, whether a particular regimen will be effective, and discontinue the treatments that will not be successful in treating the disease burden. Furthermore, it has potential to reduce the overall cost burden of ineffective treatments. These aspects are important as precision medicine becomes a reality, and new personalized medicine agents become a fundamental component of neoadjuvant breast cancer treatment. Implementation of quantitative imaging into clinical practice, in both academic medical centers and community settings, is essential in driving clinical cancer care forward.26–28 With personalized treatments becoming available, there is increased motivation to correctly identify which imaging modality most accurately assesses tumor size throughout treatment, as well as demonstrates early predictions of eventual response to various types of NAT.29,30 Currently, the response of breast tumors to NAT is monitored by changes in tumor size as measured by physical exam, mammography, ultrasound, and/or MRI. Unfortunately, these methods are difficult to quantify and often do not correlate with tumor status. However, several specialized imaging methods have matured to the point where they offer quantitative and objective information on tumor characteristics that directly relate to tumor response. The discussion now turns to several of the most promising imaging methods in development and the key tissue characteristics they report on (see Table 1 for an abbreviated description of these imaging strategies). Furthermore, as emerging imaging strategies continue to arise, and quantitative imaging related to pharmacokinetic assessment and molecular status of tumors develop, collaborations between all imaging fields will need to be adopted and standardized in order to develop an environment for optimal patient care.
Table 1.
Emerging imaging strategies and reported characteristics
| Imaging Modality | Tissue Characteristics Reported | References | |
|---|---|---|---|
| Modality | Technology | ||
| Ultrasound | Contrast-enhanced ultrasound | Vascular perfusion, vascular density | 31–34 |
| Shear-wave elastography | Stiffness | 35 | |
| Optical Imaging | Diffuse optical tomography | Oxy- and deoxyhaemoglobin water and lipid | 36 |
| Diffuse optical spectroscopy | Oxygenation | 37 | |
| MRI | Diffusion-weighted MRI (DW-MRI) | Cellularity | 38–44 |
| Dynamic contrast enhanced MRI (DCE-MRI) | Vascular perfusion and permeability, extravascular extracellular volume fraction, plasma volume fraction | 45,46 | |
| PET | 18F-FDG-PET | Glucose, tissue metabolism | 47–53 |
| 18F-FMISO-PET | Hypoxia | 54 | |
| Multimodality Imaging | FDG-PET/DW-MRI | Glucose, tissue metabolism and cellularity | 55 |
| FDG-PET/DCE-MRI | Glucose, tissue metabolism and vascular function | 56 | |
| MRI/Optical | Fibroglandular density | 57 | |
| Multiparametric Imaging | DW-MRI/DCE-MRI | Cellularity and vascular function | 45,58 |
Ultrasound
Ultrasound is an integral component of breast cancer care as it provides a method for assessing atypical or malignant lesions in real-time.59 Although ultrasound has many advantages, according to revised RECIST 1.1 guidelines, ultrasound examinations should not be used in clinical trials to measure tumor regression or progression of lesions because the examination is subjective and operator dependent.17 Ultrasound has been evaluated for its ability to assess tumor burden prior to surgery.60,61 Roubidoux et al. demonstrated that in tumors larger than 7 mm, ultrasound has 100% sensitivity for assessing response to NAT, with an overall sensitivity of 87%. False-positive results from ultrasound are typically caused by fibrosis or biopsy-related changes.62 To our knowledge, there has not been a prospective study evaluating early prediction of response using ultrasound in breast NAT prior to changes in tumor size, though there have been studies evaluating end-stage assessment of response prior to surgery.62,63 Additional early work from Huber et al. evaluated computer-assisted texture analysis to aid in assessing response to NAT.19 Keune et al. examined 196 primary breast tumors in response to NAT and revealed that ultrasound had an area under the receiver operating characteristic (ROC) of 0.741 for measuring pCR, with a sensitivity and specificity of 45.8% and 93.8%, respectively.63 Additionally, and potentially more importantly, ultrasound correctly assessed residual tumor size in 30% more tumors than mammography. This also shows potential for longitudinal and early imaging predictions of response to therapy throughout NAT. There has been a multicenter study to evaluate the sensitivity of ultrasound to assess axillary lymph node status; however, the results were shown to have poor diagnostic accuracy.64
Ultrasound has real-time functional capabilities besides anatomical imaging, including vascular perfusion imaging through Doppler or with the addition of microbubble contrast agents to create contrast-enhanced ultrasound imaging. These applications have been evaluated in clinical studies for delineation of benign and malignant breast lesions31 and evaluation of response assessment to NAT;33 however, these technologies are currently rarely applied in clinical practice. Jia et al. recently assessed three-dimensional (3D) contrast-enhanced ultrasound in response assessment for breast cancer tumors undergoing NAT and showed significant correlations with DCE-MRI in evaluation of pCR.33 3D- contrast enhanced ultrasound has been shown to correlate with biological factors within breast cancers, including levels of vascular endothelial growth factor (VEGF) and microvessel density.32,65 This technique has also been shown to be advantageous in other cancer types.66 Corcioni et al. evaluated contrast-enhanced ultrasound in comparison with MRI, and identified 83% of complete responders in a small sample size of 16 patients, indicating that the signal intensity versus time curves might be a valid index of response to therapy.34 Furthermore, another ultrasonic technique of shear-wave elastography was evaluated for prediction of response to NAT, and found that pre-treatment tumor stiffness was significantly correlative with pCR as measured at the conclusion of NAT.35
Magnetic Resonance Imaging (MRI)
MRI is frequently used in the care of breast cancer patients due to its relatively high spatial resolution and soft tissue contrast. A number of quantitative MRI techniques that can describe tumor characteristics have shown the ability to predict the response of locally advanced breast cancer to NAT.67 Furthermore, MRI may be essential in evaluating axillary lymph node involvement in breast cancer treatments,68 and in identification of residual ductal carcinoma in situ following NAT.45,69 One limitation of the quantitative MRI techniques currently available is that there are no multi-site, multi-vendor studies presently validating them.27
DW-MRI measures the apparent diffusion coefficient (ADC) of water in tissue, which has been shown to correlate with tumor cellularity.70–75 Changes in ADC before and after NAT were found to be better predictors of pCR than changes in tumor size.38,39 A multisite trial found that increased ADC observed early in the course of NAT is predictive of response.40 These changes are better predictors of eventual pathological tumor response to therapy than measurements of tumor diameter or volume.41 Furthermore, breast cancer patients with lower ADC prior to the initiation of NAT were more likely to exhibit a decrease in tumor volume during treatment.42–44 The predictive value of ADC has been shown to be further strengthened based on breast cancer subtype stratification.76,77
DCE-MRI utilizes the serial acquisition of T1-weighted images before, during, and after injection of a gadolinium-based contrast agent to assess semi-quantitative parameters, such as the signal enhancement ratio, or quantitative pharmacokinetic parameters, such as Ktrans (the volume transfer rate which is related to vascular permeability and perfusion), ve (extravascular extracellular volume fraction), vp (plasma volume fraction) and kep (Ktrans/ve). In semiquantitative studies it has been shown that the accuracy of imaging complete response with DCE-MRI has high sensitivity; however, it varies depending on breast cancer subtype.39,78 Furthermore, when quantitative pharmacokinetic DCE-MRI is performed following one cycle of NAT, parameters were shown to be excellent predictors of pCR prior to any significant changes in RECIST criteria.45,46 Figure 1 reveals how quantitative DCE-MRI parameter, kep, can show early response during NAT.
Figure 1.
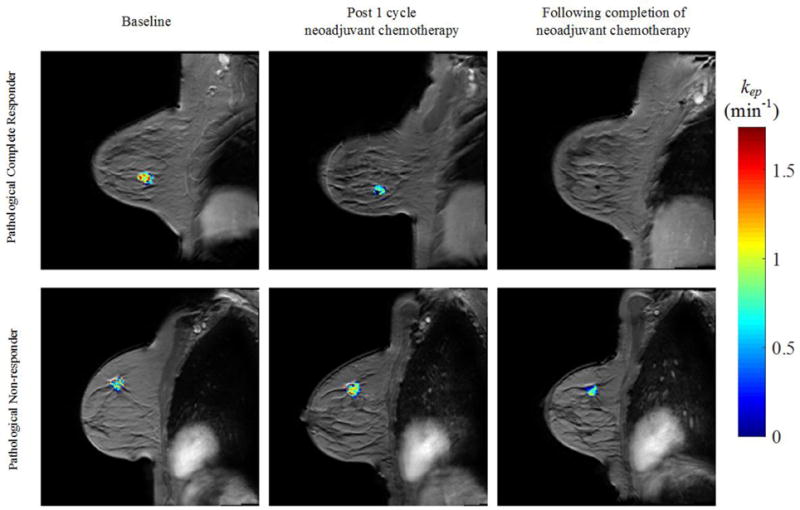
Quantitative MRI of breast cancer response to NAT. Shown are representative example images of a pathological complete responder (top row) and a pathological non-responder (bottom row) before (first column), after the first cycle (second column), and at conclusion of all NAT (third column) assessed with quantitative DCE-MRI. The parametric map of the rate constant kep is overlaid on a high resolution anatomical MRI scan.
Optical Imaging
Optical imaging methods present a noninvasive and biocompatible opportunity for real-time information on various breast cancer characteristics. Diffuse optical imaging uses near-infrared light to probe tissue absorption and scattering properties up to several centimeters, making it very useful in cancers near the skin, including breast, head and neck, and melanoma.79 One of its greatest advantages is the ability to noninvasively monitor tissue on a daily basis, without the need of an exogenous contrast agent.80 Cerussi et al. revealed that optical imaging has potential to clinically and longitudinally measure imaging biomarkers such as oxy- and deoxy-haemoglobin water and lipid, in response to targeted NAT, in addition to traditional chemotherapy.36 A recent multi-center trial showed that diffuse optical spectroscopic imaging of tumor metabolism in combination with baseline functional properties of oxygenation, as measured through tumor oxygen saturation levels, shows promise for clinical outcome prediction in breast cancer NAT.37 Specifically, in the most recent published study, 34 patients were evaluated and the responders (about 30% attained pCR) demonstrated a greater decrease in the tissue optical index than non-pCR at the half way point of therapy. Other parameters investigated in single-center studies evaluating response to NAT in breast cancers were concentrations of deoxy-hemoglobin, oxy-hemoglobin, water, and lipids, which have shown sensitivity to microvasculature, cellular metabolism, angiogenesis, edema, hypoxia, and necrosis.36,81–83 Combining the tumor oxygen saturation with the function properties of tissue optical index, demonstrated an area under the ROC of 0.83.37
Nuclear Imaging
In clinical practice, PET is utilized to characterize the whole-body distribution of a molecularly targeted radiotracer, which is then used to tailor an appropriate treatment plan for the patient.48,84 18F-FDG-PET (combined with x-ray computed tomography (CT) for anatomical imaging) is commonly used in the staging of breast cancer patients and evaluation of metastatic disease.47,48,84 Alterations in tumor metabolic activity, as quantified by 18F-FDG uptake, has been identified as a potential predictive biomarker for NAT response in breast cancer patients.48–53 A meta-analysis pooling results of 19 breast cancer patient studies demonstrated that 18F-FDG-PET had a sensitivity of 84% and specificity of 66% in predicting histopathological response to NAT,85 a finding consistent with other meta-analyses.86,87 In a prospective study investigating the early prediction of NAT response in breast cancer patients using 18F-FDG-PET, Hatt et al. found that the change in maximum standard uptake value (ΔSUVmax) and total lesion glycolysis (ΔTLG) after two cycles of NAT showed a significant difference between pathological non-responders and responders, with an area under the AUC curve of 0.82 for ΔSUVmax and 0.91 for ΔTLG.51 These studies indicate the potential for 18F-FDG-PET/CT for early assessment of NAT response, evaluating metabolic changes in the tumor that occur prior to alterations in tumor size. However, differences across studies in definitions of pathological response, time points for interim 18F-FDG-PET/CT evaluations, and 18F-FDG uptake thresholds for response, have prevented clinical translation of interim 18F-FDG-PET/CT evaluation in breast cancer patients.47,49,88 18F-FDG-PET/CT evaluation of NAT response has yet to gain wide acceptance due to limited accuracy and specificity of the technique; these limitations have been attributed to significant differences in 18F-FDG uptake between breast cancer subtypes, both at baseline and after treatment.48–50,88 While the ability of 18F-FDG-PET/CT to predict NAT response may be receptor status dependent and requires further investigation, the technique shows promise for early evaluation and prediction of response to NAT prior to downstream alterations in tumor size. Figure 2 reveals how quantitatively assessing treatment response with 18F-FDG-PET imaging during NAT can provide indications of response. For the pathological complete responder, the tumors reveal a decrease in glucose metabolic activity (as designated with standardized uptake limit (SUL)) following one round of NAT.
Figure 2.
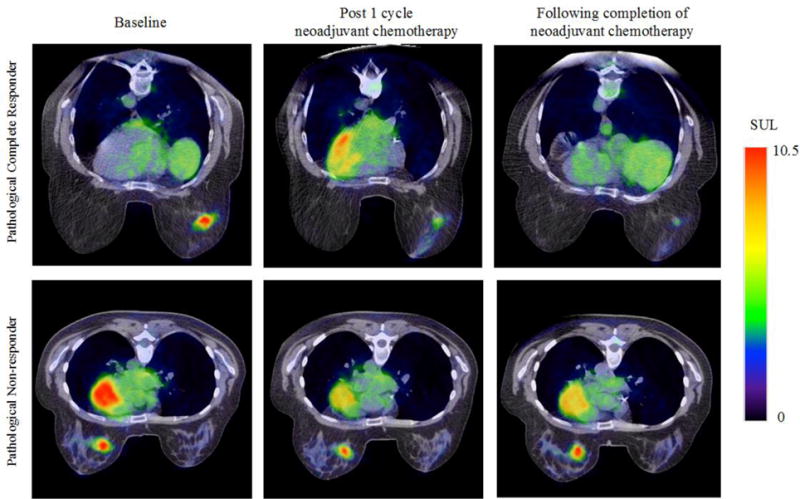
PET quantitative imaging has been shown to be a novel imaging strategy for assessing response to NAT through molecular imaging. Shown are representative example images of a pathological complete responder (top row) and a pathological non-responder (bottom row) before (first column), after the first cycle (second column), and at conclusion of all NAT (third column) assessed with FDG-PET. The parametric map of the standardized uptake limit (SUL) is overlaid on a high-resolution CT scan.
Radiotracers that characterize other aspects of tumor biology (besides glucose metabolism) have been developed and applied in describing the response of breast cancers to NAT. For example, 18F-FMISO accumulation corresponds to hypoxia within a tumor, a phenotype that is associated with more aggressive cancers and treatment resistance.89 In a study investigating the ability of 18F-FMISO-PET/CT to predict therapy resistance in estrogen receptor positive (ER+) breast cancer, 18F-FMISO uptake at baseline was shown to significantly correlate positively to progressive disease after endocrine therapy.54 Radiolabeled antibodies and antibody fragments provide specific in vivo location and expression information of protein targets in a noninvasive manner.90 These novel tracers allow for molecular characterization of a tumor and can guide targeted therapy selection for optimal patient outcome. For example, when considering targeted therapies, information regarding expression levels could be key in therapy selection. In a clinical study of radiolabeled bevacizumab, an anti-VEGF antibody, 89Zr-bevacizumab uptake with PET imaging was shown to correlate with VEGF-A expression in primary breast cancer tumors, that could then be used to plan the therapeutic regimen for the patient.87,91 Additionally, HER2 antibodies, such as trastuzumab, have been radiolabeled for SPECT and PET imaging for detection of HER2 positive lesions.88,91–95 Recent studies have also reported successful HER2-positive lesion detection using alternative radiolabels, such as 64Cu.92,93 Preliminary results from a 89Zr-trastuzumab-PET/CT study of HER2 positive metastatic breast cancer suggests that 89Zr-trastuzumab uptake could be predictive of HER2-targeted therapy response.96 These novel radiotracers could lead to improved whole-tumor in vivo characterization of breast cancer receptor expression and be used to predict treatment response of targeted therapies, leading to improved treatment selection and planning.
Multimodality Imaging
Multimodality imaging provides a pathway to combine the strengths of imaging strategies, while potentially overcoming an individual technique’s weaknesses.26 The integration of multiple imaging modalities results in the combination of different functional imaging metrics, allowing for enhanced characterization of tumor physiology and microenvironment, before and after treatment.26,97 Beginning with the development of hybrid PET/CT and SPECT/CT scanners, early multimodality imaging technologies aimed to pair molecular imaging data with high-resolution anatomical data for improved lesion localization and detection.26,97 Studies evaluating 18F-FDG-PET prediction of breast cancer NAT response predominantly use PET/CT scanners to image radiotracer uptake, and demonstrate improved lesion detection over CT evaluation alone.87,98 More recently, there has been interest in pairing PET with MRI, to determine if the additional anatomical and functional characterizations derived from MRI can improve prediction of NAT response in breast cancer patients. A few studies have demonstrated the complementary nature of PET and MRI metrics in the evaluation of breast cancer NAT response, showing correspondence in SUV values with anatomical and vascular metrics from DCE-MRI.99–102 Park et al. investigated the combined use of DW-MRI and 18F-FDG-PET/CT in prediction of pCR in breast cancer patients receiving NAT, and demonstrated improved predictive ability of the two modalities combined, with an area under the ROC of 0.944, over either technique alone.55 Multifunctional assessment of lesions can additionally provide more accurate assessment of NAT response, as breast lesions with low 18F-FDG avidity have been shown to be visualized using 23Na MR and DCE-MRI.56
While multimodality imaging allows for the longitudinal assessment of multiple functional characteristics over the whole tumor volume, these assessments are usually summarized to a tumor ROI with spatial heterogeneity information discarded. With increasing interest to evaluate tumors at a sub-anatomical level, there have been efforts to develop methods and technology that enable the spatiotemporal registration of data across imaging modalities. Atuegwu et al. developed a method to spatially and temporally register DCE-MRI, DW-MRI, and 18F-FDG-PET breast data acquired from separate imaging systems.103 Furthermore, hybrid PET/MRI scanners have been developed enabling concurrent PET and MRI data acquisition for easier registration of data and simultaneous molecular, functional, and morphological data acquisition.104,105 Given the enhanced soft-tissue contrast achieved using MRI, hybrid PET/MRI scanners show potential for improved characterization and anatomical localization of lesions.106–111 Hybrid PET/MRI scanners hold promise for enriched assessment of changes in the tumor microenvironment in response to NAT, enabling simultaneous imaging of alterations in tumor cellularity, vascularity, metabolic activity, and oxygenation prior to downstream changes in tumor size. Additional multimodality techniques include combining optical imaging with MRI, which has been shown to be of value when evaluating tissue response, as diffuse optical spectroscopy imaging correlates with MRI fibroglandular density both prior to and during NAT.57 As density changes are a strong independent risk factor for breast cancer, these modalities may provide insight into underlying biological origin of disease as well as risk of recurrence.57
Multifunctional evaluations of cellularity and vascularity from MRI alone have been used for the prediction of NAT response in breast cancer patients.45,58 The use of MRI for multiparametric assessments is inherently advantageous as multimodal imaging data can be collected during a single examination and with a single imaging system. Combined use of DCE-MRI and DW-MRI parametric data has been shown to achieve superior predictive ability of pCR after the first cycle of NAT over either modality alone, with an area under the ROC of 0.8645 Multiparametric MRI also allows for analysis of spatial heterogeneity across modalities, as Li et al. demonstrated, using a voxel based analysis of heterogeneity across DCE-MRI and DW-MRI breast data from patients receiving NAT. It was concluded that combined DCE- and DW-MRI voxel based assessments had the best prediction of pCR to NAT with an area under the ROC of 0.87.58
Interprofessional Collaborations in Emerging Strategies
All clinical trials evaluating response to a new therapy or regimen requires longitudinal noninvasive imaging to monitor response to treatment. This requires interprofessional collaborations between oncologists, radiologists, surgeons, pathologists, researchers, and clinical trials nurses. One example of a collaborative prospective clinical study that has been developed as a joint effort between radiologists, medical oncologists, radiation oncologists, surgeons, pathologists and imaging and oncology scientists to evaluate early prediction of NAT response in breast cancer is the multicenter American College of Radiology Imaging Network (ACRIN) 6655 I-SPY TRIAL, “Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And moLecular Analysis”112–114 This collaborative study evaluated early prediction of response to NAT in locally advanced breast cancer with lesion size, shape, extent, distribution, kinetics, T2 appearance, breast density, and morphologic pattern in 216 patients. Patients received standard-of-care treatment of anthracycline-cyclophosphamide chemotherapy, followed by a taxane therapy, and underwent up-to-four research quantitative MRI scans throughout NAT, prior to surgical resection of any residual disease. Early prediction of response was correlated with pathological complete response at the time of surgery. The highest predictive value of 0.84 was obtained by using a multivariate model including both MR imaging and clinical measurements.114 The knowledge acquired through this study provides direction on developing personalized strategies for breast cancer patients undergoing NAT and may motivate a shift in existing paradigms of therapy monitoring and selection in breast cancer. Furthermore, MRI assessment of early response could be broadly applicable to other solid tumors where NAT is appropriate.
Nursing considerations
Nurses caring for patients in all settings must remain current on emerging imaging techniques. Oncology nurses bridge the education gap for patients by providing information about testing procedures, ensuring the patient has appropriate pre-testing instructions, and managing patient expectations about availability of the results. Nurse navigators are especially adept at coaching women through the diagnostic testing period and may be able to share guidance to imaging staff to support women with abnormal test results who are undergoing investigational imaging testing.
Future directions
As the options for therapeutics continue to rise for all cancers at various stages of disease, it is essential to correctly identify the appropriate imaging modality and quantitative parameter that best describes response. Quantifying receptor status and metabolic activity can provide insight into which therapies might be most successful for an individual tumor. One current disadvantage of quantitative imaging strategies is the lack of standardization in both image acquisition and processing. To combat these shortcomings, efforts are needed to harmonize the novel quantitative acquisition and analysis methods that are currently being explored in clinical studies. The Quantitative Imaging Network (QIN) is striving to address these limitations by standardizing methods for all imaging modalities for image acquisition, analysis, and data sharing in order for these methods to be implemented in multi-site trials.27,115 Additionally, the Quantitative Imaging Biomarkers Alliance (QIBA), organized by the Radiological Society of North America, is seeking to improve the value and practicality of quantitative imaging biomarkers of quantifiable features from medical imaging, by reducing variability across devices and patients in order to assess disease status or degrees of change over time.116 These initiatives seek to create collaborations in order to identify the needs, barriers, and solutions to create consistent and reliable imaging results across a multitude of imaging platforms and sites.
Multiparametric or multimodality imaging which combines quantitative molecular, cellular, and physiological imaging strategies provides a unique approach to predict downstream biological responses prior to changes in tumor size with high sensitivity and high specificity. As the hardware for combination imaging continues to develop, implementation will become more homogenous. Furthermore, as novel therapeutic research continues to develop, it will be necessary to identify appropriate noninvasive imaging strategies to assess response. Immunotherapy is one example of a novel treatment that is currently being explored in a vast range of oncology clinical trials. To date there are 252 breast cancer treatment trials with immunotherapy currently enrolling in 2016 as noted by the Cancer Research Institute; these studies will require advanced imaging technique for assessing response. As there are more theranostic procedures (i.e., methods for combining imaging and therapy) in clinical trials (e.g., high intensity focused ultrasound (HIFU) ablation and embolization, and photodynamic therapy) that combine imaging techniques with treatment, we are at a unique crossroads to implement advanced quantitative monitoring strategies into clinical standard-of-care.
Conclusions
Imaging plays a fundamental role in the care of cancer patients and specifically, breast cancer patients in the neoadjuvant setting. Quantitative imaging is needed to fully assess the responses induced by cytotoxic therapies and targeted agents, and may play a significant role in other treatments, such as immunotherapy and ablation therapy. Developing and evaluating novel imaging approaches may also play a role in assisting the short and long term effects of cancer treatment. However, these emerging imaging strategies need standardization to be applied in multi-site clinical trials and standard-of-care settings. Communication and interprofessional collaboration between all members of the breast cancer care team is essential in driving novel imaging research forward to improve detection, evaluation, and standardization. The ability to predict eventual response early in the course of therapy and determine which breast cancer patients will achieve a pCR, continues to be a highly relevant clinical objective. Accurate and early response assessment provided through noninvasive imaging strategies would provide the opportunity to replace an ineffective treatment with an alternative regimen, reducing unnecessary toxicities or side effects from unsuccessful treatments, reducing health care costs, and further personalizing treatments to improve overall therapeutic efficacy.
Acknowledgments
We thank Jason Williams for his help acquiring the FDG-PET breast imaging data. We thank the National Cancer Institute for support through U01CA174706 and U01CA142565 and the National Institute of Biomedical Imaging and Bioengineering for support through T32EB007507. We thank the Cancer Prevention and Research Institute of Texas (CPRIT) for funding through RR160005. T.E.Y. is a CPRIT Scholar of Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.DeMichele A, Yee D, Berry DA, et al. The neoadjuvant model is still the future for drug development in breast cancer. Clin Cancer Res. 2015;21(13):2911–2915. doi: 10.1158/1078-0432.CCR-14-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu SV, Melstrom L, Yao K, Russell CA, Sener SF. Neoadjuvant therapy for breast cancer. J Surg Oncol. 2010;101(4):283–291. doi: 10.1002/jso.21446. [DOI] [PubMed] [Google Scholar]
- 3.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G. Neoadjuvant chemotherapy in breast cancer-insights from the German experience. Breast Cancer. 2012;19(4):282–288. doi: 10.1007/s12282-012-0393-7. [DOI] [PubMed] [Google Scholar]
- 7.Hamy-Petit AS, Belin L, Bonsang-Kitzis H, et al. Pathological complete response and prognosis after neoadjuvant chemotherapy for HER2-positive breast cancers before and after trastuzumab era: results from a real-life cohort. Br J Cancer. 2016;114(1):44–52. doi: 10.1038/bjc.2015.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gradishar W, Salerno KE. NCCN Guidelines Update: Breast Cancer. J Natl Compr Canc Netw. 2016;14(5 Suppl):641–644. doi: 10.6004/jnccn.2016.0181. [DOI] [PubMed] [Google Scholar]
- 9.Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(3):324–354. doi: 10.6004/jnccn.2016.0037. [DOI] [PubMed] [Google Scholar]
- 10.Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008;107(1):1–14. doi: 10.1007/s10549-007-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sripathi S, Ayachit A, Kadavigere R, Kumar S, Eleti A, Sraj A. Spectrum of Imaging Findings in Paget’s Disease of the Breast-A Pictorial Review. Insights Imaging. 2015;6(4):419–429. doi: 10.1007/s13244-015-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009;7(2):193–201. doi: 10.6004/jnccn.2009.0013. [DOI] [PubMed] [Google Scholar]
- 13.Rautiainen S, Masarwah A, Sudah M, et al. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology. 2013;269(1):54–60. doi: 10.1148/radiol.13122637. [DOI] [PubMed] [Google Scholar]
- 14.Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004;14(8):1371–1379. doi: 10.1007/s00330-004-2246-z. [DOI] [PubMed] [Google Scholar]
- 15.Abraham DC, Jones RC, Jones SE, et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996;78(1):91–100. doi: 10.1002/(SICI)1097-0142(19960701)78:1<91::AID-CNCR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Akazawa K, Tamaki Y, Taguchi T, et al. Preoperative evaluation of residual tumor extent by three-dimensional magnetic resonance imaging in breast cancer patients treated with neoadjuvant chemotherapy. Breast J. 2006;12(2):130–137. doi: 10.1111/j.1075-122X.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 17.Semiglazov V. RECIST for Response (Clinical and Imaging) in Neoadjuvant Clinical Trials in Operable Breast Cancer. J Natl Cancer Inst Monogr. 2015;2015(51):21–23. doi: 10.1093/jncimonographs/lgv021. [DOI] [PubMed] [Google Scholar]
- 18.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 19.Huber S, Medl M, Vesely M, Czembirek H, Zuna I, Delorme S. Ultrasonographic tissue characterization in monitoring tumor response to neoadjuvant chemotherapy in locally advanced breast cancer (work in progress) J Ultrasound Med. 2000;19(10):677–686. doi: 10.7863/jum.2000.19.10.677. [DOI] [PubMed] [Google Scholar]
- 20.Bosch AM, Kessels AG, Beets GL, et al. Preoperative estimation of the pathological breast tumour size by physical examination, mammography and ultrasound: a prospective study on 105 invasive tumours. Eur J Radiol. 2003;48(3):285–292. doi: 10.1016/s0720-048x(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 21.Pennisi A, Kieber-Emmons T, Makhoul I, Hutchins L. Relevance of Pathological Complete Response after Neoadjuvant Therapy for Breast Cancer. Breast Cancer (Auckl) 2016;10:103–106. doi: 10.4137/BCBCR.S33163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Minckwitz G, Martin M. Neoadjuvant treatments for triple-negative breast cancer (TNBC) Ann Oncol. 2012;23(Suppl 6):vi35–39. doi: 10.1093/annonc/mds193. [DOI] [PubMed] [Google Scholar]
- 23.Saini KS, Loi S, de Azambuja E, et al. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39(8):935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Munagala R, Aqil F, Gupta RC. Promising molecular targeted therapies in breast cancer. Indian J Pharmacol. 2011;43(3):236–245. doi: 10.4103/0253-7613.81497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piva R, Spandidos DA, Gambari R. From microRNA functions to microRNA therapeutics: novel targets and novel drugs in breast cancer research and treatment (Review) Int J Oncol. 2013;43(4):985–994. doi: 10.3892/ijo.2013.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yankeelov TE, Abramson RG, Quarles CC. Quantitative multimodality imaging in cancer research and therapy. Nat Rev Clin Oncol. 2014;11(11):670–680. doi: 10.1038/nrclinonc.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yankeelov TE, Mankoff DA, Schwartz LH, et al. Quantitative Imaging in Cancer Clinical Trials. Clin Cancer Res. 2016;22(2):284–290. doi: 10.1158/1078-0432.CCR-14-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dialani V, Chadashvili T, Slanetz PJ. Role of imaging in neoadjuvant therapy for breast cancer. Ann Surg Oncol. 2015;22(5):1416–1424. doi: 10.1245/s10434-015-4403-9. [DOI] [PubMed] [Google Scholar]
- 29.Atkins JJ, Appleton CM, Fisher CS, Gao F, Margenthaler JA. Which imaging modality is superior for prediction of response to neoadjuvant chemotherapy in patients with triple negative breast cancer? J Oncol. 2013;2013:964863. doi: 10.1155/2013/964863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz-Wendtland R. Neoadjuvant chemotherapy-monitoring: clinical examination, ultrasound, mammography, MRI, elastography: only one, only few or all? Eur J Radiol. 2012;81(Suppl 1):S147–148. doi: 10.1016/S0720-048X(12)70061-X. [DOI] [PubMed] [Google Scholar]
- 31.Daniela Stanzani LFC, de Barros Nestor, Giovanni G, Cerri Maria Cristina Chammas. Can Doppler or contrast-enhanced ultrasound analysis add diagnostically important information about the nature of breast lesions? Clinical Science. 2014;69(2):87–92. doi: 10.6061/clinics/2014(02)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia WR, Chai WM, Tang L, et al. Three-dimensional contrast enhanced ultrasound score and dynamic contrast-enhanced magnetic resonance imaging score in evaluating breast tumor angiogenesis: correlation with biological factors. Eur J Radiol. 2014;83(7):1098–1105. doi: 10.1016/j.ejrad.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Jia WR, Tang L, Wang DB, et al. Three-dimensional Contrast-enhanced Ultrasound in Response Assessment for Breast Cancer: A Comparison with Dynamic Contrast-enhanced Magnetic Resonance Imaging and Pathology. Sci Rep. 2016;6:33832. doi: 10.1038/srep33832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corcioni B, Santilli L, Quercia S, et al. Contrast-enhanced US and MRI for assessing the response of breast cancer to neoadjuvant chemotherapy() J Ultrasound. 2008;11(4):143–150. doi: 10.1016/j.jus.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans A, Armstrong S, Whelehan P, et al. Can shear-wave elastography predict response to neoadjuvant chemotherapy in women with invasive breast cancer? Br J Cancer. 2013;109(11):2798–2802. doi: 10.1038/bjc.2013.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerussi AE, Tanamai VW, Hsiang D, Butler J, Mehta RS, Tromberg BJ. Diffuse optical spectroscopic imaging correlates with final pathological response in breast cancer neoadjuvant chemotherapy. Philos Trans A Math Phys Eng Sci. 2011;369(1955):4512–4530. doi: 10.1098/rsta.2011.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tromberg BJ, Zhang Z, Leproux A, et al. Predicting Responses to Neoadjuvant Chemotherapy in Breast Cancer: ACRIN 6691 Trial of Diffuse Optical Spectroscopic Imaging. Cancer Res. 2016;76(20):5933–5944. doi: 10.1158/0008-5472.CAN-16-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin HJ, Baek HM, Ahn JH, et al. Prediction of pathologic response to neoadjuvant chemotherapy in patients with breast cancer using diffusion-weighted imaging and MRS. NMR Biomed. 2012;25(12):1349–1359. doi: 10.1002/nbm.2807. [DOI] [PubMed] [Google Scholar]
- 39.Wu LM, Hu JN, Gu HY, Hua J, Chen J, Xu JR. Can diffusion-weighted MR imaging and contrast-enhanced MR imaging precisely evaluate and predict pathological response to neoadjuvant chemotherapy in patients with breast cancer? Breast Cancer Res Treat. 2012;135(1):17–28. doi: 10.1007/s10549-012-2033-5. [DOI] [PubMed] [Google Scholar]
- 40.Galban CJ, Ma B, Malyarenko D, et al. Multi-site clinical evaluation of DW-MRI as a treatment response metric for breast cancer patients undergoing neoadjuvant chemotherapy. PLoS One. 2015;10(3):e0122151. doi: 10.1371/journal.pone.0122151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22(1):104–113. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 42.Park SH, Moon WK, Cho N, et al. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology. 2010;257(1):56–63. doi: 10.1148/radiol.10092021. [DOI] [PubMed] [Google Scholar]
- 43.Iacconi C, Giannelli M, Marini C, et al. The role of mean diffusivity (MD) as a predictive index of the response to chemotherapy in locally advanced breast cancer: a preliminary study. Eur Radiol. 2010;20(2):303–308. doi: 10.1007/s00330-009-1550-z. [DOI] [PubMed] [Google Scholar]
- 44.Bufi E, Belli P, Costantini M, et al. Role of the Apparent Diffusion Coefficient in the Prediction of Response to Neoadjuvant Chemotherapy in Patients With Locally Advanced Breast Cancer. Clin Breast Cancer. 2015;15(5):370–380. doi: 10.1016/j.clbc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Abramson RG, Arlinghaus LR, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2015;50(4):195–204. doi: 10.1097/RLI.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tudorica A, Oh KY, Chui SY, et al. Early Prediction and Evaluation of Breast Cancer Response to Neoadjuvant Chemotherapy Using Quantitative DCE-MRI. Transl Oncol. 2016;9(1):8–17. doi: 10.1016/j.tranon.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266(2):388–405. doi: 10.1148/radiol.12110853. [DOI] [PubMed] [Google Scholar]
- 48.Groheux D, Hatt M, Hindie E, et al. Estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast tumors: early prediction of chemosensitivity with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography during neoadjuvant chemotherapy. Cancer. 2013;119(11):1960–1968. doi: 10.1002/cncr.28020. [DOI] [PubMed] [Google Scholar]
- 49.Groheux D, Mankoff D, Espie M, Hindie E. (1)(8)F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl Med Mol Imaging. 2016;43(5):983–993. doi: 10.1007/s00259-015-3295-z. [DOI] [PubMed] [Google Scholar]
- 50.Avril S, Muzic RF, Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. (1)(8)F-FDG PET/CT for Monitoring of Treatment Response in Breast Cancer. J Nucl Med. 2016;57(Suppl 1):34S–39S. doi: 10.2967/jnumed.115.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatt M, Groheux D, Martineau A, et al. Comparison between 18F-FDG PET image-derived indices for early prediction of response to neoadjuvant chemotherapy in breast cancer. J Nucl Med. 2013;54(3):341–349. doi: 10.2967/jnumed.112.108837. [DOI] [PubMed] [Google Scholar]
- 52.Andrade WP, Lima EN, Osorio CA, et al. Can FDG-PET/CT predict early response to neoadjuvant chemotherapy in breast cancer? Eur J Surg Oncol. 2013;39(12):1358–1363. doi: 10.1016/j.ejso.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Gebhart G, Gamez C, Holmes E, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54(11):1862–1868. doi: 10.2967/jnumed.112.119271. [DOI] [PubMed] [Google Scholar]
- 54.Cheng J, Lei L, Xu J, et al. 18F-fluoromisonidazole PET/CT: a potential tool for predicting primary endocrine therapy resistance in breast cancer. J Nucl Med. 2013;54(3):333–340. doi: 10.2967/jnumed.112.111963. [DOI] [PubMed] [Google Scholar]
- 55.Park SH, Moon WK, Cho N, et al. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2012;22(1):18–25. doi: 10.1007/s00330-011-2236-x. [DOI] [PubMed] [Google Scholar]
- 56.Jacobs MA, Ouwerkerk R, Wolff AC, et al. Monitoring of neoadjuvant chemotherapy using multiparametric, (2)(3)Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast Cancer Res Treat. 2011;128(1):119–126. doi: 10.1007/s10549-011-1442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Sullivan TD, Leproux A, Chen JH, et al. Optical imaging correlates with magnetic resonance imaging breast density and reveals composition changes during neoadjuvant chemotherapy. Breast Cancer Res. 2013;15(1):R14. doi: 10.1186/bcr3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Kang H, Arlinghaus LR, et al. Analyzing Spatial Heterogeneity in DCE- and DW-MRI Parametric Maps to Optimize Prediction of Pathologic Response to Neoadjuvant Chemotherapy in Breast Cancer. Transl Oncol. 2014;7(1):14–22. doi: 10.1593/tlo.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feleppa EJ, Mamou J, Porter CR, Machi J. Quantitative ultrasound in cancer imaging. Semin Oncol. 2011;38(1):136–150. doi: 10.1053/j.seminoncol.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madjar H, Ladner HA, Sauerbrei W, Oberstein A, Prompeler H, Pfleiderer A. Preoperative staging of breast cancer by palpation, mammography and high-resolution ultrasound. Ultrasound Obstet Gynecol. 1993;3(3):185–190. doi: 10.1046/j.1469-0705.1993.03030185.x. [DOI] [PubMed] [Google Scholar]
- 61.Hieken TJ, Harrison J, Herreros J, Velasco JM. Correlating sonography, mammography, and pathology in the assessment of breast cancer size. Am J Surg. 2001;182(4):351–354. doi: 10.1016/s0002-9610(01)00726-7. [DOI] [PubMed] [Google Scholar]
- 62.Roubidoux MA, LeCarpentier GL, Fowlkes JB, et al. Sonographic evaluation of early-stage breast cancers that undergo neoadjuvant chemotherapy. J Ultrasound Med. 2005;24(7):885–895. doi: 10.7863/jum.2005.24.7.885. [DOI] [PubMed] [Google Scholar]
- 63.Keune JD, Jeffe DB, Schootman M, Hoffman A, Gillanders WE, Aft RL. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer. Am J Surg. 2010;199(4):477–484. doi: 10.1016/j.amjsurg.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwentner L, Helms G, Nekljudova V, et al. Using ultrasound and palpation for predicting axillary lymph node status following neoadjuvant chemotherapy - Results from the multi-center SENTINA trial. Breast. 2016;31:202–207. doi: 10.1016/j.breast.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 65.Chen M, Wang WP, Jia WR, et al. Three-dimensional contrast-enhanced sonography in the assessment of breast tumor angiogenesis: correlation with microvessel density and vascular endothelial growth factor expression. J Ultrasound Med. 2014;33(5):835–846. doi: 10.7863/ultra.33.5.835. [DOI] [PubMed] [Google Scholar]
- 66.Luo W, Numata K, Morimoto M, et al. Three-dimensional contrast-enhanced sonography of vascular patterns of focal liver tumors: pilot study of visualization methods. AJR Am J Roentgenol. 2009;192(1):165–173. doi: 10.2214/AJR.08.1107. [DOI] [PubMed] [Google Scholar]
- 67.Abramson RG, Arlinghaus LR, Weis JA, et al. Current and emerging quantitative magnetic resonance imaging methods for assessing and predicting the response of breast cancer to neoadjuvant therapy. Breast Cancer (Dove Med Press) 2012;2012(4):139–154. doi: 10.2147/BCTT.S35882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuijs VJ, Moossdorff M, Schipper RJ, et al. The role of MRI in axillary lymph node imaging in breast cancer patients: a systematic review. Insights Imaging. 2015;6(2):203–215. doi: 10.1007/s13244-015-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park S, Yoon JH, Sohn J, et al. Magnetic Resonance Imaging after Completion of Neoadjuvant Chemotherapy Can Accurately Discriminate between No Residual Carcinoma and Residual Ductal Carcinoma In Situ in Patients with Triple-Negative Breast Cancer. PLoS One. 2016;11(2):e0149347. doi: 10.1371/journal.pone.0149347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 72.Chenevert TL, Brunberg JA, Pipe JG. Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology. 1990;177(2):401–405. doi: 10.1148/radiology.177.2.2217776. [DOI] [PubMed] [Google Scholar]
- 73.Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI—a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5(4):220–233. doi: 10.1038/ncponc1073. [DOI] [PubMed] [Google Scholar]
- 74.Guo Y, Cai YQ, Cai ZL, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16(2):172–178. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 75.Aryal MP, Nagaraja TN, Keenan KA, et al. Dynamic contrast enhanced MRI parameters and tumor cellularity in a rat model of cerebral glioma at 7 T. Magn Reson Med. 2014;71(6):2206–2214. doi: 10.1002/mrm.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richard R, Thomassin I, Chapellier M, et al. Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2013;23(9):2420–2431. doi: 10.1007/s00330-013-2850-x. [DOI] [PubMed] [Google Scholar]
- 77.Liu S, Ren R, Chen Z, et al. Diffusion-weighted imaging in assessing pathological response of tumor in breast cancer subtype to neoadjuvant chemotherapy. J Magn Reson Imaging. 2015;42(3):779–787. doi: 10.1002/jmri.24843. [DOI] [PubMed] [Google Scholar]
- 78.Fukuda T, Horii R, Gomi N, et al. Accuracy of magnetic resonance imaging for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy: association with breast cancer subtype. Springerplus. 2016;5:152. doi: 10.1186/s40064-016-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoshi Y, Yamada Y. Overview of diffuse optical tomography and its clinical applications. J Biomed Opt. 2016;21(9):091312. doi: 10.1117/1.JBO.21.9.091312. [DOI] [PubMed] [Google Scholar]
- 80.Jakubowski DB, Cerussi AE, Bevilacqua F, et al. Monitoring neoadjuvant chemotherapy in breast cancer using quantitative diffuse optical spectroscopy: a case study. J Biomed Opt. 2004;9(1):230–238. doi: 10.1117/1.1629681. [DOI] [PubMed] [Google Scholar]
- 81.Cerussi A, Hsiang D, Shah N, et al. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci U S A. 2007;104(10):4014–4019. doi: 10.1073/pnas.0611058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choe R, Corlu A, Lee K, et al. Diffuse optical tomography of breast cancer during neoadjuvant chemotherapy: a case study with comparison to MRI. Med Phys. 2005;32(4):1128–1139. doi: 10.1118/1.1869612. [DOI] [PubMed] [Google Scholar]
- 83.Soliman H, Gunasekara A, Rycroft M, et al. Functional imaging of neoadjuvant chemotherapy response in women with locally advanced breast cancer using diffuse optical spectroscopy. J Clin Oncol. 2009;27(15_suppl):3591. doi: 10.1109/IEMBS.2009.5333532. [DOI] [PubMed] [Google Scholar]
- 84.Kramer-Marek G, Capala J. The role of nuclear medicine in modern therapy of cancer. Tumour Biol. 2012;33(3):629–640. doi: 10.1007/s13277-012-0373-8. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Zhang C, Liu J, Huang G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat. 2012;131(2):357–369. doi: 10.1007/s10549-011-1780-z. [DOI] [PubMed] [Google Scholar]
- 86.Mghanga FP, Lan X, Bakari KH, Li C, Zhang Y. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Clin Breast Cancer. 2013;13(4):271–279. doi: 10.1016/j.clbc.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 87.Cheng X, Li Y, Liu B, Xu Z, Bao L, Wang J. 18F-FDG PET/CT and PET for evaluation of pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Acta Radiol. 2012;53(6):615–627. doi: 10.1258/ar.2012.110603. [DOI] [PubMed] [Google Scholar]
- 88.Ulaner GA, Riedl CC, Dickler MN, Jhaveri K, Pandit-Taskar N, Weber W. Molecular Imaging of Biomarkers in Breast Cancer. J Nucl Med. 2016;57(Suppl 1):53S–59S. doi: 10.2967/jnumed.115.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajendran JG, Mankoff DA, O’Sullivan F, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10(7):2245–2252. doi: 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- 90.Rijpkema M, Boerman OC, Oyen WJ. Tumor Targeting Using Radiolabeled Antibodies for Image-Guided Drug Delivery. Curr Drug Targets. 2015;16(6):625–633. doi: 10.2174/1389450115666141029234200. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimoto M, Kurihara H, Fujii H. Theragnostic imaging using radiolabeled antibodies and tyrosine kinase inhibitors. ScientificWorldJournal. 2015;2015:842101. doi: 10.1155/2015/842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tamura K, Kurihara H, Yonemori K, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54(11):1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 93.Mortimer JE, Bading JR, Colcher DM, et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET. J Nucl Med. 2014;55(1):23–29. doi: 10.2967/jnumed.113.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perik PJ, Lub-De Hooge MN, Gietema JA, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006;24(15):2276–2282. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- 95.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 96.Gebhart G, Lamberts LE, Garcia C, et al. PET/CT with 89Zr-trastuzumab and 18F-FDG to individualize treatment with trastuzumab emtansine (T-DM1) in metastatic HER2-positive breast cancer (mBC) J Clin Oncol. 2014;32:5s. [Google Scholar]
- 97.Jacobs MA, Wolff AC, Macura KJ, et al. Multiparametric and Multimodality Functional Radiological Imaging for Breast Cancer Diagnosis and Early Treatment Response Assessment. J Natl Cancer Inst Monogr. 2015;2015(51):40–46. doi: 10.1093/jncimonographs/lgv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar A, Kumar R, Seenu V, et al. The role of 18F-FDG PET/CT in evaluation of early response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Eur Radiol. 2009;19(6):1347–1357. doi: 10.1007/s00330-009-1303-z. [DOI] [PubMed] [Google Scholar]
- 99.Chen X, Moore MO, Lehman CD, et al. Combined use of MRI and PET to monitor response and assess residual disease for locally advanced breast cancer treated with neoadjuvant chemotherapy. Acad Radiol. 2004;11(10):1115–1124. doi: 10.1016/j.acra.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Tateishi U, Miyake M, Nagaoka T, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging—prospective assessment. Radiology. 2012;263(1):53–63. doi: 10.1148/radiol.12111177. [DOI] [PubMed] [Google Scholar]
- 101.Semple SI, Staff RT, Heys SD, et al. Baseline MRI delivery characteristics predict change in invasive ductal breast carcinoma PET metabolism as a result of primary chemotherapy administration. Ann Oncol. 2006;17(9):1393–1398. doi: 10.1093/annonc/mdl136. [DOI] [PubMed] [Google Scholar]
- 102.Iagaru A, Masamed R, Keesara S, Conti PS. Breast MRI and 18F FDG PET/CT in the management of breast cancer. Ann Nucl Med. 2007;21(1):33–38. doi: 10.1007/BF03033997. [DOI] [PubMed] [Google Scholar]
- 103.Atuegwu NC, Li X, Arlinghaus LR, et al. Longitudinal, intermodality registration of quantitative breast PET and MRI data acquired before and during neoadjuvant chemotherapy: preliminary results. Med Phys. 2014;41(5):052302. doi: 10.1118/1.4870966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sauter AW, Wehrl HF, Kolb A, Judenhofer MS, Pichler BJ. Combined PET/MRI: one step further in multimodality imaging. Trends Mol Med. 2010;16(11):508–515. doi: 10.1016/j.molmed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Yankeelov TE, Peterson TE, Abramson RG, et al. Simultaneous PET-MRI in oncology: a solution looking for a problem? Magn Reson Imaging. 2012;30(9):1342–1356. doi: 10.1016/j.mri.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med. 2012;53(6):928–938. doi: 10.2967/jnumed.112.105338. [DOI] [PubMed] [Google Scholar]
- 107.Catalano OA, Rosen BR, Sahani DV, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology. 2013;269(3):857–869. doi: 10.1148/radiol.13131306. [DOI] [PubMed] [Google Scholar]
- 108.Schwenzer NF, Schraml C, Muller M, et al. Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging—pilot study. Radiology. 2012;264(2):551–558. doi: 10.1148/radiol.12111942. [DOI] [PubMed] [Google Scholar]
- 109.Boss A, Bisdas S, Kolb A, et al. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J Nucl Med. 2010;51(8):1198–1205. doi: 10.2967/jnumed.110.074773. [DOI] [PubMed] [Google Scholar]
- 110.Drzezga A, Souvatzoglou M, Eiber M, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53(6):845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 111.Pichler BJ, Kolb A, Nagele T, Schlemmer HP. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med. 2010;51(3):333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 112.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132(3):1049–1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263(3):663–672. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mountz JM, Yankeelov TE, Rubin DL, et al. Letter to cancer center directors: Progress in quantitative imaging as a means to predict and/or measure tumor response in cancer therapy trials. J Clin Oncol. 2014;32(19):2115–2116. doi: 10.1200/JCO.2014.55.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sullivan DC, Obuchowski NA, Kessler LG, et al. Metrology Standards for Quantitative Imaging Biomarkers. Radiology. 2015;277(3):813–825. doi: 10.1148/radiol.2015142202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.CRI Clinical Trials. 2017 www.cancerresearch.org. Accessed January 1, 2017.


