Abstract
CD28 is the major costimulatory receptor on T cells regulating proliferation, survival and effector function. Acquired mutations in the extracellular domain of CD28 have been identified in patients with cutaneous T cell lymphoma, angioimmunoblastic T cell lymphoma and other T cell neoplasms, suggesting it may contribute to disease pathogenesis. We used a heterologous system in which mutant human CD28 was expressed on primary murine T cells deficient in CD28 to ascertain how specific mutations identified in a genetic screen of patients with cutaneous T cell lymphoma affected normal T cell function. All three mutant CD28 proteins examined enhanced CD28-dependent T cell proliferation and effector function. These data suggest that the mutant CD28 isoforms could accelerate tumor cell growth and increase tumor burden in affected patients. Interruption of CD28:ligand interactions may be an effective, targeted therapy for a subset of patients whose tumors bear the mutant CD28 receptor.
Keywords: CD28, Costimulation, Cutaneous T cell lymphoma
1. Introduction
Binding of CD28 to either of its ligands, CD80 (B7-1) or CD86 (B7-2), initiates signaling that synergizes with TCR engagement to augment T cell proliferation, cytokine secretion and cell survival [1]. Despite its critical role in normal T cell function, acquired mutations of CD28 have not previously been shown to cause human disease although some polymorphisms have been associated with susceptibility to autoimmune conditions [2–4].
Recurrent acquired mutations in the extracellular domain of CD28, as well as fusions between CTLA-4 (CD152), ICOS (CD278) and CD28 have been identified in cells from up to 10% of patients with cutaneous T cell lymphoma (CTCL), angioimmunoblastic T cell lymphoma (AITL), peripheral T cell lymphoma (PTCL) and adult T cell lymphoma-leukemia (ATL) [5–11]. However, whether and how these mutations contribute to the disease phenotype remains unknown. We expressed human CD28 receptors with either of 2 point mutations that had been identified as occurring with the greatest frequency in CTCL as well as a fusion protein between CD28 and CTLA-4 also identified in patients with CTCL in primary T cells isolated from CD28-deficient mice and assessed for changes in T cell proliferation and effector function [5, 7, 9, 10]. Our studies show that T cells expressing the mutant CD28 isoforms have significantly higher proliferative responses and IL-2 secretion as compared to wild type CD28. Our data suggests that this is due to a higher binding affinity of the mutant proteins to CD86 and support a mechanism by which the transformed T cells in CTCL might receive an augmented CD86:CD28 mediated signal, which could drive further clonal expansion of the malignant cells. These data provide new insights into how mutations in CD28 may contribute to the pathogenesis of CTCL and possibly to other T cell neoplasms. Furthermore as approved therapies exist, such as abatacept or belatacept that interfere with engagement of CD28 by ligand [12], suggest a novel, targeted therapeutic strategy for treatment of a subset of patients.
2. Methods
2.1. Mice
CD28-deficient mice were bred into the DO11.10 strain mice in the Balb/c background, which recognize the OVA(323–339) peptide as previously described [13, 14]. All mice were bred and housed in a specific pathogen free environment at Washington University School of Medicine. All protocols were reviewed and approved by the Washington University School of Medicine Animal Studies Committee.
2.2. Cell lines, plasmids, antibodies
Full length human CD28 cDNA (originally provided by C. Thompson MD, Memorial Sloan-Kettering Cancer Center, New York, New York) was cloned into the GFP-RV vector (provided by K. Murphy, Washington University School of Medicine, St Louis, MO) and single point mutations made using the Q5 Site Directed Mutagenesis Kit (New England Biolabs, Ipswich, MA) A CTLA-4-CD28 fusion protein was constructed consisting of the extracellular domain of murine CTLA-4 (amino acids 1-162) and the transmembrane and cytoplasmic domain of human CD28 (amino acids 152-219). All constructs were verified by direct sequencing. Chinese Hamster Ovary cells (CHO) expressing IAd (gift from Dr K. Murphy, Washington University School of Medicine, St Louis, MO) were stably transfected with plasmids expressing either human CD80 or CD86 (Sino Biologicals, China). PlatinumE (PlatE) packaging cell line and the retroviral packaging vector pCMV-10A1 were kindly provided by Dr. T. Egawa (Washington University School of Medicine, St Louis, MO).
Fluorescently labeled antibodies were purchased from Biolegend (San Diego, CA) unless otherwise stated. CTLA4Ig was purchased from BioXcell (West Lebanon, NH) and human CD80Ig and CD86Ig were purchased from ACRObiosystems (Newark, DE).
2.3. Retroviral transduction
PlatE cells were co-transfected with the pCMV-10A1 plasmid and GFP-RV encoding empty vector, wild-type or mutant CD28 as indicated. Culture supernatants were harvested 48 hours later and used to transduce splenocytes from CD28-deficient DO11.10 mice that had been activated with OVA peptide (0.3 μM) for 48 hours. The activated splenocytes were resuspended in the viral supernatant in the presence of polybrene (10μg/ml, Sigma Chemical, St Louis, MO) and centrifuged at 400XG for 1.5 hour at room temperature. The process was repeated on day 3 and the cells expanded in IL-2 for an additional 24 hours. Infection efficiency was assessed by flow cytometry and ranged from 30% to 50% between experiments, but was similar for each construct within a given experiment (data not shown).
2.4. Proliferation assay
Splenocytes (1×105/well) from CD28-deficient DO11.10 mice retrovirally transduced with either empty vector, wild type human CD28 or human CD28-F51I or CD28-F51V mutant, or the CTLA-4-CD28 chimeric constructs were cocultured in 96 well plates with irradiated (1000 R) or mitomycin C treated splenocytes (2×105/well) from wild type Balb/CbyJ mice then treated with either media or OVA(323–339) peptide at the indicated doses alone or in the presence of CTLA4Ig, anti-human CD28 antibody (1.0 μg/ml, clone CD37.51, BD Biosciences, San Jose, CA) or antibodies against CD80 or CD86 (10 μg/ml, clones 16-10A1 and GL-1, BioLegend, San Diego, CA). Cultures were pulsed with 1 μCi/well of tritiated thymidine for the final 10 hours of a 48 hour culture period and proliferation assessed by tritiated thymidine incorporation. For some experiments, CD4+ cells were first enriched by magnetic bead selection (Miltenyi Biotec, San Diego, CA) followed by flow cytometric sorting on GFP (Sony iCyt Synergy BSC, Sony Biotechnology, San Jose, CA). Purified T cells expressing control, wild type or mutant CD28 constructs were incubated in a 1:1 ratio with CHO cells expressing either IAd alone or co-expressing human CD80 or CD86. For stimulation with Ig fusion proteins, purified transduced T cells were stimulated with plate bound α-CD3 (.01 μg/ml) along with graded doses plate bound Ig fusion protein. The cultures were pulsed with tritiated thymidine for the final 10 hours of a 72 hour culture. All experiments were performed a minimum of 3 times and representative data presented. Proliferation data is expressed as the mean ± standard deviation of triplicate wells.
2.5. Binding assays
Splenocytes from CD28-deficient DO11.10 mice were retrovirally transduced and incubated in PBS 1% BSA alone at 4 °C or in the presence of graded doses of CD80Ig- or CD86Ig fusion proteins followed by staining with a fluorescently conjugated anti-Ig secondary antibody. For analysis, the GFP positive cells were gated on and the mean fluorescence index (MFI) of the Ig fusion protein staining determined.
2.6. Cytokine assays
Splenocytes from CD28-deficient DO11.10 mice were retrovirally transduced with empty vector, wild type or mutant CD28. The transduced cells were plated into a 96 well plate (1×105 cells/well) and stimulated with OVA peptide (0.3 μM) for 48 hours. Culture supernatants from triplicate wells were collected and cytokine concentrations determined using the Th1/Th2/Th17 Cytometric Bead Array (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Shown is the mean ± standard deviation of the triplicate samples. Data is representative of 3 independent experiments.
2.7. Statistics
All statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software Inc, La Jolla, CA). Unless otherwise indicated, the analysis performed was a 2 tailed unpaired T-test. Multiple comparison testing was performed when appropriate.
3. Results
3.1. CD28 mutations augment primary T cell proliferation and IL-2 secretion
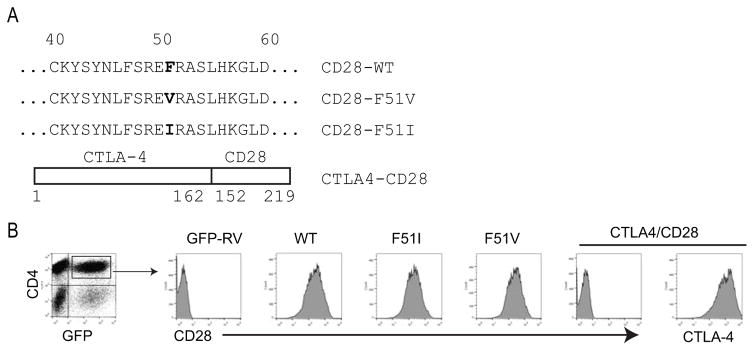
Retroviral gene transfer was used to express wild type or mutant CD28 receptors in primary, antigen specific T cells isolated from CD28-deficient, TCR transgenic mice. Point mutations at position 51 as well as a chimeric construct encoding the extracellular domain of CTLA-4 and the transmembrane and cytoplasmic domains of CD28 were examined as these had been reported in patients with CTCL and other T cell neoplasms [5, 7, 8, 10] (Figure 1A). Transduction efficiency was high, ranging between 30–50% between experiments and within a given experiment efficiency differed between constructs by no more than 5%. Staining with anti-CD28 or anti-CTLA-4 confirmed similar levels of expression for each construct (Figure 1B). As the level of expression of CD28 and CTLA-4 cannot be directly compared due to the different antibodies used, we compared the intensity of GFP expression. This was identical between all constructs further suggesting that expression levels of CD28 and CTLA-4 were similar between the CD28 and CTLA-4 encoding constructs (data not shown).
Figure 1. Sequence and expression of mutant CD28 proteins.
A) The amino acid sequences of wild type human CD28 and the CD28-F51V and –F51I mutations are shown, with the point mutations bolded. The CTLA4-CD28 chimeric construct consists of the extracellular domain of murine CTLA-4 (aa 1-162 of CTLA4) fused to the transmembrane and cytoplasmic domain of murine CD28 (aa 152-219 of CD28) Amino acid numbering includes the signal peptide. B) CD28-deficient splenocytes were transduced with either empty vector (GFP-RV), or constructs encoding wild type or mutant CD28 constructs and stained with fluorescently conjugated antibodies against CD4 and either CD28 or CTLA-4. CD4/GFP double positive cells were gated on and expression of CD28 or CTLA-4 shown. Representative data of > 5 independent experiments is shown.
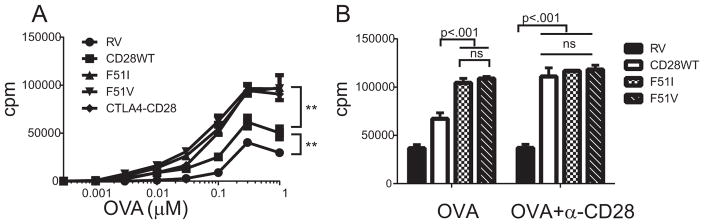
To test whether the mutations altered the T cell response to antigen stimulation, we co-cultured OVA specific T cells either lacking CD28 expression or expressing wild type CD28 or mutant forms of CD28 with autologous antigen presenting cells in the presence of cognate antigen (OVA peptide) and assayed proliferation, expression of activation markers and cytokine secretion. T cells expressing CD28- F51I, CD28-F51V or the CTLA-4-CD28 chimeric protein all proliferated significantly more than cells expressing wild type CD28 (Figure 2A). The maximal proliferative response was increased as well as the sensitivity of the cells to antigen, with the mutants responding to between a 5 and 10-fold lower dose of antigen than wild type cells. Under these experimental conditions, costimulation is being delivered by endogenous murine CD80 or CD86 expressed on APC in the culture. When costimulation was provided by α-CD28 antibody, there was no difference in proliferation between wild type and mutant cells (Figure 2B).
Figure 2. CD28 mutations augment T cell proliferation.
A) Splenocytes from CD28-deficient OVA TCR transgenic mice were transduced with either empty vector (RV), wild type or mutant CD28 constructs and stimulated with OVA peptide in the presence of autologous APC and proliferation determined by tritiated thymidine incorporation. Shown are the mean and standard deviation of triplicate wells. ** indicates p < .001 by 2-tailed unpaired T-test with adjustment for multiple comparisons for all 3 mutant constructs compared to WT CD28 and for WT CD28 as compared to RV at OVA doses from .03 μM to 1.0 μM OVA. B) Retrovirally transduced CD28-deficient splenocytes were stimulated with OVA alone (0.3 μM) alone or with the addition of anti-CD28 antibody (1.0 μg/ml), both in the presence of autologous APC and proliferation measured. All results shown are representative results from 3–5 independent experiments. Statistical significance was determined by a 2-tailed unpaired T-test with multiple comparisons.
Under the OVA plus APC conditions both mutant isoforms proliferated more than the wild type (figure 2A), whereas addition of the high affinity agonistic α-CD28 antibody augmented proliferation in cells expressing the wild type but not the mutant receptors (Figure 2B). This demonstrates that under the experimental conditions of OVA plus APC, costimulation through wild type CD28 was submaximal but that costimulation through the mutant CD28 was maximal as it was unable to be further augmented. This suggests that intrinsic differences in CD28 signaling by the mutant receptor are not the mechanism accounting for the differences in costimulation between wild type and mutant, as one would have predicted that the differences in proliferation would have persisted when antibody was added. An alternative explanation might be that the mutant CD28 is constitutively active, thus it would be irrelevant if the ligand is endogenous CD80 or CD86 or antibody. However, the subsequent data using CTLA4Ig and anti-CD80/CD86 antibodies demonstrate that in fact signaling through the mutant CD28 receptors remains ligand dependent. Together, these data suggest that signaling through CD28 is not intrinsically altered in the CD28 mutants and also confirms that any differences in proliferation are not due to differences in CD28 expression.
Malignant T cells from patients with CTCL have been shown to be skewed towards a Th2 phenotype [15], therefore we examined whether the mutant CD28 constructs differentially affected Th1 or Th2 cytokine secretion. The mutations led to a 2-fold increase in the amount of IL-2 in the culture supernatant as compared to wild type CD28 (Table 1). In contrast, there was no effect of the mutations on either the absolute amount of IL-4 or IL-10, or the ratio of IFNγ to IL-4 (expressed as Th1/Th2) suggesting that at least in primary cells, there was no effect on T cell differentiation. Similarly, there was no effect of the mutations on secretion of IL-6, IL-17A or TNFα (data not shown). We also examined expression of the activation markers CD69 and CD25. At each dose of antigen tested, the expression of each marker was greater on cells expressing the mutant CD28 as compared to wild type (supplemental figure 1)).
Table 1.
Stimulated cytokine secretion of CD28-deficient splenocytes retrovirally transduced with empty vector (RV), wild type or mutant CD28. All values in pg/ml
| RV | CD28WT | F51I | F51V | CTLA4-CD28 | |
|---|---|---|---|---|---|
| IL-2 | 326±52 | 760±19* | 1,522±104** | 1,643±116** | 1,894±205** |
| IFN-g | 1,006±125 | 1,130±217 | 1,403±263 | 1,358±347 | 1,400±52 |
| IL-4 | 3,384±189 | 3,484±211 | 3,574±100 | 3,547±301 | 3,452±91 |
| IL-10 | 3,160±521 | 4,078±700 | 4,374±746 | 4,286±737 | 4,176±740 |
| Th1/Th2 | 30% | 32% | 39% | 38% | 41% |
Splenocytes from CD28-deficient DO11.10 T cells were retrovirally transduced with empty vector, wild type or mutant CD28 and stimulated with OVA peptide. Culture supernatant was collected after 48 hours and cytokine levels assayed. P<.001 compared to RV, **p<.001 compared to CD28WT as determined by 2-tailed unpaired T testing.
3.2. CD86 preferentially binds and stimulates cells expressing mutant CD28
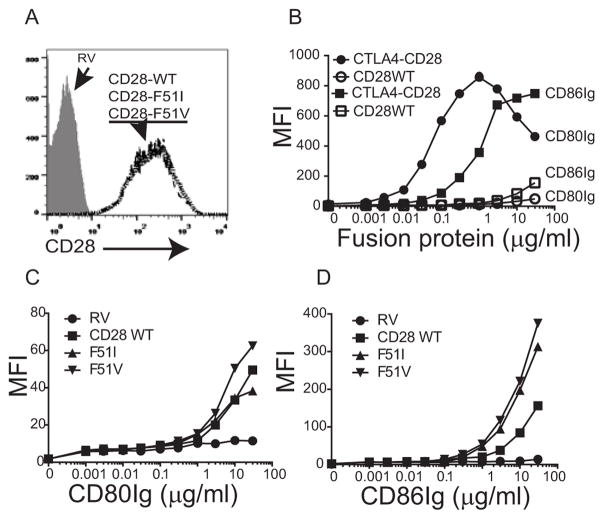
The augmented responses of the mutant isoforms of CD28 and the CTLA-4-CD28 chimera might be explained by increased binding affinity to CD80 and/or CD86 [16]. To test this, we incubated cells with either human CD80- or CD86-Ig fusion proteins at varying concentrations and measured binding by flow cytometry (Figure 3). CD28 expression was assessed by antibody staining and was identical for each construct (Figure 3A). The CTLA4-CD28 chimeric protein had substantially higher binding of CD80Ig and CD86Ig than did the wild-type or the CD28-F51I or -F51V mutant proteins (Figure 3B). Binding to CD80Ig was slightly increased in the CD28-F51V mutant compared to wild type at higher concentrations (figure 3C), however both had markedly increased binding to CD86Ig (figure 3D).
Figure 3. CD28 mutations enhance binding to CD80 and CD86.
A) Retrovirally transduced CD28-deficient splenocytes were stained with fluorescently conjugated anti-CD28 antibody and the GFP positive cells assessed for CD28 expression by flow cytometry. The histograms for WT and mutants are shown as overlays and are superimposable. B–D) CD28-deficient splenocytes were transduced with either empty vector (RV), wild type CD28, the CTLA4-CD28 chimera (panel B), or the CD28-F51I or F51V mutant constructs (panels C and D) and incubated with human CD80Ig or CD86Ig at the indicated concentrations followed by a fluorescently conjugated anti-Ig antibody. Binding was assessed by flow cytometry.
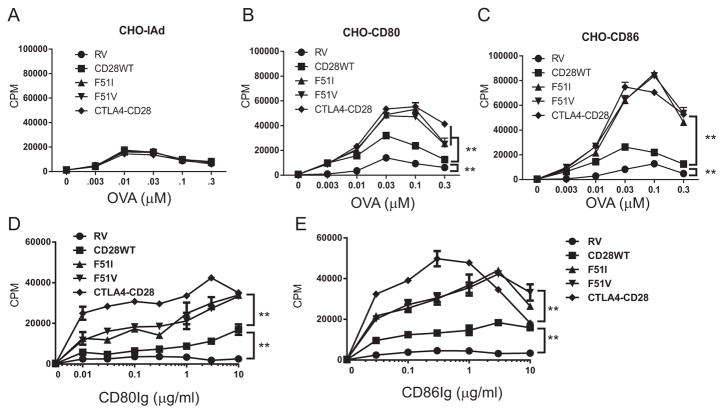
The previous experiments utilized autologous APC to present antigen which express not only CD80 and CD86, but also many other costimulatory and inhibitory proteins. To determine the effects of CD80 and CD86 in isolation, we cocultured purified T cells with CHO cells expressing IAd alone or with either human CD80 or CD86 as APC and activated the cells with OVA peptide. The expression of CD80 and CD86 was comparable between each CHO cell line, with the exception of CHO-IAd which expressed neither. IAd expression was identical between CHO cell lines (data not shown). Minimal proliferation was induced by OVA peptide in the absence of costimulation regardless of CD28 isoform expressed by the T cell (Figure 4A). In contrast, co-expression of CD80 or CD86 with IAd induced a strong proliferative response, which was augmented in cells expressing the mutant CD28 receptors as compared to wild type CD28 (Figure 4B and C).
Figure 4. CD28 mutations augment proliferation to costimulation mediated by CD86 engagement to a greater degree than CD80.
A–C) Purified CD28-deficient CD4+ T cells were retrovirally transduced with either empty vector (RV), CD28-F51I, CD28-F51V or the CTLA4-CD28 chimera and cocultured with CHO cells expressing either IAd alone (panel A), or coexpressing human CD80 (panel B) or human CD86 (panel C) and stimulated with OVA peptide at the indicated doses. D and E) Purified CD4+ cells as described above were stimulated with plate bound anti-CD3 (.01 μg/ml) alone or in combination with graded doses of plate bound human CD80Ig or CD86Ig and proliferation determined. For all panels, the data shown are the mean ± standard deviation of triplicate wells and are representative of 3–5 independent experiments. Statistical comparisons were made comparing the mutant constructs to the wild type construct, and the wild type to empty vector. **= p<.001 by 2 tailed, multiple comparison, unpaired T testing. Differences were significant at all doses.
To further exclude the participation of any other cell surface receptors, we stimulated purified T cells with a low dose of α-CD3 along with plate bound CD80Ig or CD86Ig (Figure 4D and E). Consistent with CHO cell data, both fusion proteins augmented proliferation in the mutant T cells. The response to CD86 was greater than that to CD80, although the magnitude of the difference was less than that observed when costimulation was provided by CHO.
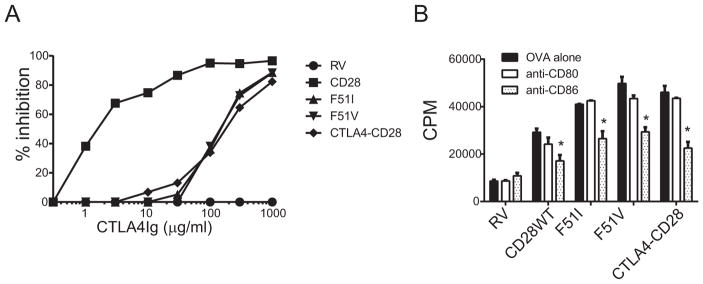
CTLA4Ig is a soluble fusion protein that binds both CD80 and CD86 and inhibits CD28-dependent T cell responses by preventing ligand engagement of CD28 [17]. It is currently marketed as an approved therapy for rheumatoid arthritis, and a related protein is approved for prevention of renal transplant rejection [18, 19]. As shown in figure 5A, proliferation of splenocytes expressing wild type or mutant CD28 was inhibited by CTLA4Ig, confirming that costimulation through the mutant CD28 isoforms was CD80- and/or CD86-dependent. However maximal inhibition of mutant T cells required a 30 fold higher concentration of CTLA4Ig than necessary to block wild type CD28, consistent with higher affinity binding of CD28 to ligand. Selective inhibition of either CD80 or CD86 using anti-blocking antibodies revealed partial inhibition with anti-CD86, but little to no effect with anti-CD80 (Figure 5B). Thus, under these conditions, the dominant ligand binding to CD28 and inducing costimulation appears to be CD86.
Figure 5. Inhibition of mutant CD28-mediated costimulation by blockade of CD80/CD86.
A) Retrovirally transduced CD28-deficient splenocytes were stimulated with OVA (0.03 μM) alone or in the presence of CTLA4Ig at doses ranging from 1–1000 μg/ml and proliferation determined. The percent inhibition at each dose of CTLA4Ig was calculated relative to proliferation in the absence of CTLA4Ig. Results shown are representative results from 3 independent experiments. B) Cells were stimulated as above with the addition of either anti-CD80 or anti-CD86 antibody (10 μg/ml), and proliferation measured. *= p <.05 by 2-tailed t-test adjusted for multiple comparisons, comparing anti-CD86 treatment to either OVA alone or OVA plus anti-CD80. No other comparisons were significantly different. Results shown are representative results from 3 independent experiments.
4. Discussion
The importance of CD28 in normal T cell function is well established, however, there has been little direct evidence of acquired mutations in CD28 leading to human disease. While polymorphisms in CD28 have been associated with autoimmune disease and it is likely these contribute to pathology, direct causation has not been established [2–4]. Interestingly, a significant proportion of patients (~ 10%) with CTCL, AITL, PTCL and ATL [5, 7, 10, 11, 20] have been shown to have acquired mutations in CD28, but there has been little additional analysis performed to determine whether CD28 plays a role in these or other malignancies. In this paper, we have provided evidence that the most frequent CD28 mutations in the extracellular domain of that were identified in patients with CTCL result in a gain of function phenotype that while not sufficient to transform the T cells, when present in combination with other oncogenic mutations have the potential to contribute to the disease pathology.
We performed a comprehensive analysis of three mutant human CD28 isoforms, described in CTCL as well as other T cell neoplasms, using retroviral reconstitution of primary murine CD28-deficient cells. By using this system we avoided a number of confounding variables that would be present in studying normal and/or malignant human T cells, including expression of endogenous human CD28 and the effect of co-existing oncogenic mutations. We found that the mutant isoforms increase binding to both CD80 and CD86 and that this resulted in significantly increased T cell proliferation and IL-2 secretion.
Although these mutations have previously been reported, there has been little if any functional analysis. In the initial paper identifying the presence of these mutations in CTCL, experiments performed in Jurkat cells also suggested that binding to CD86 and IL-2 production was increased [10]. However, there are several important limitations to that data. Jurkat cells express endogenous CD28 proteins making it impossible to cleanly separate effects of the transfected mutant CD28 isoform from the wild type. In addition, as CD28 is a homodimer the mutant protein may heterodimerize with the wild type protein further complicating the analysis. Due to a constitutive loss of PTEN, CD28 signaling is known to be defective in Jurkat cells. All of these problems confound interpretation of the data obtained in Jurkat [21, 22].
Recently, Rohr et al characterized the effects of 2 CD28 mutations, D124V and T195P, which have been described in patients with AITL [11]. The D124V mutation is in the extracellular domain and was shown by surface plasmon resonance to increase the affinity for CD86 but not CD80. Interestingly, induction of NFkB activity by the D124V mutant was increased compared to wild type when engaged by either CD80 or CD86. This is similar to our finding that binding to CD86 appears to be increased in the F51 mutants to a greater extent than CD80 binding, yet proliferation and IL-2 secretion are increased by both CD80 and CD86. Rohr also examined the consequences of the T195P mutation which effects the intracellular domain of CD28 and has also been reported by other groups [7, 8, 11, 20]. Using a cell free system they found that binding of GRB2 and GRAP2 to the T195P mutation was increased compared to wild type, consistent with an earlier report by Lee et al that also examined the T195P mutation [8].
CD28 activates a number of downstream signaling pathways that synergize with TCR signals to ultimately regulate T cell effector function [1]. While it is possible that mutations of the extracellular domain could alter properties of the signaling domain, the observation that the mutant receptors did not differ from wild type when CD28 was ligated by antibody makes this less likely. Furthermore, effector function remained dependent on ligand binding and there was no evidence of constitutive activation of CD28. Thus, although we did not directly examine downstream signaling intermediates, our studies did not suggest that these mutations altered intrinsic aspects of CD28 signal transduction. However, it is intriguing that mutations of either the intracellular domain or extracellular domain lead to similar effects on proliferation and cytokine secretion. This suggests that the end phenotype is a result of enhanced signaling which may be the result of either enhanced ligand binding or direct alterations in the intracellular signaling motifs. Our data together with the above described studies of additional CD28 mutations data imply that a gain of function in CD28, whether caused by changes in ligand binding or intracellular signaling, may be an important mechanism contributing to some T cell neoplasms.
Although there is some overlap in the CD28 mutations identified in CTCL and other T cell neoplasms, there also appears to be some distinctions in spectrum of mutations observed in AITL as opposed to CTCL. The reason for this is not clear, but may possibly relate to the pattern of DNA injury caused by the initial mutagenic stimulus, for example, UV light verses chemical carcinogen.
In the normal immune response, CD28 is most important in the initial activation of a naïve T cell. Retroviral infection of T cells requires that the cells are activated in order to be successfully infected, thus our model does not recapitulate the effects that might be seen in naïve T cells with complete accuracy. However, the mutations we are studying are acquired mutations identified in malignant cells, not germline mutations that would be expressed on naïve T cells. The finding that these mutations enhance the proliferative response of activated T cells supports that a similar effect might also be expected in cells from CTCL patients, in which the malignant T cells bear an activated phenotype [8, 10, 20].
Additional support that these mutations are important in the pathogenesis of CTCL and other T cell lymphomas will require detailed studies of cells isolated from patients with the disease. However, this analysis will be complicated by the fact that there is significant heterogeneity between patients and that the malignant T cells carry a number of additional mutations, making identification of appropriate controls and direct comparisons between patients difficult [5, 10, 11]. Thus, the work presented in this paper, while reductionist in nature, is a necessary step to understand the possible mechanisms by which these mutations contribute to disease and sets the stage for important future work.
One model of the disease pathogenesis is that in vivo, interactions with typically innocuous, low affinity self-antigen may provide a sufficient signal to the malignant cells to proliferate [23]. Expression of the mutant CD28 isoforms is likely to amplify that proliferative signal as well as lower the threshold at which the TCR-mediated signal becomes mitogenic, thus increasing overall tumor cell responsiveness to antigen and expansion. This in turn suggests a potential therapeutic strategy to interrupt this process. Abatacept and belatacept are approved drugs that bind CD80 and CD86, which would prevent interaction with either mutant or wild type CD28 [18, 19]. Alternatively, a reagent that preferentially binds the mutant CD28 in favor of the wild type without activating the receptor, such as a CD86Ig, may selectively inhibit interaction with the mutant isoform over wild type CD28, perhaps resulting in less immunosuppression than by strategies that globally block CD28 signaling.
Supplementary Material
Highlights.
Recurrent CD28 mutations identified in cutaneous T cell lymphoma increase binding to the ligands for CD28.
These mutations confer a gain of function phenotype, resulting in increased ligand dependent growth and IL-2 secretion
Interference with ligand binding may be a novel treatment strategy for patients with cutaneous T cell lymphoma expressing mutant CD28 isoforms.
Acknowledgments
This work was supported in part by grants from the NIH (HL 062683) awarded to JMG and the JC (K08 CA191019) and ACS-Institutional research Grant (IRG-15-173-21) awarded to JC, and NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center. We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Mo., for the use of the Siteman Flow Cytometry Core, which provided cell sorting service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boomer JS, Green JM. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2:a002436. doi: 10.1101/cshperspect.a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, Graham RR, Manoharan A, Ortmann W, Bhangale T, Denny JC, Carroll RJ, Eyler AE, Greenberg JD, Kremer JM, Pappas DA, Jiang L, Yin J, Ye L, Su DF, Yang J, Xie G, Keystone E, Westra HJ, Esko T, Metspalu A, Zhou X, Gupta N, Mirel D, Stahl EA, Diogo D, Cui J, Liao K, Guo MH, Myouzen K, Kawaguchi T, Coenen MJ, van Riel PL, van de Laar MA, Guchelaar HJ, Huizinga TW, Dieude P, Mariette X, Bridges SL, Jr, Zhernakova A, Toes RE, Tak PP, Miceli-Richard C, Bang SY, Lee HS, Martin J, Gonzalez-Gay MA, Rodriguez-Rodriguez L, Rantapaa-Dahlqvist S, Arlestig L, Choi HK, Kamatani Y, Galan P, Lathrop M, Eyre S, Bowes J, Barton A, de Vries N, Moreland LW, Criswell LA, Karlson EW, Taniguchi A, Yamada R, Kubo M, Liu JS, Bae SC, Worthington J, Padyukov L, Klareskog L, Gregersen PK, Raychaudhuri S, Stranger BE, De Jager PL, Franke L, Visscher PM, Brown MA, Yamanaka H, Mimori T, Takahashi A, Xu H, Behrens TW, Siminovitch KA, Momohara S, Matsuda F, Yamamoto K, Plenge RM R consortium, G consortium. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner M, Sobczynski M, Karabon L, Bilinska M, Pokryszko-Dragan A, Pawlak-Adamska E, Cyrul M, Kusnierczyk P, Jasek M. Polymorphisms in CD28, CTLA-4, CD80 and CD86 genes may influence the risk of multiple sclerosis and its age of onset. J Neuroimmunol. 2015;288:79–86. doi: 10.1016/j.jneuroim.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Pruul K, Kisand K, Alnek K, Metskula K, Reimand K, Heilman K, Peet A, Varik K, Peetsalu M, Einberg U, Tillmann V, Uibo R. Differences in B7 and CD28 family gene expression in the peripheral blood between newly diagnosed young-onset and adult-onset type 1 diabetes patients. Mol Cell Endocrinol. 2015;412:265–271. doi: 10.1016/j.mce.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka K, Nagata Y, Kitanaka A, Shiraishi Y, Shimamura T, Yasunaga J, Totoki Y, Chiba K, Sato-Otsubo A, Nagae G, Ishii R, Muto S, Kotani S, Watatani Y, Takeda J, Sanada M, Tanaka H, Suzuki H, Sato Y, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Iwanaga M, Ma G, Nosaka K, Hishizawa M, Itonaga H, Imaizumi Y, Munakata W, Ogasawara H, Sato T, Sasai K, Muramoto K, Penova M, Kawaguchi T, Nakamura H, Hama N, Shide K, Kubuki Y, Hidaka T, Kameda T, Nakamaki T, Ishiyama K, Miyawaki S, Yoon SS, Tobinai K, Miyazaki Y, Takaori-Kondo A, Matsuda F, Takeuchi K, Nureki O, Aburatani H, Watanabe T, Shibata T, Matsuoka M, Miyano S, Shimoda K, Ogawa S. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Ni X, Covington KR, Yang BY, Shiu J, Zhang X, Xi L, Meng Q, Langridge T, Drummond J, Donehower LA, Doddapaneni H, Muzny DM, Gibbs RA, Wheeler DA, Duvic M. Genomic profiling of Sezary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426–1434. doi: 10.1038/ng.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo HY, Kim P, Kim WS, Lee SH, Kim S, Kang SY, Jang HY, Lee JE, Kim J, Kim SJ, Ko YH, Lee S. Frequent CTLA4-CD28 gene fusion in diverse types of T-cell lymphoma. Haematologica. 2016;101:757–763. doi: 10.3324/haematol.2015.139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, Kim JS, Kim J, Kim SJ, Kim WS, Lee S, Ko YH, Yoo HY. A highly recurrent novel missense mutation in CD28 among angioimmunoblastic T-cell lymphoma patients. Haematologica. 2015;100:e505–507. doi: 10.3324/haematol.2015.133074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekulic A, Liang WS, Tembe W, Izatt T, Kruglyak S, Kiefer JA, Cuyugan L, Zismann V, Legendre C, Pittelkow MR, Gohmann JJ, De Castro FR, Trent J, Carpten J, Craig DW, McDaniel TK. Personalized treatment of Sezary syndrome by targeting a novel CTLA4:CD28 fusion. Molecular genetics & genomic medicine. 2015;3:130–136. doi: 10.1002/mgg3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Goh G, Walradt T, Hong BS, Bunick CG, Chen K, Bjornson RD, Maman Y, Wang T, Tordoff J, Carlson K, Overton JD, Liu KJ, Lewis JM, Devine L, Barbarotta L, Foss FM, Subtil A, Vonderheid EC, Edelson RL, Schatz DG, Boggon TJ, Girardi M, Lifton RP. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011–1019. doi: 10.1038/ng.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohr J, Guo S, Huo J, Bouska A, Lachel C, Li Y, Simone PD, Zhang W, Gong Q, Wang C, Cannon A, Heavican T, Mottok A, Hung S, Rosenwald A, Gascoyne R, Fu K, Greiner TC, Weisenburger DD, Vose JM, Staudt LM, Xiao W, Borgstahl GE, Davis S, Steidl C, McKeithan T, Iqbal J, Chan WC. Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. Leukemia. 2016;30:1062–1070. doi: 10.1038/leu.2015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229:307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 14.Burr JS, Savage NDL, Messah GE, Kimzey SL, Shaw AS, Arch RH, Green JM. Cutting Edge: Distinct Motifs Within CD28 Regulate T Cell Proliferation and Induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 15.Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, Fisher DC, Kupper TS, Clark RA. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19:3755–3763. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse B, Hunig T, Sorensen P, Stuart DI, Davis SJ. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- 17.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by the soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 18.El-Charabaty E, Geara AS, Ting C, El-Sayegh S, Azzi J. Belatacept: a new era of immunosuppression? Expert Rev Clin Immunol. 2012;8:527–536. doi: 10.1586/eci.12.42. [DOI] [PubMed] [Google Scholar]
- 19.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 20.Rohr J, Guo S, Hu D, Bouska A, Gascoyne RD, Rosenwald A, Simone P, Zhang W, Xiao W, Wang C, Fu K, Greiner TC, Weisenburger DD, Vose JM, Staudt LM, Berger F, Borgstahl G, Davis S, McKeithan T, Iqbal J, Chan WC. CD28 Mutations in Peripheral T-Cell Lymphomagenesis and Progression. Blood. 2014;124:1681–1681. [Google Scholar]
- 21.Shan X, Czar MJ, Bunnell SC, Liu P, Liu Y, Schwartzberg PL, Wange RL. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein PH, Fraser JD, Weiss A. The Cytoplasmic Domain of Cd28 Is Both Necessary and Sufficient for Costimulation of Interleukin-2 Secretion and Association with Phosphatidylinositol 3′-Kinase. Molecular and Cellular Biology. 1994;14:3392–3402. doi: 10.1128/mcb.14.5.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–1988. doi: 10.1056/NEJMra032810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.