Abstract
Background
Idiosyncratic drug induced liver injury (DILI) is a rare but potentially serious liver disorder and a major cause of significant liver injury. Limited data exist on racial differences in DILI incidence, presentation and course.
Aim & Methods
We compared the causative agents, clinical features and outcomes of DILI among self-described African-Americans and non-Hispanic whites (Caucasians) enrolled in the DILIN Prospective Study. Individuals with definite, highly likely, or probable DILI enrolled between Sept 2004 and Feb 2016 were included in this analysis.
Results
144 African-Americans and 841 Caucasian patients met the eligibility criteria. Causal medications varied by race: trimethoprim/sulfamethoxazole being the most common cause among African-Americans (7.6% vs 3.6%) followed by methyldopa (4% vs <1%), phenytoin (5% vs <1%), isoniazid (4% vs 4%) and amoxicillin/clavulanate (4.1% vs 13.4%). The severity of illness, however, tended to be greater in African-Americans than Caucasians as determined by peak mean bilirubin (14.3 vs 12.8 mg/dL), INR (1.9 vs 1.6) and DILIN severity score (3.0 vs 2.6). The frequency of severe cutaneous reactions was significantly higher in African-Americans (2.1 vs. 0.36% in Caucasians, p=0.048). African-Americans also had higher rates of hospitalization (76.7% vs 57.6%, p<0.001), liver transplantation or liver related death by 6 months (10.2% vs 5.8%, p=0.02 after controlling for selected covariates) and chronic DILI (24% vs. 16%, p=0.06).
Conclusions
The most common DILI causative agents differ between African-Americans and Caucasians. African-Americans are more likely to have severe cutaneous reactions and more severe liver injury leading to worse outcomes, including death and liver transplant. [Word Count 250]
Keywords: Drug Induced Liver Injury, Drug Induced Liver Injury Network, Severe Skin Reactions, RUCAM, Chronic DILI
Introduction
Idiosyncratic drug induced liver injury is a rare but clinically important entity with a recent population frequency of 15 events per 100,000 inhabitants in the Western world (1). The prompt discontinuation of the implicated agent improves the liver injury event in the majority suffering from DILI, but there is a high rate of hospitalization and rarely, acute liver failure leading to liver transplantation or death (2,3). Furthermore, recent reports suggest that sizable number of acute DILI events may lead to chronic liver disease with demonstrable long-term histological damage. A recent report from the DILIN observed that severe cutaneous reactions accompanied one percent of DILI events and were associated with high mortality rate (2).
Established in 2003, the United States Drug-Induced Liver Injury Network (DILIN) is conducting a prospective study of individuals who are ≥2 years of age with suspected DILI at several clinical centers across the United States (5). Individuals enrolled into this study are from varying ethnic and racial backgrounds and the DILIN Prospective Study has enrolled over 100 individuals who are of self-reported African-American race. This diversity provides an opportunity to characterize the causative agents and the characteristics and outcomes of DILI in this important demographic. During the conduct of this study, we anecdotally observed some differences in terms of causative agents or skin reactions among various racial and ethnic groups. There is a suggestion in the literature that certain adverse drug reactions (ADRs) have different frequency and clinical phenotypes in different racial and ethnic groups. For example, it was recently observed that African-Americans are at significantly higher risk for allopurinol-induced Stevens-Johnson syndrome, an ADR which shares overlapping features with DILI (6). In a report from the United States Acute Liver Failure Study Group (ALFSG), Forde et al. have shown that blacks (24.4%) were more likely to have acute liver failure due to drugs than whites (14.9%, p=0.015) (7). Recent studies have also highlighted racial and ethnic differences in the prevalence and characteristics of nonalcoholic fatty liver disease (8,9). This prompted us to carefully analyze DILI in self-reported African-Americans enrolled in the DILIN Prospective Study. The objectives of this report are to compare the profile of causative agents, the clinical characteristics and the outcomes of DILI in African Americans in comparison to Caucasians.
Methods
The DILIN Prospective Study (NCT 00345930) is conducted by the United States Drug Induced Liver Injury Network which is funded as a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health. DILIN consists of 6 to 8 medical centers, a central data coordinating center and a sample repository. The DILI Prospective Study design, method of causality assessment and various scoring systems have been described in detail elsewhere (5). The study was approved by the appropriately convened institutional review boards at the participating clinical centers and data coordinating center as well as by a Data Safety and Monitoring Board appointed for this purpose by the NIDDK. All participants provided written informed consent before enrollment. The DILIN Prospective Study has been the source for multiple publications on topics which ranged from overall patient population characteristics to phenotypes and sub-phenotypes and select implicated agents (10–17). Participants who form the basis for this report were included in other reports published by the DILIN. All participants completed a questionnaire that captured self-reported racial and ethnic information. Cases of DILI that underwent formal causality assessment and were judged to be definite, highly likely, or probable were eligible for inclusion in this analysis.
Data Management and Analyses
Demographic and clinical data for subjects enrolled into the DILIN Prospective Study between September 2004 and February 2016 were extracted on February 12, 2016. Descriptive statistics, such as means with SD, median with interquartile ranges and frequency distributions were used to describe the cohort. Between group difference were tested using the χ2 test for categorical variables and Wilcoxon/Kruskal-Wallis test for the continuous variables. In the logistic regression analyses, the relationship between African-American race and DILIN Severity Score and patient outcomes (liver transplant/death or chronic DILI) was adjusted for age, body mass index, and diabetes. Statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Inc, Cary, NC) and P value <.05 was considered statistically significant.
Results
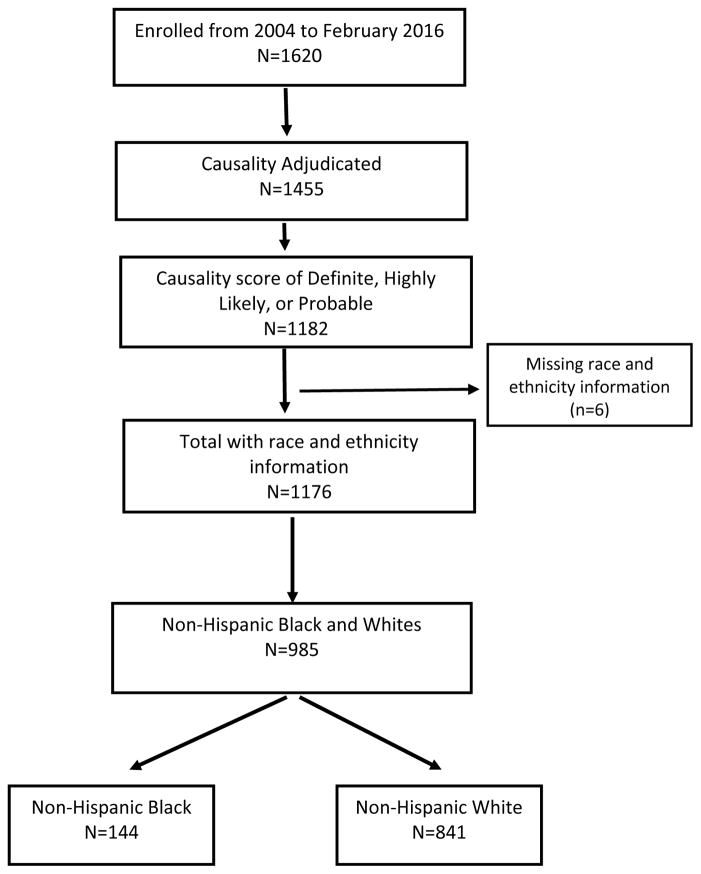
Between September 2014 and February 2016, 1620 individuals were enrolled into the DILIN Prospective Study and 1455 had undergone six months of follow up and formal causality assessment of whom 1182 were scored as definite, highly likely, or probable. These confirmed DILI cases included 144 individuals who self-reported their ethnicity and race as non-Hispanic Black or African-American and 841 who reported being non-Hispanic Whites or Caucasians. The remaining 191, reported their ethnicity and race as Asian (n=41), Hispanic (n=86) or other (n=36) or did not provide ethnic and racial information (n=6). The construction of the patient cohort for analysis is shown diagrammatically in Figure 1. The analysis was limited to cases occurring among non-Hispanic whites (Caucasians) and blacks (African Americans).
Figure 1.
Study Population: Flow Diagram
A comparison of the demographic, clinical and biochemical features of the two study groups are shown in Table 1. In comparison to Caucasians, African-Americans with DILI tended to be younger with significantly higher BMI and higher prevalence of diabetes but lower frequency of prior drug allergies or alcohol consumption history. Gender and the frequency of preexisting liver disease were not different between two groups. There were more African Americans with DILI who lacked any health insurance or were without at least a high school education.
Table 1.
Demographic and Selected Clinical Features for the African-American and Caucasians with definite, highly likely, or probable Drug-Induced Liver Injury
| African-Americans (n=144) | Caucasians (n=841) | P value | |
|---|---|---|---|
|
| |||
| Age (years, mean [SD]) | 46.9 ± 15.7 | 50.7 ± 17 | 0.005 |
|
| |||
| Females (%) | 63 | 57 | 0.17 |
|
| |||
| High School or Higher Education (%) | 83 | 93 | <0.001 |
|
| |||
| Health Insurance (%) | 88 | 95 | 0.005 |
|
| |||
| BMI (kg/m2, mean [SD]) | 29.9 ± 7.6 | 27.3 ± 6.6 | <0.001 |
|
| |||
| Prior drug allergies (%) | 38 | 49 | 0.02 |
|
| |||
| Alcohol Use (%) | 32 | 55 | <0.001 |
|
| |||
| Preexisting Liver Disease (%) | 12 | 9 | 0.20 |
|
| |||
| Diabetes mellitus (%) | 31 | 22 | 0.03 |
|
| |||
| Latency (days in median, IQR) | 49 (24, 103) | 42 (22, 99) | 0.60 |
|
| |||
| Days from earliest sign to primary implicated drug stop (days in median, IQR) | 2 (0.5, 5.5) | 3 (0, 12) | 0.40 |
|
| |||
| Severe skin reactions (%) | 2.1 | 0.4 | 0.04 |
|
| |||
| Jaundice (%) | 69 | 69 | 0.9 |
|
| |||
| Liver Test Results – Onset | |||
| ALT (U/L, mean [SD]) | 835 ± 893 | 773 ± 1095 | 0.10 |
| Alk P (U/L, mean [SD]) | 262 ± 199 | 292 ± 261 | 0.50 |
| Total bilirubin (mg/dL, mean [SD]) | 7.5 ± 7.2 | 6.4 ± 6.3 | 0.30 |
| INR | 1.4 ± 0.8 | 1.4 ± 1.0 | 0.19 |
|
| |||
| Liver Test Results – Peak values | |||
| ALT (U/L, mean [SD]) | 1117 ± 1126 | 930 ± 1180 | 0.01 |
| Alk P (U/L, mean [SD]) | 409 ± 383 | 403 ± 373 | 0.70 |
| Total bilirubin (mg/dL, mean [SD]) | 14.2 ± 11.5 | 12.7 ± 11.6 | 0.06 |
| INR | 1.9 ± 1.8 | 1.6 ± 1.5 | <0.001 |
|
| |||
| Eosinophilia (>500/μL) (%) | 13.4 | 11.6 | 0.56 |
|
| |||
| Improvement in liver tests (median days) | |||
| Peak ALT to below ULN | 66 | 62 | 0.40 |
| Peak Alk P to below ULN | 142 | 77 | 0.06 |
| Peak bilirubin to ≤ 2.5 mg/dL | 36 | 33 | 0.14 |
|
| |||
| R-ratio (mean, SD) | 12 ± 14 | 12 ± 26 | 0.14 |
|
| |||
| Pattern of Liver Injury (%) | |||
| Hepatocellular | 61 | 51 | 0.08 |
| Cholestatic | 22 | 25 | |
| Mixed | 17 | 24 | |
Abbreviations: ALT, serum alanine aminotransferase; AP, serum alkaline phosphatase; BMI, body mass index; Chol, cholestatic; DILI, drug-induced liver injury; HC, hepatocellular; INR, international normalized ratio; IQR, interquartile range (25–75%); ULN, upper limit of normal
The five leading therapeutic classes and top 10 single prescription agents implicated among the 144 African-Americans and 841 Caucasians are shown in Table 2. In general, there were no significant differences in the classes of agents implicated between two groups, with antimicrobials, herbal and dietary supplements, cardiovascular system agents, and central nervous system agents being the most commonly implicated classes. However, there appeared important differences in the individual agents that were implicated in causing DILI between the African-American and Caucasian groups(Table 2). The combination of trimethoprim and sulfamethoxazole (TMP/SMZ) was the most common agent implicated in causing DILI in African-Americans (7.6%) and its frequency was significantly higher than in Caucasians (3.6%, p=0.04). In contrast, the combination of clavulanic acid with amoxicillin (Clav/Amox) was the most commonly implicated agent in DILI among Caucasians (13.4%), but it ranked fourth and its frequency was significantly lower among African-Americans (4.1%, p<0.001). Additionally, compared to Caucasians, African-Americans had significantly higher frequency of liver injury due to phenytoin (4.8% vs. 0.8%, p=0.002), methyldopa (4.1% vs. 0.5%, p=0.001), and allopurinol (2.7% vs. 0.4%, p=0.01). These 3 agents ranked 3rd, 5th and 8th among African Americans but only 24th, 41th and 51th among Caucasians.
Table 2.
Implicated Classes and Individual Agents in African-Americans (n=144) and Caucasians (n=841) with definite, highly likely, or probable DILI
| African-Americans (n=144) | Caucasians (n=841) | |
|---|---|---|
| Top 5 Classes of Implicated Agents |
|
|
| Top 10 individual agents implicated |
|
|
HDS: Herbal and dietary supplements; CNS: Central nervous system; Amox-Clav: Amoxicillin and clavulanate; TMP-SMX: Trimethoprim and sulfamethoxazole
The time between initiating the implicated medication or herbal agent and onset of DILI (latency) and the interval between earliest symptoms of liver injury and discontinuation of the suspected medication (delay in stopping) were not different between the African-Americans and Caucasian groups. In addition, the degrees of likelihood as assessed by RUCAM, DILIN causality scores and DILIN causality categories were similar between the two groups (Table 3). The R-ratio or the patterns of liver injury at presentation were minimally different between the two groups, although there was a trend towards the “mixed hepatocellular-cholestatic” pattern of liver injury being less frequent among African-Americans with DILI than Caucasians (17% vs 24%, p=0.078). While the degree of elevation of serum enzymes at onset of DILI was not different between the two groups, peak levels of ALT and INR were significantly higher in African Americans than in Caucasians, and the peak total bilirubin was higher but these differences were not statistically significant. The time to improvement in serum liver biochemical tests was similar in the two racial groups.
Table 3.
Causality and Severity Scores and Outcomes of African-American and Caucasians with definite, highly likely, or probable Drug-Induced Liver Injury
| Feature | African-Americans (n=144) | Caucasians (n=841) | P value |
|---|---|---|---|
|
| |||
| DILIN Causality Score (mean, SD) | 2.1 ± 0.7 | 2.0 ± 0.7 | 0.08 |
|
| |||
| DILIN Causality (%): Definite | 19 | 25 | |
| Highly likely | 53 | 53 | 0.20 |
| Probable | 28 | 23 | |
|
| |||
| DILIN Severity Score (mean, SD) | 3.0 ± 1.2 | 2.6 ± 1.2 | <0.001 |
|
| |||
| DILIN Severity (%) | |||
| Mild | 15 | 23 | |
| Moderate | 12 | 24 | <0.001 |
| Moderate-Hospitalized | 38 | 29 | |
| Severe | 24 | 18 | |
| Fatal (within 6 months of onset) | 10 | 6 | |
|
| |||
| Hospitalization or non-DILI hospitalization was prolonged (%) | 77 | 56 | <0.001 |
|
| |||
| Death, any cause (%) | 9.0 | 5.7 | 0.10 |
|
| |||
| DILI-related death or Liver Transplantation (%) | 10.2 | 5.8 | 0.16 |
|
| |||
| Chronicity (%) | 24 | 16 | 0.06 |
DILIN: Drug Induced Liver Injury Network; DILI: Drug Induced Liver Injury
Assessment of severity using the DILIN scoring system indicated that the liver injury was on average more severe among African-Americans than Caucasians (Table 3). Thus, the mean DILIN severity scores were significantly higher in African-Americans than Caucasians (3.0 vs 2.6), and the distribution of scores was skewed to a higher frequency of severe and fatal cases among African-Americans compared to Caucasians (34% vs 24%). Among age, BMI, and diabetes, only BMI had statistically significant univariate relationship with the DILIN severity score. When we controlled for BMI, the relationship between African-American and DILIN severity score statistically significant (OR 1.98, 95% CI: 1.42–2.75, p<0.001). The frequency of severe liver injury leading to death or requiring liver transplantation was higher in African-Americans (10.2%) than in Caucasians (5.8%). Among age, BMI, and diabetes, both age and BMI had statistically significant univariate relationship with the DILIN severity score. When we controlled for age and BMI, the relationship between African-American and the likelihood of death or liver transplantation statistically significant (OR 2.12, 95% CI: 1.12–3.9, p=0.02).
In addition, chronicity was more frequent in the DILIN cases among African-Americans than Caucasians (24% vs. 16%), but this difference was of borderline statistical significance (p=0.06). Finally, severe cutaneous reactions occurred in 0.6% of patients in this cohort and were more frequent in African-Americans than Caucasians (2.1 vs. 0.36%, p=0.048). Two of the cases with severe cutaneous reactions were attributed to lamotrigine (n=2) and one each to carbamazepine, azithromycin, and nitrofurantoin.
There were no significant differences in the latency, liver biochemical tests at presentation or their peak values, R-ratio, or outcomes (such as death or liver failure needing liver transplantation) of liver injury caused by TMP/SMX, amoxicillin-clavulanate, isoniazid, nitrofurantoin, or phenytoin between African-Americans and Caucasians. While the severity of liver injury due to TMP/SMX, amoxicillin-clavulanate, isoniazid, or nitrofurantoin was not different between African-Americans and Caucasians, there were significantly higher number of severe/fatal liver injury events due to phenytoin in African-Americans than in Caucasians (86% vs. 14%, p=0.008).
Discussion
Our study adds to a growing body of literature showing racial and ethnic differences in the frequency and nature of adverse drug reactions (ADRs). A recent systematic review found no consistent relationship between race and ADRs in general but noted that certain racial groups may be at higher risk for ADRs due to certain classes of agents (18). Asians were found to be higher risk for ADRs from oral anticoagulants whereas blacks were at higher risks from antidiabetic agents and whites from opiate analgesics (18). In a study of United States Vital Statistics, deaths due to ADRs were significantly more frequent in African-Americans than Caucasians, Hispanics, or Asians (19). Asians and blacks appear to be at significantly higher risk for severe cutaneous reactions due to allopurinol and this phenomenon is strongly associated with higher frequency of HLA-B*5801 in Asians and blacks (6). A few studies investigated the relationship between race and ethnicity and hepatotoxicity from anti-tuberculosis (anti-TB) medications (20–22). In the United States Public Health Service study published in 1979, the frequency of liver injury due to anti-TB medications was significantly lower in African-American males compared to white males, but there existed no such difference among females (20). However, this observation was not reproduced in subsequent studies (21,22). In our study, we did not find significant difference in the frequency of liver injury due to isoniazid between African-Americans and Caucasians (6.2% vs. 3.6%, p=0.16).
The major causes of DILI among African Americans in the DILIN Prospective Study were somewhat distinctive. Compared to common causes among Caucasians, the major causes of DILI among African-Americans were more likely to be agents that are associated with acute and sometimes severe hypersensitivity reactions or those typically present with a “mixed hepatocellular-cholestatic” pattern of injury. Thus, TMP/SMZ, phenytoin and allopurinol are all associated with immune-allergic type of injury that is commonly “mixed”, and methyldopa with an autoimmune phenotype that is commonly hepatocellular. When, analyzed by individual agents, African-Americans, who made up 13% of the DILIN cohort, represented 27% of TMP/SMZ, 50% of phenytoin, 43% of allopurinol and 55% of methyldopa cases. In contrast, African-Americans were underrepresented in the cases of amox-clav associated liver injury. Amox-clav has repeatedly been shown to be the most common cause of DILI in the western world (2, 10, 23, 24). It is interesting to note that the frequency of HLA A*02:01 and HLA DRB1*15:01 alleles which previously have been reported as risk factors for liver injury due to amoxicillin-clavulanate is much lower in African Americans than in Caucasians (25–27). In one study, the frequency of HLA A*02:01 among Caucasians residing in the United States was 27.2% whereas its frequency in African Americans was only 12.1% (26). Similarly, the frequency of HLA DRB1*15:01 in African-Americans was only 3% compared to 25% among Caucasians residing in the United States (27). However, the underrepresentation of amoxicillin-clavulanate induced liver injury among African-Americans could be due to other factors such as differences in underlying infections for which this medication was prescribed, or differences in prescription patterns of health care providers.
The liver injury in African-Americans was more severe as reflected by higher DILIN severity scores and more frequent hospitalization and was associated with higher frequency of worse outcomes such as liver related mortality/liver transplantation and chronic DILI. The reasons for this phenomenon are unclear. The duration between earliest DILI symptoms and stopping the implicated agent was comparable between the two groups and similarly liver biochemistries at DILI presentation were not different between two groups. This suggests that greater severity and worse outcomes associated with DILI in African Americans is not likely due to a delay in seeking care by African-Americans. Furthermore, except for phenytoin, none of the other common causes of DILI in this cohort (TMP/SMX, isoniazid, nitrofurantoin, herbal and dietary supplements, or amox-clav) per se were associated with more severe liver injury in African-Americans.
The higher frequency of severe cutaneous reactions we observed in African-Americans with DILI is intriguing. This does not appear to be differences in the DILI causative agents between two groups. Antiepileptic drugs are known to be associated with severe cutaneous reactions but the frequency of liver injury due to antiepileptics was not different between African-Americans and Caucasians was not different. Trimethoprim/sulfamethoxazole (TMP/SMZ) and allopurinol are known to cause severe cutaneous reactions. Although there were more cases of TMP/SMZ and allopurinol associated liver injury among African-Americans, none were associated with severe cutaneous reactions. It needs to be explored if African-Americans have an increased genetic predisposition to severe cutaneous reactions.
In summary, this report summarizes causative agents, clinical aspects and outcomes of well characterized DILI in a large cohort of African-Americans. The causative agents, certain patterns) and (b) DILI in African Americans may have worse outcomes and thus they may be followed closely with intensified follow-up. Further research is needed to better understand the basis for these differences. Larger population based studies should be conducted to confirm if African-Americans are at increased risk (or lower risk) for liver injury from well-recognized hepatotoxic medications. Adequately powered genomic studies with optimal control groups should also be conducted to understand if there are genetic factors that at least in part explain our observations. clinical features (e.g., frequency of severe cutaneous reactions) and outcomes of DILI are distinct in African-Americans. We believe that our observations add to the growing body of literature on DILI. It is of value to providers to know that (a) some compounds are more likely to cause DILI in AA than in Caucasian (may influence their prescription
Study Highlights.
1. What is currently known?
Drug induced liver injury (DILI) is a rare but potentially devastating adverse reaction to medications or supplements
The causes and outcomes of DILI in African-Americans are not well studied.
2. What is new here?
The most common DILI causative agents differ between African-Americans and Caucasians. Trimethoprim/sulfamethoxazole is the most common cause for DILI in African-Americans whereas Amoxicillin-clavulanic acid is the most common cause for DILI in Caucasians.
African-Americans are more likely to have severe cutaneous reactions and more severe liver injury leading to worse outcomes, including death and liver transplant.
Acknowledgments
Financial support: The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants: 2U01-DK065176-06 (Duke), 2U01-DK065201-06 (UNC), 2U01-DK065184-06 (Michigan), 2U01-DK065211-06 (Indiana), 5U01DK065193-04 (UConn), 5U01-DK065238-08 (UCSF/CPMC), 1U01-DK083023-01 (UTSW), 1U01-DK083027-01 (TJH/UPenn), 1U01-DK082992-01 (Mayo), 1U01-DK083020-01 (USC). Additional funding is provided by CTSA grants: UL1 RR025761 (Indiana), UL1TR000083 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and in part by the Intramural Research Program of the NIH, National Cancer Institute.
DILIN Clinical Sites:
Indiana University-Purdue: Naga Chalasani, MD, PI; Marwan S. Ghabril, MD, Sub-I; Raj Vuppalanchi, MD, Sub-I; [Audrey Corne, RN, EdD, Study Coord; Wendy Morlan, RN, Study Coord];
University of Michigan-Ann Arbor: Robert J. Fontana, MD, PI; Hari Conjeevaram, MD, Sub-I; Frank DiPaola, MD, Sub-I; [Cassandra Coffman, Study Coord; Sophana Mao, Study Coord];
-
University of North Carolina-Chapel Hill: Paul Watkins, MD, PI; Jama Darling, MD, Sub-I; Michael Fried, Sub-I; Paul H. Hayashi, MD, Sub-I; Steven Lichtman, MD, Sub-I; Steven Zacks, MD, MPH, Sub-I; [Tracy Russell, CCRP, Study Coord];
Satellite Sites:
Asheville: William Harlan, MD, PI; [Tracy Russell, CCRP, Study Coord];
Wake Forest Baptist Medical Center: Herbert Bonkovsky, MD, PI; Pradeep Yarra, MD, Sub-I; [Denise Faust, Study Coord];
-
University of Southern California: Andrew Stolz, MD, PI; Neil Kaplowitz, MD, Sub-I; John Donovan, MD, Sub-I; [Susan Milstein, RN, BSN, Study Coord];
Satellite Sites:
University of California-Los Angeles (Pfleger Liver Institute): Francisco A. Durazo, MD, PI; [Yolanda Melgoza, Study Coord; Val Peacock, RN, BSN, Co-Coord];
Albert Einstein Medical Center: Victor J. Navarro, MD, PI; Simona Rossi, MD, Sub-I; [Maricruz Vega, MPH, Study Coord; Manisha Verma, MD, MPH, Study Coord];
Icahn School of Medicine at Mount Sinai: Joseph Odin, MD, PhD, PI; Jawad Ahmad, MD, PI; Nancy Bach, Sub-I; Meena Bansal, MD, Sub-I; Charissa Chang, MD, Sub-I; Douglas Dieterich, MD, Sub-I; Priya Grewal, MD, Sub-I; Lawrence Liu, MD, Sub-I; Thomas Schiano, MD, Sub-I; [Monica Taveras, Study Coord];
National Institutes of Health Clinical Center: Christopher Koh, MD, PI; [Beverly Niles, Study Coord];
DILIN Data Coordinating Center at Duke Clinical Research Institute: Huiman X. Barnhart, PhD, PI; Katherine Galan, RN, Project Lead; Theresa O’Reilly, Lead CRA; Elizabeth Mocka, CRA; Olivia Pearce, CTA; Michelle McClanahan-Crowder, Data Management; Coleen Crespo-Elliott, Data Management; Hoss Rostami, Data Management; Qinghong Yang, Programmer-Statistics; Jiezhun (Sherry) Gu, PhD, Statistician; Tuan Chau, Lead Safety Associate;
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): José Serrano, MD, Project Scientist; Rebecca J. Torrance, RN, MS, Clinical Trials Specialist; Rebekah Van Raaphorst, MPH, LT, USPHS, Health Research Administrator; Francisco O. Calvo, PhD, COC Contact; James E. (Jay) Everhart, MD, MPH, Scientific Advisor; Jay Hoofnagle, MD; Scientific Advisor; Averell H. Sherker, MD, FRCP(C), Program Official.
Abbreviations
- ALK P
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- DILI
Drug induced liver injury
- DILIN
Drug induced liver injury network
- HLA
Human leukocyte antigen
- INR
International normalized ratio
- RUCAM
Rousell Uclaf Causality assessment method
- ULN
Upper limit of normal
Footnotes
Conflicts of Interests: Dr. Chalasani has ongoing consulting activities (or had in preceding 12 months) with NuSirt, Abbvie, Eli Lilly, Afimmune (DS Biopharma), Tobira (Allergan), Madrigal, Shire, Cempra, Ardelyx, Gen Fit and Amarin. These consulting activities are generally in the areas of nonalcoholic fatty liver disease and drug hepatotoxicity. Dr. Chalasani receives research grant support from Intercept, Lilly, Gilead, Galectin Therapeutics and Cumberland where his institution receives the funding. Over the last decade, Dr. Chalasani has served as a paid consultant to more than 30 pharmaceutical companies and this outside activities have regularly been disclosed to his institutional authorities. Dr Reddy serves on ad hoc advisory board for Abbvie, Merck and Gilead. He has research support from Abbvie, Merck, Gilead, BMS, Janssen, and Conatus where his institution is the recipient of the funding. Dr. Fontana has received research support from BMS, Janssen, and Gilead and served as a consultant to Regulus. Drs. Barnhart, Gu, Stolz, Hayashi, Ahmad, Navarro, and Hoofnagle have no conflicts of interest to disclose.
References
- 1.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug induced liver injury in general population. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Bonkovsky HL, Fontana RJ, Vuppalanchi R, Hayashi PH, Navarro V, Reddy KR, Lee WM, Davern T, Barnhart H, Atolz A, Kleiner DE, Talwalkar J, Serrano J, Gu J, Watkins PB, Hoofnagle J. Drug-induced liver injury in the United States: A report of 899 events prospectively assessed. Gastroenterology. 2015;148:1340–1352. [Google Scholar]
- 3.Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N, et al. Persistent liver injury is more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110:1450–1459. doi: 10.1038/ajg.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medina-Caliz I, Robles-Diaz M, Garcia-Munoz B, Stephens C, Ortega-Alonso A, et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol. 2016;65:532–542. doi: 10.1016/j.jhep.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, et al. Drug induced liver injury network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu N, Rai SK, Terkeltaub R, Kim SC, Menendez ME, Choi HK. Racial disparities in the risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis as urate-lowering drug adverse events in the United States. Seminars in Arthritis and Rheumatism. 2016;46:253–258. doi: 10.1016/j.semarthrit.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forde KA, Reddy KR, Troxel AB, Sanders CM, Lee WM for the Acute Liver Failure Study Group. Racial and ethnic differences in presentation, etiology, and outcomes of acute liver failure in the United States. Clin Gastroenterol Hepatol. 2009;7:1121–1126. doi: 10.1016/j.cgh.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider AL, Lazo M, Selvin E, Clark JM. Racial differences in nonalcoholic fatty liver disease in the U.S. population. Obesity. 2014;22:292–299. doi: 10.1002/oby.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster T, Anania FA, Li D, Katz R, Budoff M. The prevalence and clinical correlates of nonalcoholic fatty liver disease (NAFLD) in African-Americans: the multiethnic study of atherosclerosis (MESA) Dig Dis Sci. 2013;58:2392–2398. doi: 10.1007/s10620-013-2652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011 Nov;141(5):1665–72. e1–9. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalasani N, Vuppalanchi R, Navarro VJ, et al. Acute liver injury due to Flavocoxid (limbrel), a medical food for osteoarthritis. A case series. Ann Intern Med. 2013;156:857–60. doi: 10.7326/0003-4819-156-12-201206190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleiner DE, Chalasani N, Lee W, Fontana R, Bonkovsky HL, Watkins PB, Hayashi P, Davern T, Navarro V, Reddy KR, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2013 Aug 28; doi: 10.1002/hep.26709. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molleston JP, Lopez J, Fontana RJ, Chalasani N for the Drug Induced Liver Injury Network. Characteristics of Drug Induced Liver Injury in Children: Interim results from the DILIN Prospective Study. J Pedia Gastro Hepatol. 2011;53:182–18. doi: 10.1097/MPG.0b013e31821d6cfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro V, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano K, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R. Liver injury due to herbal and dietary supplements in the U.S.. Drug Induced Liver Injury Network. Hepatology. 2014 Jul 12; doi: 10.1002/hep.27317. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghabril M, Fontana R, Rockey D, Jiezhun G, Chalasani N. Drug induced liver injury caused by intravenously administered medications: The Drug Induced Liver Injury Network (DILIN) experience. J Clinical Gastroenterology. 2013 Feb 5; doi: 10.1097/MCG.0b013e318276bf00. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, Serrano J, Lee WM, Chalasani N, Stolz A, Davern T, Talwalkar TA. Idiosyncratic Drug-Induced Liver Injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147:96–108. doi: 10.1053/j.gastro.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baehr A, Pena JC, Hu DJ. Racial and ethnic disparities in adverse drug events: a systematic review of the literature. Journal of Racial and Ethnic Health Disparities. 2015;2:527–536. doi: 10.1007/s40615-015-0101-3. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd G, Mohorn P, Yacoub K, May DW. Adverse drug reaction deaths in United States vital statistics, 1999–2006. Ann Pharmacother. 2012;46:169–175. doi: 10.1345/aph.1P592. [DOI] [PubMed] [Google Scholar]
- 20.Kopanoff DE, Snider D, Caras G. Isoniazid related hepatitis: A U.S. Public Health Service Cooperative Surveillance Study. Am Rev Respir Dis. 1979;117:991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 21.Nolan CM, Goldberg S, Buskin S. Hepatotoxicity associated isoniazid preventive therapy: a 7-year evaluation from a public health tuberculosis clinic. JAMA. 1999;281:1014–1018. doi: 10.1001/jama.281.11.1014. [DOI] [PubMed] [Google Scholar]
- 22.Fountain FF, Tolley E, Chrisman CE, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis treatment: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128:116–123. doi: 10.1378/chest.128.1.116. [DOI] [PubMed] [Google Scholar]
- 23.Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Lucena MI, Andrade RJ, Fernandez MC, et al. Determinants of the clinical expression of amoxicillin-clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44:850–6. doi: 10.1002/hep.21324. [DOI] [PubMed] [Google Scholar]
- 25.Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, et al. Susceptibility to amoxicillin-clavulanate induced liver injury s influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton D, Menchaca L, Rood H, Komerofsky R. New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens. 2003;61(5):403–7. doi: 10.1034/j.1399-0039.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 27.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Human Immunology. 2007;68:779–788. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]