Abstract
Citrobacter rodentium is a murine mucosal pathogen used as a model to elucidate the molecular and cellular pathogenesis of infection with two clinically important human gastrointestinal pathogens, enteropathogenic Escherichia coli (EPEC) and enterohaemorrhagic E. coli (EHEC). C. rodentium infection provides an excellent model to study different aspects of host-pathogen interaction in the gut, including intestinal inflammatory responses during bacteria-induced colitis, mucosal healing and epithelial repair, the induction of mucosal immune responses and the role of the intestinal microbiota in mediating resistance to colonization by enteric pathogens. In this unit, we provide detailed protocols to grow this bacterium, infect mice by intragastric inoculation, measure bacterial loads in feces and organs and monitor the intestinal pathology induced by this infection. Additional protocols describe steps needed to create frozen stocks, establish a growth curve of C. rodentium, perform ex vivo organ cultures, isolate immune cells from the large intestine and measure immune response by flow cytometry.
Keywords: Citrobacter rodentium, intestinal infection, colitis, mouse model
INTRODUCTION
Citrobacter rodentium is a Gram-negative enteric bacterium whose natural host is mice, where infection causes transmissible colonic hyperplasia and is responsible for high mortality in suckling mice (Barthold et al., 1978; Schauer and Falkow, 1993; Schauer et al., 1995). C. rodentium is a member of the attaching and effacing (A/E) family of bacterial pathogens, which are characterized by intimate adherence to host intestinal epithelial cells, effacement of the brush border microvilli, and reorganization of the host actin cytoskeleton to form pedestal-like extensions of epithelial cells beneath the adherent bacteria called A/E lesions (Collins et al., 2014; Wales et al., 2005). C. rodentium is the only known A/E pathogen to naturally infect mice and therefore is a valuable model organism for the study of pathogenesis of the clinically significant human pathogens: enteropathogenic Escherichia coli (EPEC), a leading cause of infantile diarrhea in developing countries, and enterohaemorrhagic E. coli (EHEC) that is responsible for kidney failure and highly prevalent in developed countries (Bhinder et al., 2013; Borenshtein et al., 2008; Luperchio et al., 2000; Luperchio and Schauer, 2001; Mallick et al., 2012).
C. rodentium infection-induced colitis is one of the rare models of infectious colitis that has been well characterized (Bhinder et al., 2013; Collins et al., 2014; Eckmann, 2006; Nell et al., 2010). Enteric bacteria have been linked to Inflammatory Bowel Diseases (IBD) consisting of Crohn’s disease and ulcerative colitis that are characterized by chronic intestinal inflammation. Recent studies have established a link between the development of chronic inflammatory responses present in patients genetically susceptible to IBD and abnormal exposure of the intestinal immune system to enteric bacteria (Belkaid and Hand, 2014; Ignacio et al., 2016; Silva et al., 2016). C. rodentium infection of mice has become an excellent tool for the analysis of host responses to enteric bacteria and further understanding of potential mechanisms of IBD pathogenesis, an essential step in developing novel preventative and therapeutic treatment strategies (Borenshtein et al., 2008; Eckmann, 2006; Higgins et al., 1999; Nell et al., 2010).
In this unit, we provide detailed protocols to establish a robust mouse model of infection with C. rodentium and characterize the colitis and immune responses induced by this infection. Basic Protocol 1 outlines the steps required to culture and prepare inoculum of the antibiotic-resistant strain of C. rodentium ICC169. The Alternate Protocol provides a method to grow the non-antibiotic resistant strain of C. rodentium DBS100. Support Protocols 1 and 2 describe methods to prepare frozen stocks used to start bacterial cultures and to create a growth curve for liquid culture of C. rodentium, respectively. Basic Protocol 2 outlines the procedures to perform intragastric infection of mice with C. rodentium by oral gavage. Basic Protocol 3 describes the steps required to measure the bacterial numbers in the feces and in different organs, while Basic Protocol 4 provides the methods used to assess histologically the inflammation in the large intestine following C. rodentium infection. Support Protocol 3 provides methods to perform ex vivo cultures of colonic tissue for quantification of secreted cytokines. Support Protocol 4 outlines the steps required to extract immune cells from the large intestine. Finally, Support Protocol 5 describes procedures used to stain cell surface and intracellular antigens to analyze immune responses to C. rodentium infection by flow cytometry. Additional parameters, such as measurement of colonic epithelial barrier permeability or RNA extraction and analysis of gene expression levels, can be assessed to characterize more thoroughly responses to C. rodentium infection. Methods to perform such analysis can be found in other excellent articles (Crepin et al., 2016; Koroleva et al., 2015).
Caution: Citrobacter rodentium is a Biosafety Level 2 (BSL-2) pathogen. All appropriate guidelines and regulations for use and handling of pathogenic microorganisms should be followed. All C. rodentium infections and housing of infected mice should be carried out in a Biosafety Level 2 animal facility.
Note: All protocols using live animals require pre-review and pre-approval by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for care and use of laboratory animals.
BASIC PROTOCOL 1 – PREPARATION OF Citrobacter rodentium INOCULUM
The proper cultivation of C. rodentium is an essential first step for any experiment using C. rodentium infection as a model of intestinal infection and infectious colitis in mice. It is essential that C. rodentium cultures be performed with standardized methods, using the same culture medium and cultural conditions, to ensure reproducibility of experiments and to be able to compare data obtained from different experiments. This protocol outlines the basic cultivation of C. rodentium and the preparation of the inoculum to infect mice.
Materials
Frozen stock of the nalidixic-acid-resistant strain ICC169 of Citrobacter rodentium (see Support Protocol 1 in this unit).
Luria-Bertani (LB) broth (see reagents and solutions).
LB agar plates (see reagents and solutions).
Nalidixic acid sodium salt (Sigma-Aldrich).
125 ml polycarbonate Erlenmeyer flasks for bacterial culture, sterile (VWR International).
17 × 100 mm 14 ml polystyrene round-bottom tube, sterile (Corning Falcon).
10 μl inoculation loops, sterile (VWR International).
Phosphate-buffered saline (PBS), sterile (Corning Cellgro).
37°C shaking incubator.
Spectrophotometer.
Disposable polymethyl methacrylate cuvettes for spectrophotometer (Brandtech Scientific).
Eppendorf 5810R refrigerated centrifuge (or equivalent).
Inoculum preparation
Take a frozen stock cryovial of C. rodentium strain ICC169 out of the −80°C freezer and place on dry ice to prevent excessive thawing.
Slightly thaw the frozen stock of C. rodentium at room temperature for about 30 seconds before gently scraping the surface of the stock with a sterile inoculation loop.
-
Using the technique described in Figure 1, spread the bacteria across an LB Agar plate supplemented with nalidixic acid (50 μg/ml). Incubate the plate overnight in a dry incubator at 37°C.
LB Agar plate should be warmed up to room temperature prior to spreading the bacteria.
-
The following morning, isolate a single nalidixic-acid resistant colony using a sterile inoculation loop. Use this colony to inoculate 25 ml of LB broth supplemented with nalidixic acid (50 μg/ml) in a sterile Erlenmeyer flask.
Plates with C. rodentium can be stored at 4°C up to 1 week.
A culture tube with 3 ml of sterile LB broth should also be cultured overnight to ensure that there is no bacterial contamination of the broth itself. The LB broth should appear clear after overnight culture.
-
Allow the bacteria to grow overnight at 37°C with shaking at 200 rpm in a benchtop incubation shaker.
C. rodentium should reach a stationary phase of growth overnight.
The following day, measure the optical density (OD) of the overnight culture at 600 nm with a spectrophotometer using a disposable plastic cuvette.
In Using the overnight culture material, inoculate a new batch of LB broth (supplemented with nalidixic acid (50ug/ml)) in a new Erlenmeyer flask or a 14-ml polystyrene round-bottom tube by diluting the overnight culture to an OD600 nm = 0.1. Incubate at 37°C with shaking at 200 rpm in a benchtop incubation shaker.
-
After 3–3.5 hours, check the OD600 nm of the culture.
The OD600 nm of the culture should be between 1 and 1.5. This corresponds to the logarithmic or exponential phase of bacterial growth in which cell numbers increase in a logarithmic fashion, and each cell generation occurs in the same interval as the preceding ones. during The number of colony-forming units (CFU) can be predicted accurately during the logarithmic phase.
Using a pre-established growth curve for liquid culture of C. rodentium (see Support Protocol 2), calculate the concentration of bacteria in CFU/ml of culture from the OD600 nm.
-
Calculate the desired number of CFU required to infect mice and transfer the corresponding volume of C. rodentium culture into a sterile centrifuge tube.
The standard dose used for C. rodentium via the intragastric route is 108 – 109 CFU per mouse, delivered in a volume of 0.2 ml.
Centrifuge the bacteria for 10 min at 3,000 × g and 4°C and discard supernatant.
Wash the bacteria in sterile PBS 10 min at 3,000 × g and 4°C. Discard supernatant.
Wash again as in step 12.
Re-suspend in the appropriate volume of sterile PBS for oral inoculation (gavage) so each mouse receives the desired dose of bacteria in a volume of 0.2 ml (see Basic Protocol 2). Keep C. rodentium suspension on ice until use. Aim to use inoculum immediately.
Figure 1.
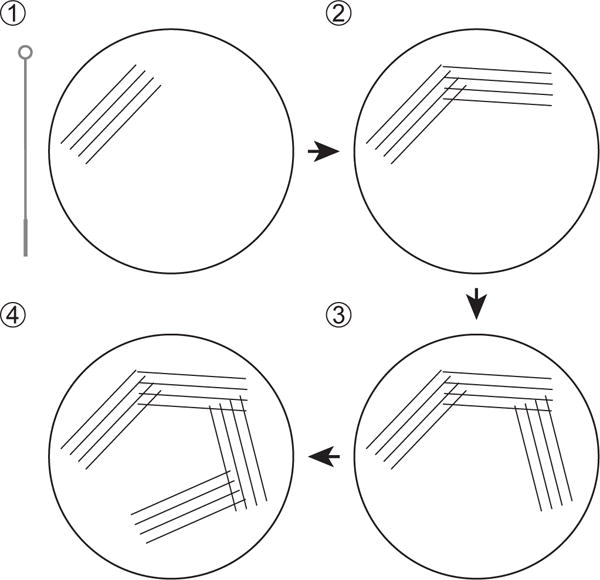
Streaking a frozen stock of C. rodentium on a LB or MacConkey agar plate for overnight culture.
ALTERNATE PROTOCOL – CULTIVATION OF NON-ANTIBIOTIC RESISTANT STRAIN OF Citrobacter rodentium
This alternate protocol is a slight variation of Basic Protocol 1, used to culture strain DBS100 of C. rodentium that is not resistant to any antibiotic.
Materials
Frozen stock of Citrobacter rodentium DBS100 (Schauer and Falkow, 1993) (see Support Protocol 1 in this unit).
MacConkey agar plates (see reagents and solutions).
Luria-Bertani (LB) broth (see reagents and solutions).
LB agar plates (see reagents and solutions).
Sterile 125 ml polycarbonate Erlenmeyer flasks for bacterial culture (VWR International).
Sterile 17 × 100 mm 14 ml polystyrene round-bottom tube (Corning Falcon).
Sterile 10 μl inoculation loops (VWR International).
Phosphate-buffered saline (PBS), sterile (Corning Cellgro).
37°C shaking incubator
Spectrophotometer
Disposable polymethyl methacrylate cuvettes for spectrophotometer (Brandtech Scientific).
Eppendorf 5810R refrigerated centrifuge (or equivalent).
Slightly thaw a frozen glycerol stock of C. rodentium DBS100 at room temperature for about 30 seconds. Gently scrape the surface of the stock with a sterile inoculation loop and streak a MacConkey agar plate as described in Figure 1. Incubate the plate overnight in a dry incubator at 37°C.
-
The following morning, inoculate 25 ml of LB broth in a sterile Erlenmeyer flask with a single C. rodentium colony from the plate using an inoculation loop. Allow the bacteria to grow overnight at 37°C with shaking at 200 rpm in a benchtop incubation shaker.
C. rodentium colonies are identified as pink colonies with narrow white trim on a MacConkey agar plates.
The following day, measure the optical density (OD) of the overnight culture at 600 nm with a spectrophotometer using a disposable plastic cuvette.
Using the overnight culture, start a fresh culture of C. rodentium DBS100 at an OD600 nm = 0.1 by inoculating some LB broth in an Erlenmeyer flask or a round-bottom tube. Incubate at 37°C with shaking at 200 rpm in a benchtop incubation shaker.
Proceed with steps 8–14 of Basic protocol 1 to prepare the inoculum of C. rodentium strain DBS100 that will be used to infect mice by oral gavage.
BASIC PROTOCOL 2 – INTRAGASTRIC INFECTION OF MICE
Citrobacter rodentium is transmitted via the fecal-oral route. Most infection studies involve the intragastric inoculation of mice by oral gavage with laboratory-cultured bacteria, which results in a highly reproducible infection cycle (Borenshtein et al., 2008; Luperchio and Schauer, 2001; Mundy et al., 2005). However, infection can also be performed by cohousing naïve mice with previously infected mice leading to the natural transmission of C. rodentium via coprophagy. This basic protocol details how to perform intragastric infection of mice with C. rodentium. For infection of mice by cohousing, refer to Crepin et al. (Crepin et al., 2016).
Materials
6- to 8-week-old sex-matched mice.
Bacterial inoculum (see Basic Protocol 1).
Phosphate-buffered saline (PBS), sterile (Corning Cellgro).
Housing cages for the mice.
Personal protective equipment (e.g., laboratory coat, gloves, and eye protection).
1 ml syringe, sterile (Covidien Monoject).
Sterile reusable stainless animal feeding needle, 22-G × 1 in., straight, with a 1.25 mm-diameter bulbous tip (Braintree Scientific).
Additional reagents and equipment for the anesthesia of rodents (see Unit 1.4).
Prepare animals
-
1
Acclimate 6- to 8-week-old sex-matched mice to the animal facility environment for at least 1 week prior to infection.
Animals should be acclimated under the standard lighting and temperature conditions in individually HEPA-filtered cages with sterile bedding and nesting and free access to food and water provided ad libitum.
-
2
Ear tag or tattoo the mice for identification.
-
3
The day prior to infection, weigh the mice and record their initial weight.
-
4
Fast mice (giving only water) for 8–12 hours before infection.
Fasting is performed to avoid the delivery of the inoculum into a full stomach (which could lead to aspiration of the inoculum in the lung) and to decrease the variability between animals.
-
5
Just before infection, fit the animal feeding needle on the syringe and fill the syringe with 0.2 ml of bacterial inoculum prepared as described in Basic Protocol 1.
To ensure accuracy of the volume of inoculum injected, get rid of any air bubbles that could be present in the syringe and wipe the outside of the animal feeding needle with an alcohol swab and a paper towel after each syringe filling to eliminate any excess liquid that could be present on the outside of the needle. In addition, gently agitate the culture within the tube prior to loading the syringe for gavage so that an equal dose of bacteria is delivered to each mouse in step 11. The group of mock-infected animals should be inoculated with 0.2 ml of sterile PBS.
Perform inoculation
-
6
Remove the mouse from the cage by its tail.
Sedation or anesthesia for intragastric inoculation is unnecessary for trained and experienced personnel. However, at this point, mice can be lightly anesthetized before gavage if needed, using the standard anesthetic for laboratory animals, isoflurane, delivered to effect in concentrations up to 5% for initial induction.
-
7
Scruff the mouse by holding its tail and allowing it to grasp the stainless wire bar cage top with its forelegs. Pull gently on the tail with one hand, and grasp the mouse firmly by the scruff at the base of the neck with the other hand, using thumb and index, so that the forelegs are extended out to the side, preventing the front feet from pushing the gavage needle away.
-
8
Lift the mouse from the cage top and secure the tail between the palm and little finger of the hand holding the mouse, freeing the other hand for gavage.
-
9
Gently pull the head back in order to extend the mouse’s neck and align the oral cavity and pharynx with the esophagus.
-
10
Maintain the mouse in an upright position and direct the bulbous tip of the feeding needle along the side of its mouth and over its tongue. Gently pass the needle along the roof of the mouth and into the esophagus and stomach.
If there is any resistance felt during this procedure, remove and then re-insert the feeding needle. and re-insert it.
-
11
Slowly inject the inoculum (in 0.2 ml) and carefully withdraw the needle after completing the injection, following the same angle as used for insertion.
-
12
Observe the mouse for regular breathing pattern for 1 min and return the animal to its cage. Proceed with infection of additional mice. When all inoculations are completed, check the mice for normal behavior, looking for any sign of labored breathing or distress; and return them to their housing location with food if this is not present. Euthanize mice with labored breathing or distress.
If mice were anesthetized, make sure to monitor them for full recovery from the anesthesia.
-
13
Clean the work area with appropriate disinfectant. Discard the syringe and disinfect the feeding needle with ethanol 70% prior to re-sterilization.
After oral inoculation, mice are monitored daily for determination of their weight or euthanized and dissected at different time points for determination of bacterial counts from organs (see Basic Protocol 3), histological assessment of intestinal inflammation (see Basic Protocol 4), or isolation of lymphoid cells from the large intestine and analysis of immune response by flow cytometry (see Support Protocols 4 and 5).
-
14
Optional: after inoculation of the mice, count the number of live bacteria within the inoculum by plating 1:10 dilution of the excess inoculum on LB Agar plates supplemented with nalidixic acid (50 μg/ml in water). Incubate for 12–24 hours and count CFU to determine viable bacteria concentration in the inoculum.
BASIC PROTOCOL 3 – Organ recovery and bacterial enumeration
After oral inoculation, C. rodentium typically colonizes the caecum during the first day of infection, followed by progression to the distal colon (Collins et al., 2013; Wiles et al., 2004). In immunocompromised mice, the bacteria can disseminate systemically and can be detected in peripheral organs, such as the spleen and the liver (Collins et al., 2014; Simmons et al., 2003; Vallance et al., 2002). Enumeration of C. rodentium over the course of the infection is performed by plating viable bacteria from stool samples and/or from excised tissues collected at various time points after infection.
This protocol describes the procedures for harvesting stool samples and mouse organs to be analyzed after infection by C. rodentium (i.e the caecum, the colon, the mesenteric lymph nodes, the spleen, and the liver), and for bacterial enumeration after homogenization of the feces or organ homogenization.
Caution: All harvesting of tissues derived from C. rodentium-infected animals must be done in an appropriate laboratory equipped with a Class II biohazard hood.
Important note: The success of this protocol is dependent on proper use of aseptic techniques.
Materials
C. rodentium-infected sex-matched mice (see Basic Protocol 2).
5% (v/v) Lyzol
70% (v/v) ethanol in a spray bottle.
Phosphate-buffered saline (PBS), sterile (Corning Cellgro).
LB agar plates, sterile (see reagents and solutions).
Nalidixic acid sodium salt (Sigma-Aldrich).
2 ml Safe-Lock microcentrifuge tubes, sterile (Eppendorf).
100 × 15 mm Petri dishes, sterile (VWR International).
Sterile 2 ml or 4.5 ml homogenization tubes containing sterile 1.4 mm ceramic beads (MP Biomedicals Lysing Matrix D).
FastPrep® Tissue homogenizer (MP Biomedicals).
Personal protective equipment (disposable laboratory coat, gloves and eyes protection).
Sterile dissection instruments: dissecting pins, dressing forceps (curved and narrow at the tip), iris scissors (straight and curved), and tweezers (curved and thin at the tip).
Dissection cutting board, sterile.
Additional reagents and equipment for the euthanasia of rodents (see Unit 1.8).
Measurement of bacterial load in the feces
-
1
Label and pre-weigh 2 ml sterile centrifuge tubes.
-
2
Collect fresh stool samples (1–2 fecal pellets) from each infected animal daily or at chosen time points after bacterial inoculation. Place the tube on ice until ready to proceed with step 3.
-
3
Weigh the tubes containing the fecal pellets and calculate the weight of the stools.
-
4
Add the appropriate volume of sterile PBS in each tube so the stools are re-suspended at a ratio of 0.1 g of stool per 1 ml of PBS.
-
5
Allow the stools to soften in PBS for 15 min. Disrupt the fecal pellet by shaking or vortexing.
Mashing with the end tip of a 200 μl sterile pipet tip in addition of shaking or vortexing can also help disperse the stools.
-
6
Centrifuge each tube for 1 min at 400 × g and collect supernatant.
-
7
Perform serial dilutions of the supernatant in sterile PBS and plate each dilution on LB agar plates supplemented with nalidixic acid (50 μg/ml). Incubate for 18–24 hours at 37°C.
For the serial dilution, dilute 10 μl of the supernatant in 990 μl of PBS up to 10−8 dilution. Plate 100 μl of each dilution in duplicate.
-
8Count the number of colonies and calculate the bacterial load within the feces as colony forming units (CFU)/g of stool using the following formula:
Where CC is the mean colony count, VT is the total volume of the plated sample, VP is the volume plated, DF is the dilution factor of the plated sample for which colony are counted, and W is the weight of the stool sample.
Measurement of bacterial load in tissues
-
9
Prepare in advance sterile homogenization tubes to collect the different organs. Label the tubes and weigh them after adding appropriate volume of sterile PBS.
Caecum, colon, mesenteric lymph nodes and spleen are homogenized in 2 ml tubes with 1 ml of PBS. Liver is homogenized in a 4.5 ml tube with 2–3 ml of PBS.
-
10
Disinfect the work surfaces of a Class II biosafety cabinet using 5% Lysol followed by 70% ethanol. On a sterile surgical field, prepare a cleaned dissection board previously disinfected with 70% ethanol and all sterile dissection instruments under the Class II biohazard cabinet.
-
11
Euthanize the infected mouse according to guidelines approved by your Institutional Animal Care and Use Committee using CO2 inhalation followed by cervical dislocation according to guidelines approved by your Institutional Animal Care and Use Committee. Carefully check that the mouse is dead.
-
12
In a Class II biohazard hood, pin the mouse on to a dissection board with the abdomen facing up. Wet the fur of the chest and abdomen by spraying 70% ethanol to reduce the possibility of contamination by hair.
-
13
With sterilized forceps, elevate the skin above the urethral opening and cut along the ventral midline from the groin to the sternum using fine point sharp straight scissors. Gently peel the skin back from the peritoneal wall underneath using forceps.
-
14
Using another pair of sterilized scissors and forceps, carefully open up the abdomen by cutting through the peritoneal wall.
-
15
If required for measurement of bacterial load, carefully remove the spleen, located behind the stomach, and the whole liver and place them in two distinct homogenization tubes on ice.
-
16
Fold back the caecum to expose the mesenteric lymph nodes (MLN) located along the blood vessels in the intestinal mesentery, the fatty tissue that attaches the small intestine to the posterior wall of the abdomen. Grasp the lymph nodes with curved tweezers and pull them free of the mesentery. Ensure complete removal of associated adipose tissue and place the MLNs in a 2 ml homogenization tube on ice.
-
17
Identify the bottom end of the small intestine and cut through the small intestine at the junction with the caecum. Carefully and slowly draw the large intestine (caecum and colon) out of the peritoneal cavity using forceps and tweezers. Make sure to dissect away any associated adipose tissue. Cut the large intestine at the lowest extent of the distal colon near the rectum.
-
18
Separate the caecum from the colon. Gently push the stools out of the colon using forceps. Cut the caecum and the colon open longitudinally and remove the luminal content by washing the tissue in sterile PBS in a Petri dish. Place the rinsed caecum and colon in two distinct 2 ml homogenization tubes.
Stools and luminal content can be collected in sterile tubes to enumerate bacterial load. Proceed as described above for measurement of bacterial load in the feces.
Scissors should be thoroughly cleaned with 70% ethanol between cutting open the caecum and the colon to avoid cross-contamination of the two tissues.
-
19
Dispose of carcasses according to appropriate guidelines and regulations in place in your institution for the disposal of mice infected with a Biosafety Level 2 (BSL-2) pathogen.
-
20
Weigh the tubes containing tissue and calculate the weight of tissue for each tube.
-
21
Homogenize the samples using MP Biomedicals FastPrep® tissue homegenizer for 40 seconds at a speed setting of 6.
Be careful that each tube is closed tightly before performing homogenization. Make sure each sample is uniformly homogenized before proceeding to step 22.
-
22
Determine the number of viable bacteria released from the organs by performing serial 1:10 dilutions of the tissue homogenates in sterile PBS (final volume = 1 ml).
The number of dilutions depends on the expected bacterial loads within the organs, the time points post-infection, as well as the susceptibility of the mouse strain to infection. In general, up to 10−8 dilutions should be plated for colon homogenates. Early after infection, colon homogenates should be diluted up to 105 times, whereas they should be plated undiluted and diluted up to 103 times at later time points. Homogenates from the spleen, the liver and the MLNs should be plated undiluted.
-
23
Apply 0.1 ml of each dilution in duplicate on LB agar plates nalidixic acid (50 μg/ml). Incubate for 18–48 hours at 37°C.
-
24
After incubation, count the number of colonies and calculate the bacterial load in each organ as colony forming units (CFU)/gram of tissue (use formula similar to the one described in step 8).
Organs may not be uniformly colonized by C. rodentium. It is therefore recommended that bacterial load measurement be performed on whole organs or that the same portion of an organ is always collected for this analysis.
BASIC PROTOCOL 4 – HISTOLOGICAL ASSESSMENT OF Citrobacter rodentium-MEDIATED INTESTINAL INFLAMMATION
Following infection with C. rodentium, mice develop colitis, including crypt hyperplasia, immune cell infiltration and goblet cell depletion. Susceptibility to infection and disease progression/severity can be monitored by evaluating the histological damages in the large intestine at different stages of infection. This Basic Protocol describes the methods required to perform histological assessment of intestinal inflammation mediated by C. rodentium.
Materials
C. rodentium-infected sex-matched mice (see Basic Protocol 2).
70% ethanol in a spray bottle.
Phosphate-buffered saline (PBS), sterile (Corning Cellgro).
Petri dishes (VWR International)
Whatman paper (GE Healthcare).
10% (v/v) neutral-buffered formalin (Sigma-Aldrich).
Hematoxylin solution (Mayer’s; Sigma-Aldrich).
Eosin Y solution (Sigma-Aldrich).
Tissue-processing histology cassettes (VWR International).
Histology pencil (VWR International).
Personal protective equipment (disposable laboratory coat, gloves and eyes protection).
Dissecting instruments (see Basic Protocol 3).
Dissection cutting board.
Additional reagents and equipment for the euthanasia of rodents (see Unit 1.8) and optional for paraffin embedding of tissues, sectioning of paraffin-embedded tissues, and hematoxylin/eosin staining of tissue sections (see Unit 21.4).
At critical time points after infection (day 4, 7, 14 and 21 after intragastric gavage for C57BL/6 mice) or upon passing a preset morbidity/weight loss criterion, euthanize the mice using an approved humane killing method such as CO2 inhalation followed by cervical dislocation. Carefully check that the mouse is dead.
Wet the fur with 70% ethanol. Open the abdomen and dissect out the entire large intestine (caecum + colon) as described in Basic Protocol 3. Place lengthwise on a paper towel moistened with PBS. Dissect away attached mesentery and adipose tissue. Separate the caecum from the colon using sharp scissors and place them in Petri dish containing PBS.
Cut the caecum longitudinally on its internal side and fold open to form a triangular shape.
Gently wash the caecum in PBS to remove caecal content and then carefully dab dry on a paper towel.
-
Place the caecal tissue, luminal side facing up, onto Whatman paper and carefully stretch in order to lay flat in contact with Whatman paper. Place mounted caecum into histology cassette.
This step should be performed with particular care, in order to not damage the tissue, nor to overstretch it as such action will distort tissue histology.
-
Using a scalpel blade, cut a small portion (1 cm) of proximal and distal colon, keeping the colonic tube intact. Gently push the luminal content out of the colon pieces using the back of the curved tips of forceps. Transfer tissue pieces to a histology cassette.
To obtain comparable data, always collect the same portion of the colon for histopathological analysis.
-
Assign each sample a code and label using a histology pencil. Fix in 10% (v/v) neutral-buffered formalin for at least 24 hours.
Samples can be stored in buffered formalin for up to two weeks prior to processing.
-
Paraffin embed samples and cut two to three 4–5 μm sections per samples. Mount on a microscope slide and stain with hematoxylin and eosin.
Paraffin embedding, histological processing and staining can be performed in-house (see Unit 21.4) or samples can be sent to a histology lab.
-
Analyze stained section by light microscopy to determine the extent of intestinal inflammation according to the semi-quantitative scoring scheme described in Table 1.
Slides should be assessed in a blinded fashion (i.e. by somebody who does not know the identity of the sample) and who is familiar with the normal and diseased architecture of the colon.
For each category described in Table 1 (epithelium, inflammation in lamina propria, area affected, and markers of severe inflammation), a score should be given on a scale from 0 to 3, resulting in a total score for each tissue section between 0 and 12. For each mouse, scores from sections of proximal colon, distal colon and caecum should be averaged to give a mean overall intestinal inflammation score. Differences between groups should be analyzed using a nonparametric statistical test (e.g. Mann-Whitney U test, also called Wilcoxon rank-sum test).
Table 1.
Semi-quantitative scoring scheme used to assess histopathological changes in the large intestine after C. rodentium infection.
| A – Epithelium | |||
|---|---|---|---|
|
| |||
| Score | Hyperplasia | Goblet cell depletion | |
| 0 | None | None | |
| 1 | Mild (1.5 × normal length) | AND/OR | Mild (25%) |
| 2 | Moderate (2–3 × normal length) | Moderate (25–50%) | |
| 3 | Severe (>3 × normal length) | Severe (>50%) | |
| B – Lamina propria inflammation | |
|---|---|
|
| |
| Score | Description |
| 0 | None – few leukocytes |
| 1 | Mild – some leukocytes at villi tips OR many lymphoid follicles |
| 2 | Moderate – marked infiltrate (crypt broadening) |
| 3 | Severe – dense leukocyte infiltrate |
| C – Area affected (% of section) | |
|---|---|
|
| |
| Score | Description |
| 0 | None |
| 1 | 0–25% |
| 2 | 25–50% |
| 3 | >50% |
| D – Severe markers | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Score | Submucosal inflammation | Crypt abscesses | Crypt branching | Ulceration or Extensive fibrosis | ||
| 0 | None | 0 | No | No | ||
| 1 | Mild | OR | <5 | No | No | |
| 2 | Mild | AND | <5 | No | No | |
| 2 | Severe | OR | >5 | OR | Yes | No |
| 3 | Severe | AND | >5 | OR | Yes | No |
| 3 | – | – | – | Yes | ||
SUPPORT PROTOCOL 1 – PREPARATION OF Citrobacter rodentium BACTERIAL STOCKS
This support protocol describes how to prepare frozen bacterial stocks of C. rodentium.
Materials
Citrobacter rodentium [nalidixic-acid-resistant strain ICC169 or strain DBS100 (ATCC 51549)].
Luria-Bertani (LB) broth (see reagents and solutions).
Luria-Bertani (LB) agar plates (see reagents and solutions).
McConkey agar plates (see reagents and solutions).
Glycerol, sterile (Sigma-Aldrich).
125 ml polycarbonate Erlenmeyer flasks for bacterial culture, sterile (VWR International).
14 ml polystyrene round-bottom tube, sterile (Corning Falcon).
10 μl inoculation loops, sterile (VWR International).
50 ml conical tubes, sterile (e.g. Greiner).
1.5 ml cryovial, sterile (e.g. Corning).
Eppendorf 5810R refrigerated centrifuge (or equivalent).
Grow the bacteria at 37°C overnight on a MacConkey agar plate (for strain DBS100) or a LB agar plate supplemented with nalidixic acid (for strain ICC169).
The following morning, pick a single C. rodentium colony on the plate and inoculate 50 ml of LB broth (supplemented with nalidixic acid for strain ICC169) in an Erlenmeyer flask using an inoculation loop. Allow the bacteria to grow overnight at 37°C with shaking at 200 rpm in a benchtop incubation shaker.
The following day, measure the OD600 nm to check that the bacteria grew efficiently and determine the concentration in CFU/ml.
Transfer the bacterial culture in a 50 ml conical tube and centrifuge 10 min at 3,000 × g and 4°C.
-
Aspirate the supernatant. Prepare frozen stocks by re-suspending the pellet in 15% (v/v) glycerol in LB broth and distributing the bacterial suspension in 1.5 ml sterile cryovials. Store for years at −80°C or in liquid nitrogen.
Alternatively, frozen stocks can be generated by dispersing single colonies from an overnight cultured plate (see Step 1) in a Microbank vial (Pro-Lab Diagnostics) as described by the manufacturer. Cultures can then be started from a single frozen microbank bead.
SUPPORT PROTOCOL 2 – CREATING A GROWTH CURVE FOR LIQUID CULTURE OF Citrobacter rodentium
The first step in creating a growth curve for liquid culture of C. rodentium is to inoculate a flask containing LB broth with a single bacterial colony picked up on a LB agar plate cultured overnight (see Basic Protocol 1). The flask is placed in a benchtop incubation shaker at 37°C with shaking at 200 rpm. Then, at regular intervals (at least every 30–60 min), the OD600 must be determined using an aliquot of the liquid culture. This aliquot also needs to be serially diluted and inoculated onto a fresh series of LB agar plates. The plates are then incubated at 37°C for 18–24 hours or until individual colonies are visible. If no colonies can be seen, the plates are returned to the incubator. If colonies are visible, they must be counted. This is repeated for every time point on the growth curve. Establishing a growth curve is a time-consuming and labor-intensive process. Therefore, one should take great care in doing it and think twice before altering culture conditions in a way that would require creating a new growth curve.
SUPPORT PROTOCOL 3 – EX VIVO ORGAN CULTURES FOR QUANTIFICATION OF SECRETED CYTOKINES
The ex vivo organ culture allows investigation of biological processes in the context of the intact tissue architecture. This support protocol describes a method of ex vivo culture of the colon, which can be used to analyze the immune response after infection with C. rodentium.
Materials
C. rodentium-infected sex-matched mice (see Basic Protocol 2).
70% ethanol in a spray bottle.
Phosphate-buffered saline (PBS), sterile (Corning Cellgro).
100 × 15 mm Petri dishes, sterile (VWR International).
48-well plate, polystyrene, sterile (Corning Costar).
1.5 ml Safe-Lock microcentrifuge tubes (Eppendorf).
Complete media (see reagents and solutions).
Thermostat-controlled cell culture incubator large enough to contain stir plate, 5% CO2 and 37°C.
Personal protective equipment (disposable laboratory coat, gloves and eyes protection).
Dissecting instruments (see Basic Protocol 3).
Dissection cutting board.
Additional reagents and equipment for the euthanasia of rodents (see Unit 1.8)
Euthanize the mouse with CO2 asphyxiation followed by cervical dislocation. As described in Basic Protocol 3, remove the large intestine from the abdominal cavity using pre-sterilized dissecting scissors and forceps.
Using scissors, collect a portion (~1–2 cm) of the terminal colon and cut it open longitudinally. Wash the tissue in a Petri dish with sterile PBS to eliminate any feces and fecal material.
Rinse with additional sterile PBS in a new Petri dish and weigh the tissue.
Place the tissue in 400 μl of complete media in a 48-well plate.
Incubate for 16–24 hours in a cell culture incubator at 37°C, 5% CO2.
-
Collect the media from each well into sterile 1.5 ml tubes. Centrifuge the tubes at 12,000 × g, 4°C for 5 min.
Colon pieces can be collected in place in 1.5 ml RNase/DNase-free tubes containing RNAlater for subsequent RNA extraction using commercial TRIzol reagent. RNA can then be used to measure expression by RT-PCR of targeted genes of interest (such as antimicrobial genes, cytokines, chemokines).
Collect the supernatant into fresh 1.5 tubes and store at −20°C or −80°C until performing analysis of cytokine levels by ELISA according to the manufacturer’s instructions.
SUPPORT PROTOCOL 4 – ISOLATION OF IMMUNE CELLS FROM THE LARGE INTESTINE
This support protocol described the procedure to isolate intraepithelial lymphocytes and lamina propria lymphocytes from the large intestine. In this procedure, intraepithelial lymphocytes are first isolated from the epithelial layer of the large intestine prior to enzymatic digestion of the tissue used to release the lymphocytes from the lamina propria.
Materials
Naïve and C. rodentium-infected sex-matched mice (see Basic Protocol 2).
70% ethanol in a spray bottle.
Phosphate-buffered saline (PBS), sterile (Corning Cellgro).
Harvest media (see reagents and solutions).
Strip media (see reagents and solutions).
Shake media (see reagents and solutions).
Digest media (see reagents and solutions).
Complete media (see reagents and solutions).
37.5% Percoll (see reagents and solutions).
100 × 15 mm Petri dishes, sterile (VWR International).
15 ml and 50 ml polypropylene centrifuge tubes, sterile (Greiner).
125 ml glass Erlenmeyer flask (VWR International).
3 × 12 mm magnetic stirring bars, sterile (VWR International).
Multi-position magnetic stirring plate (VWR International).
50 ml and 600 ml glass beaker (VWR International).
10 cm diameter fine mesh stainless steel strainers.
70 μm and 40 μm cell strainers, sterile (Corning Falcon).
1 ml syringe, sterile (Covidien Monoject).
Thermostat-controlled cell culture incubator large enough to contain stir plate, 5% CO2 and 37°C.
Eppendorf 5810R refrigerated centrifuge (or equivalent).
Personal protective equipment (disposable laboratory coat, gloves and eyes protection).
Dissecting instruments (see Basic Protocol 3).
Dissection cutting board.
Additional reagents and equipment for the euthanasia of rodents (see Unit 1.8)
Isolation of colonic intraepithelial lymphocytes
-
1
Euthanize the mouse according to guidelines approved by your Institutional Animal Care and Use Committee using CO2 asphyxiation followed by cervical dislocation. Perform a midline incision, retract the skin. Open the abdominal cavity and dissect out the entire large intestine (caecum + colon) as described in Basic Protocol 3. Place the large intestine in a Petri dish containing harvest media on ice.
Care should be taken to remove all adipose tissue associated with the large intestine.
-
2
Using fine sharp straight scissors with a blunt end on one point, insert the blunt end into the large intestine and cut the large intestine open longitudinally. Remove caecal and colonic fecal material by grasping the large intestine with forceps and shaking it vigorously in the harvest media.
-
3
Transfer the tissue to a new Petri dish and wash the tissue in PBS warmed up at room temperature to remove any remaining caecal and colonic contents.
-
4
Carefully dab dry the cleaned large intestine on paper towels. Laterally cut the opened large intestine into ~2 cm pieces. Transfer the pieces in a 125-ml glass Erlenmeyer flask containing 20 ml of strip media. Add a magnetic stir bar and cover the flask with aluminum foil.
-
5
Place the Erlenmeyer flask on a multi-position stirring plate placed inside a cell culture incubator (5% CO2 and 37°C). Stir at ~250 rpm for 25 min.
-
6
During incubation period, prepare 600 ml glass beaker with 20 ml of harvest media on ice. Place a kitchen strainer on top of each beaker. Label 50 ml centrifuge tubes (2 for each large intestine).
-
7
After the incubation period, take the Erlenmeyer flasks out of the incubator. Pour off the content of each Erlenmeyer flask into a 600-ml beaker through a kitchen strainer. Remove the magnetic stir bar and keep aside for use in step 16.
-
8
Tap the kitchen strainer on the beaker to remove any remaining media. Transfer the tissue pieces into a 50 ml centrifuge tube containing 10 ml of shake media. Close the lid of the tube tightly and shake vigorously for ~30 seconds. Carefully open the tubes and strain its content on the kitchen strainer. Repeat the straining-tapping-shaking process a total of 3 times.
-
9
Wash intestinal pieces on the kitchen strainer with some PBS and tap the kitchen strainer to remove excess of PBS.
At this stage, perform steps 15 onwards to isolate lymphocytes from the lamina propria using the intestinal pieces. To finish the isolation of the intraepithelial lymphocytes, proceed with step 10 onwards.
-
10
Transfer the content of the 600-ml beaker by pipetting through a 70 μm cell strainer into 50 ml centrifuge tubes. Centrifuge cell suspension at 450 × g, 4°C for 5 min.
-
11
Aspirate supernatant and re-suspend the pellet in 4 ml of 37.5% Percoll warmed to room temperature. Transfer the cell suspension to a 15 ml centrifuge tubes. Centrifuge at 650 × g, room temperature for 18 min.
Make sure acceleration is minimal and centrifuge brake is turned off.
-
12
Aspirate the top layer of cells and the Percoll solution being careful to avoid disrupting the pellet.
Intraepithelial lymphocytes are in the pellet at the bottom of the tube. Dead cells and some epithelial cells are in the cell layer at the top of the tube.
-
13
Rinse the pellet with 10 ml of cold harvesting media and centrifuge at 450 × g, 4°C for 5 min.
-
14
Discard the supernatant and re-suspend the intraepithelial lymphocytes in 0.5–1 ml of complete media.
Count the viable cells using trypan blue dye exclusion with a hematocytometer and a microscope (Appendix 3B). Keep cells on ice until further use.
Isolation of colonic lamina propria lymphocytes
-
15
Transfer the intestinal pieces from step 9 from the kitchen strainer to a 50-ml glass beaker containing 1 ml of digest media. Using sharp fine tip scissors, mince the intestinal pieces until they measure ~3 × 3 mm.
-
16
Rinse the scissors above the beaker using an additional 4 ml of digest media. Add the magnetic stirring bar put aside in step 7 into the beaker and cover it with aluminum foil. Place the beaker on a multi-position stirring plate placed inside a cell culture incubator (5% CO2 and 37°C). Stir at ~250 rpm for 25 min.
-
17
During incubation period, label and prepare 50 ml centrifuge tubes (one for each large intestine) on ice. Place a 70 μm cell strainer on top of each tube.
-
18
Following incubation period, take the beakers out of the incubator and place them on ice. Add 20 ml of harvest media into each beaker to stop the digestion.
-
19
Pass the digested intestine/digest media through a 70-μm cell strainer into a 50-ml centrifuge tube on ice. Use the rubber end of a 1 ml syringe plunger to lightly mash the remaining tissue.
-
20
Rinse the 50-ml beaker with 10 ml of harvest media and wash through the cell strainer.
-
21
Centrifuge cell suspension at 450 × g, 4°C for 5 min.
-
22
Discard the supernatant and re-suspend the pellet in 10 ml of cold harvest media. Pipet the cell suspension and filter through a 40-μm cell strainer into the same centrifuge tube. Rinse the cell strainer with an additional 10 ml of cold harvest media.
-
23
Centrifuge cell suspension at 450 × g, 4°C for 5 min.
-
24
Aspirate the supernatant and re-suspend the cells in 0.5–1 ml of cold complete media.
Count the viable cells using trypan blue dye exclusion with a hematocytometer and a microscope (Appendix 3B). Keep cells on ice until further use.
SUPPORT PROTOCOL 5 – IMMUNOPHENOTYPING OF LYMPHOID CELLS IN THE LARGE INTESTINE BY FLOW CYTOMETRY
The immune responses to C. rodentium infection can be evaluated through analysis of the different cell-surface expression of markers and the intracellular staining of cytokines by flow cytometry. This support protocol describes the main steps involved in performing staining of cell surface and intracellular antigens by flow cytometry. For more detailed protocol, refer to Unit 5.3.
Materials
Single-cell suspension of intraepithelial lymphocytes or lamina propria lymphocytes from the large intestine (see Support Protocol 4).
Complete media (see reagents and solutions).
Ionomycin (Sigma-Aldrich).
Phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich).
Brefeldin A (GolgiPlug™; BD Biosciences).
Hanks’ Balanced Salt Solution (HBSS), 4°C (Corning Cellgro).
Unlabeled and fluorochrome-labeled antibodies (see Table 2).
Fluorescent fixable viability dye (Molecular Probes).
Fixation/Permeabilization solution, 4°C (eBioscience or BD Biosciences).
Permabilization solution, 4°C (eBioscience or BD Biosciences).
96-well round-bottom microtiter plates.
Multi-channel pipette.
Eppendorf 5810R refrigerated centrifuge (or equivalent).
Table 2.
Antibodies typically used to assess intestinal immune response by flow cytometry following C. rodentium infection.
| Antigen | Antibody clone | Antigen | Antibody clone |
|---|---|---|---|
| CD4 | RM4-5 | CD335 (NKp46) | 29A1.4 |
| CD8α | 53.6.7 | F4/80 | BM8 |
| CD8β | eBioH35-17.2 | Foxp3 | FJK-16s |
| CD11b | M1/70 | Gr1 | RB6-8C5 |
| CD11c | N418 | IFN-γ | XMG1-2 |
| CD16/32 (FcBlock) | 93 | IL-17A | eBio17B7 or TC11-18H10.1 |
| CD19 | 6D5 or eBio1D3 | IL-22 | 1H8PWSR or IL22JOP |
| CD44 | IM7 | Ly6G | 1A8 |
| CD45 | 30-F11 | RORγ(t) | B2D |
| CD45R (B220) | RA3-6B2 | TCRβ | H57-597 |
| CD90.2 (Thy1.2) | 53-2.1 | γδTCR | eBioGL3 |
| CD127 (IL-7R) | A7R34 | Ter119 | TER-119 |
| CD196 (CCR6) | 29-2L17 | TNF-α | MP6-XT22 |
In vitro re-stimulation
-
1
Transfer 2–4 × 106 live intraepithelial lymphocytes or lamina propria lymphocytes from the large intestine (obtained as described in Support Protocol 4) in each well of a 96-well round-bottom microtiter plate. Cover the plate with a lid and centrifuge at 450 × g, 4°C for 5 min.
-
2
Discard the supernatant by inverting the plate above a waste tray and re-suspend cell pellets in 200 μl of complete media supplemented with 50 ng/ml of PMA, 5 μg/ml of ionomycin, and brefeldin A (GolgiPlug™) diluted per manufacturer’s recommendations). Cover the plate with a lid and incubate for 2.5 hours in a cell culture incubator (5% CO2, 37°C).
-
3
After incubation period, centrifuge the plate at 450 × g, 4°C for 5 min.
Immunofluorescence staining of cell surface antigens
-
4
Wash cells by adding 200 μl of ice cold HBSS. Centrifuge plate at 450 × g, 4°C for 5 min.
-
5
Repeat wash step 4 two times.
-
6
During centrifugation, prepare staining cocktail by mixing appropriate dilution of each antibody against cell surface antigens of interest (see Table 2) in ice cold HBSS.
Surface staining cocktail should contain FcR blocking reagent (anti-CD16/32) and fluorescent fixable viability dye to exclude dead cells during flow cytometry analysis. The appropriate dilution for each antibody should be determined by titration before using the antibody (see Unit 5.4).
-
7
Discard the supernatant by inverting plate above a waste tray and add 100 μl of surface staining cocktail into each well of the 96-well microtiter plate. Re-suspend cells by mixing gently with a multi-channel pipette. Wrap plate in aluminum foil to protect from light exposure and incubate for 20 min on ice.
-
8
Wash cells by adding 200 μl of ice cold HBSS. Centrifuge plate at 450 × g, 4°C for 5 min.
-
9
Repeat step 8 two times.
Immunofluorescence staining of intracellular antigens (cytokines, transcription factors)
-
10
Fix and permeabilize cells by adding 100 μl of ice cold fixation/permeabilization solution into each well. Re-suspend the cells by mixing gently with a multi-channel pipette. Wrap plate in aluminum foil to protect from light exposure and incubate for 30 min on ice.
-
11
Wash cells by adding 200 μl of ice cold permeabilization solution. Centrifuge plate at 450 × g, 4°C for 5 min.
-
12
Repeat wash step 11 two times.
-
13
During centrifugation, prepare intracellular staining cocktail by mixing appropriate dilution of each antibody against intracellular antigens of interest (see Table 2) in ice cold permeabilization solution.
Intracellular staining cocktail should contain FcR blocking reagent (anti-CD16/32).
-
14
Discard the supernatant by inverting plate above a waste tray and add 100 μl of intracellular staining cocktail into each well of the 96-well microtiter plate. Re-suspend cells by mixing gently with a multi-channel pipette. Incubate for 1 hour on ice, protected from exposure to light.
-
15
Wash the cells by adding 200 μl of ice cold permeabilization solution. Centrifuge plate at 450 × g, 4°C for 5 min.
-
16
Repeat step 15 two times.
-
17
Re-suspend cells in 200 μl of ice cold HBSS. Keep samples at 4°C, protected from light, until flow cytometric analysis.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. Media should be prepared aseptically to ensure sterility.
Luria-Bertani (LB) broth
20 g dehydrated Luria Bertani broth Miller (Sigma-Aldrich)
1 L deionized, distilled water
To prepare broth, weigh the LB broth powder in a large Erlenmeyer glass flask; stir and dissolve on hot plate until just boiling (make sure to watch carefully). Autoclave on liquid cycle at 121°C for 15 min. Allow to cool down before storing at 4°C for up to 1 month. Supplement LB broth with required amount of nalidixic acid sodium salt diluted in sterile water just before culture set up.
Luria-Bertani (LB) agar plates
20 g dehydrated Luria Bertani broth Miller (Sigma-Aldrich)
15 g bacteriological agar (Sigma-Aldrich)
1 L deionized, distilled water
Nalidixic acid sodium salt 50 mg/ml in water (Sigma-Aldrich)
To prepare plates, mix the ingredients above in a large Erlenmeyer glass flask; stir and dissolve on hot plate until just boiling (make sure to watch carefully). Autoclave on liquid cycle at 121°C for 15 min and allow to cool down to 45–50°C before aseptically adding nalidixic acid sodium salt at a final concentration of 50 μg/ml. Mix and dispense quickly in a biosafety cabinet into sterile Petri dishes (about 20–25 ml for a 100 × 15 mm plate). Allow the plate to solidify with the lids half-way off, then dry thoroughly, until no visible moisture remains on the surface of the agar. Place the plates in a plastic sleeve and seal. Store upside down up to 1 month at 4°C. Make sure to warm up and dry the plates again before use.
MacConkey agar plates
50.03 g dehydrated MacConkey agar (HiMedia)
1 L deionized, distilled water.
To prepare plates, weigh the MacConkey agar powder in a large Erlenmeyer glass flask; stir and dissolve on hot plate until just boiling (make sure to watch carefully). Autoclave on liquid cycle at 121°C for 15 min. Allow to cool down before storing at 4°C for up to 1 month.
Harvest media
454.5 ml of RPMI 1640
15 ml of fetal bovine serum, heat-inactivated (3% FBS)
10 ml of HEPES 1M
5 ml of L-glutamine 200 mM
5 ml of penicillin/streptomycin (5,000 IU/5,000 μg/ml)
5 ml of sodium pyruvate 100 mM
5 ml of non-essential amino acids 100X
0.5 ml β-mercaptoethanol 55 mM
Store at 4°C.
Strip media (20 ml/large intestine)
20 ml of Harvest media
0.2 ml of EDTA 0.5M
0.145 mg DL-dithiothreitol (Sigma-Aldrich)
Prepare fresh.
Shake media (30 ml/large intestine)
500 ml of RPMI 1640.
10 ml of HEPES 1M.
5 ml of L-glutamine 200 mM
5 ml of penicillin/streptomycin (5,000 IU/5,000 μg/ml)
5 ml of sodium pyruvate 100 mM
5 ml of non-essential amino acids 100X.
0.5 ml β-mercaptoethanol 55 mM
2 ml of EDTA 0.5M
Store at 4°C.
Digest media (5 ml/large intestine)
93.9 ml of RPMI 1640
2 ml of HEPES 1M
1 ml of L-glutamine 200 mM
1 ml of penicillin/streptomycin (5,000 IU/5,000 μg/ml)
1 ml of sodium pyruvate 100 mM
1 ml of non-essential amino acids 100X
0.1 ml β-mercaptoethanol 55 mM
0.1 mg/ml Liberase™ TL research grade (Roche Diagnostics)
0.5 mg/ml DNase I (Sigma-Aldrich)
Prepare fresh.
Complete media
419.5 ml of RPMI 1640
50 ml of fetal bovine serum, heat-inactivated (10% FBS)
10 ml of HEPES 1M
5 ml of L-glutamine 200 mM
5 ml of penicillin/streptomycin (5,000 IU/5,000 μg/ml)
5 ml of sodium pyruvate 100 mM
5 ml of non-essential amino acids 100X
0.5 ml β-mercaptoethanol 55 mM
Store at 4°C.
37.5% Percoll (4 ml/large intestine)
Prepare a 1X stock solution by mixing 90 ml Percoll (GE Healthcare) with 10 ml of 10X PBS. Keep sterile at room temperature. Prepare 37.5% fresh solution by mixing 37.5 ml of 1X Percoll stock and 62.5 ml of RPMI 1640.
COMMENTARY
Background information
Infections with gastrointestinal bacteria such as enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) are a major cause of morbidity and mortality worldwide (Croxen et al., 2013). EPEC is an important cause of infantile diarrhea, that leads to high rates of morbidity and mortality in developing countries (Croxen et al., 2013; Kaper et al., 2004; Nataro and Kaper, 1998). EHEC, in particular the serotype O157:H7 which express the highly potent Shiga toxin, is highly prevalent in developed countries and causes bloody diarrhea and sudden kidney failure known as hemolytic uremic syndrome (Croxen et al., 2013; Kaper et al., 2004; Nataro and Kaper, 1998). Our understanding of the pathogenesis of EPEC and EHEC in vivo has been hampered for a long time, as mice are naturally resistant to infection with those bacteria (Mundy et al., 2006; Mundy et al., 2005). Citrobacter rodentium is a natural mouse Gram-negative enteric pathogen which is similar to EPEC and EHEC in that it utilizes attaching and effacing (A/E) lesions in the intestinal epithelium to colonize the gastrointestinal tract of the host (Collins et al., 2014; Wales et al., 2005). A/E lesions are characterized by the tight and intimate attachment of the bacteria to the plasma membrane of the host epithelial cells, the localized effacement of the brush border microvilli, and the formation of pedestal-like structures underneath the adherent bacterium, all three phenomena leading to the remodeling of the intestinal epithelium. C. rodentium shares with both EPEC and EHEC several of the key virulence genes involved in the strategies used by those mucosal pathogens to colonize the gastrointestinal tract and induce mucosal pathogenesis. In particular, those three pathogens carry the locus for enterocyte effacement, a highly-conserved pathogenicity island, which is required for the development of A/E lesions (Collins et al., 2014). Therefore, C. rodentium infection of mice has become the gold standard model for investigating the mechanisms involved in the pathogenesis of attaching and effacing bacteria (Borenshtein et al., 2008; Luperchio et al., 2000; Luperchio and Schauer, 2001). Studies of the regulation of virulence genes in the C. rodentium mouse model have opened the possibility to test new hypothesis regarding the regulation of virulence gene in EPEC and EHEC, and are likely to provide better understanding of the molecular pathogenesis of those important human pathogens.
Following infection with C. rodentium, mice develop mild colitis accompanied by mild diarrhea. The hallmark pathological feature of the intestinal inflammation observed during C. rodentium-induced colitis is transmissible murine crypt hyperplasia, which is defined macroscopically by a thickening of the colonic mucosa and histologically by a marked elongation of the colonic crypt (hyperplasia), caused by an excessive induction of epithelial regeneration and repair mechanisms (Luperchio and Schauer, 2001; Mundy et al., 2005; Schauer and Falkow, 1993). Transmissible murine crypt hyperplasia typically lasts 2–3 weeks in C57BL/6 mice that are resistant to C. rodentium, and follows a biphasic response with progressive and regressive phases (Collins et al., 2014; Luperchio and Schauer, 2001). During the progressive phase, the loss of epithelial barrier integrity results in the transit of bacteria into the colonic lamina propria and induction of inflammation. The regressive phase involves C. rodentium clearance, resolution of colitis and inflammation and return to a normal intestinal homeostasis. The epithelial regenerative and repair pathways associated with transmissible murine crypt hyperplasia after C. rodentium infection are similar to the pathology and cellular signaling events observed in human inflammatory bowel diseases (IBD), such as ulcerative colitis and Crohn’s disease (Higgins et al., 1999). Thus, C. rodentium-induced transmissible murine crypt hyperplasia is a suitable model for the study of certain aspects of such diseases (Eckmann, 2006; Higgins et al., 1999).
C. rodentium infection and the subsequent colitis cause a pronounced dysbiosis resulting from the remarkable ability of this bacterium to colonize the colon. C. rodentium levels can reach up to 3% of the total intestinal microbiota (109 colony forming units per gram in the colon) and this overgrowth results in major alterations to the composition, structure and diversity of the intestinal microbiota (Hoffmann et al., 2009; Lupp et al., 2007). Interestingly, recent studies have shown that the composition of the intestinal microbiota influences the susceptibility to C. rodentium (Ivanov et al., 2009; Willing et al., 2011). As dysregulated immune response to the microbiota can contribute to the pathogenesis of IBD (Belkaid and Hand, 2014), infection of mice with C. rodentium can help elucidate several of the potential mechanisms of IBD pathogenesis. In addition, it provides a natural relevant model to investigate under physiological conditions the mechanisms involved in intestinal inflammation and mucosal healing, epithelial cell physiology and regulation of commensal microbiota (Bhinder et al., 2013; Borenshtein et al., 2008; Eckmann, 2006; Higgins et al., 1999; Nell et al., 2010).
C. rodentium infection has also proven to be an ideal murine model to investigate pathogen-host immune interactions in the gut and in particular mucosal immune responses to enteric infection. As a non-invasive microorganism in immunocompetent mice, C. rodentium allows the study of how the host recognized and eliminates pathogens in the intestinal lumen, while distinguishing such pathogens from the normal flora. Studies in mice with targeted deletions of the immune system have provided significant advances in understanding the particular arms of the immune response involved both in the immune response and the pathology after C. rodentium infection. Both the innate and adaptive immune responses play a critical role in protection against this bacterium (for a review of the immune components involved in protection against C. rodentium, see (Collins et al., 2014; Koroleva et al., 2015)). In recent years, the use of C. rodentium infection has been at the heart of the discovery and functional characterization of new immune cell subsets in mice, such as group 3 innate lymphoid cells (ILC3), Th17 and Th22 lymphocytes, innate γδ T cells, and IgA+ plasma cells (Basu et al., 2012; Cella et al., 2009; Fritz et al., 2011; Geddes et al., 2011; Ivanov et al., 2009; Sonnenberg et al., 2011; Tumanov et al., 2011). In particular, C. rodentium has been key to unravelling the function of Th17 cells, a type of CD4+ T helper cell producing IL-17 and playing a critical role in mucosal immune defenses (Basu et al., 2012; Geddes et al., 2011). Th17 cells, but also T helper cells secreting IL-22 (Th22), play a central role in driving host resistance to C. rodentium infection via the production of IL-17 which recruits neutrophils, and the anti-inflammatory cytokine IL22 which upregulates the expression of anti-microbial peptides by colonic epithelial cells and promote epithelial barrier integrity (Ishigame et al., 2009; Zheng et al., 2008). Both IL-17 and IL-22 have been shown to be crucial for the complete resolution of C. rodentium infection (Basu et al., 2012; Collins et al., 2014; Geddes et al., 2011; Ishigame et al., 2009; Torchinsky et al., 2009; Zheng et al., 2008). In addition to Th17 and Th22 cells, ILC3 innate lymphoid cells also are an important source of IL-17 and IL22, particularly in the caecum early after infection, and have been shown to contribute to the clearance of C. rodentium infection (Geddes et al., 2011).
Critical parameters and Troubleshooting
Several steps described in this unit are critical when working with the mouse model of infection with C. rodentium. Mice should be allowed to recover from the stress of transport and to acclimatize to their new environment for at least a week before experiments are carried out. As much as possible, animals of the same sex and age/weight must always be used to ensure experiment homogeneity and reproducibility. Even though the C. rodentium model is highly reproducible, robust power calculation should be made to determine the minimal number of mice per experimental group for each experiment. Mice inoculated with PBS and therefore not infected should always be used as controls. As the intestinal microbiota has recently been shown to influence the immune response and susceptibility to C. rodentium (Ivanov et al., 2009; Willing et al., 2011), this factor should be taken into account when changing the commercial supplier of mice or when working with mice from other research institutes. It is also recommended to co-house animals for at least a week when performing any experiment comparing the susceptibility of different mouse strain, in order to equilibrate their intestinal flora. Finally, animals should be monitored closely to determine a humane end-point for the experiment when infecting a new strain for the first time.
The infection of mice with C. rodentium via the intragastric route requires as a prerequisite the preparation of a fresh bacterial culture. If the culture is left in the shaker or on the bench top at room temperature for extended periods of time, there is a chance that not enough viable bacteria remains in the culture to induce a proper infection in mice. Intragastric inoculation (oral gavage) is a relatively simple and quick procedure allowing the administration of a specific volume of an agent only to the GI tract. However, it should only be performed by trained and experienced personnel. The most common technical problems related to this procedure are perforation of esophagus or stomach during gavage or accidental delivery of fluid into the lungs. Several technical precautions have been recommended in this unit to reduce these risks. These include wiping the outside of the feeding needle with an alcohol swab after each syringe filling and pulling back slightly on the syringe plunger before withdrawing the gavage needle from the animal. In addition, if any resistance is felt while inserting the gavage needle into the esophagus, it is recommended to remove the needle and try to re-insert it. The mice should be closely monitored for 1 to 2 min immediately after inoculation by oral gavage for their general appearance and activity. Animals displaying labored breathing or other signs of respiratory distress should be euthanized immediately as this will reflect the inadvertent introduction of bacterial suspension into the trachea and the lungs. The presence of any trace of blood on the gavage needle should also be a reason for the immediate euthanasia of the animals. To ensure accuracy of the dose of bacteria delivered to each animal, one should make sure to properly agitate the bacterial suspension before loading the syringe for infection and to get rid of any air bubble present in the syringe.
Following infection with C. rodentium, daily animal observation is a necessary part of animal husbandry. Information such as general behavior, feeding habits, appearance of stools and daily weights can provide useful information for evaluating the disease progression. Signs of illness as well as mortality rate and survival time should also be recorded. Disease progression and susceptibility to infection can also be monitored by measuring bacterial loads in the feces and different organs, as well as by evaluating intestinal inflammation at different time point after infection. Feces should be collected fresh. To avoid contamination and accurate measurement of bacterial loads in tissue, it is crucial to use aseptic techniques for the recovery of organs and their homogenization, as well as during the dilution and plating of organ homogenates. When using a strain of C. rodentium that does not possess resistance to any antibiotic, plating of fecal suspensions or tissue homogenates on LB agar plates is not an entirely selective method to determine bacterial loads, as other bacteria can grow and give rise to colonies with similar appearance than C. rodentium colonies. PCR for a C. rodentium-specific gene on bacterial colonies can then be perform to confirm that colonies growing on LB agar plates are C. rodentium. Alternatively, one can plate organs homogenates on MacConkey agar, as this medium is more selective for C. rodentium than LB agar. C. rodentium colonies will also specifically appear as pink colonies with narrow white trim on MacConkey agar plates.
Additionally, when collecting tissues for histopathological analysis, one should make sure that they are properly and entirely submerged in 10% neutral-buffered formalin for at least 24 hours to ensure proper fixation. Scoring of intestinal pathology should be performed in a blinded fashion by a person familiar with the normal and inflamed architecture of the colon, in order to avoid any bias and ensure proper evaluation. Several parameters can be assessed using the caecum and colon from an individual mouse to characterize thoroughly the responses to C. rodentium infection. In such experiments, it is recommended that the experimenter always collects the same portion of an organ for the same analysis between animals and experiments (see Figure 2).
Figure 2.
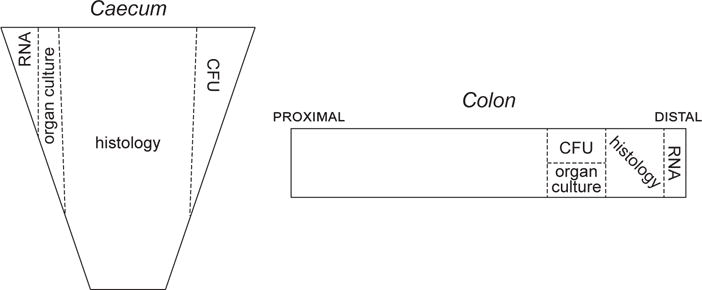
Diagram illustrating the collection of tissue samples from the caecum and the colon to perform various analysis: CFU measurement, histological assessment, ex vivo organ culture, RNA extraction.
Anticipated results
During a standard infection experiment, mice are typically infected with 108–109 CFU of C. rodentium by oral gavage. Susceptibility to C. rodentium infection is influenced by the genetic background of the host. In immunocompetent mice, such as C57BL/6, infection results in only mild disease, characterized by a modest and transient weight loss and diarrhea due to only minimal inflammation and tissue damage in the large intestine. Although a rare occurrence, C57BL/6 may however become ill and require to be euthanized. In contrast, various genetically modified mice display severe diarrhea and colon inflammation and ultimately succumb to infection (for a list of susceptible mice, see Table 1 in (Koroleva et al., 2015)). It is therefore important to monitor the degree of weight loss and symptoms of distress such as ruffled fur and hunched posture, to determine the extent to which different strains of mice are affected by the infection. Initially after oral inoculation, C. rodentium is mainly detected in the cecum, particularly the cecal patch. From day 3–4 post infection, C. rodentium migrates to the distal colon where it undergoes rapid expansion. In C57BL/6 mice, the infection plateaus at day 7 post infection and bacterial loads in the colon are generally measured to peak around 109 CFU/g one week after infection. By day 10 post infection, the infection starts to clear and complete clearance of the pathogen in the stool occurs 2–3 weeks post infection. Mouse strains sensitive to infection may display similar bacterial titers in the colon or the feces during the first 10 days of infection when compared to C57BL/6 mice. However, the kinetics of bacterial clearance from the colon at later time points (after day 12) can be a valuable parameter to determine the susceptibility of mice to infection. In addition, in the absence of proper protective immune mechanisms, C. rodentium disseminates systemically and can be detected in the blood and peripheral organs, such as the mesenteric lymph nodes, the liver and the spleen, whereas C57BL/6 mice typically do not display detectable levels of C. rodentium outside of the colon.
C. rodentium infection induces colitis characterized macroscopically by a shrunken caecum, a shortening in length of the colon and thickening of the mucosa. On histopathology, C. rodentium-associated colonic inflammation is defined by immune cell infiltration, goblet cell depletion, and a profound crypt hyperplasia (elongation of colonic crypts). In C57BL/6 mice, most of the intestinal pathology observed during C. rodentium infection occurs in the distal 2 cm of the colon. Signs of colitis, in particular colonic crypt hyperplasia, are first observed around day 6 post infection and typically last 2–3 weeks. At the point of peak hyperplastic responses, the bacteria can no longer be isolated from the colon. Two months after infection, lesions have resolved and colonic mucosa appears normal.
C. rodentium infection also triggers robust inflammatory responses that involve release of multiple cytokines, antimicrobial peptides, innate lymphoid cells, neutrophils, macrophages, B cells and T cells. Neutrophils are first recruited in the colon around day 4 post infection, when C. rodentium starts colonizing this tissue, whereas macrophages and dendritic cells are first infiltrating the colon on day 8 post infection. The numbers of those three populations of immune cells peak around day 14 post infection before declining and returning to levels seen in naïve mice by day 21. B cells are considerably increased on day 8 post infection, CD4+ and CD8+ T lymphocytes peak on day 14 post infection, with levels of both T cell subtypes declining on day 21 post infection, although the numbers remain much higher compared with numbers in uninfected mice. IL-17 and IL-22 are two key cytokines which are essential for protection against C. rodentium infection. These two cytokines can be produced not only by group 3 innate lymphoid cells (ILC3) but also by CD4+ T cells. ILC3s function as the main source of IL-17 and IL-22 early after infection (up until day 4 post infection), whereas CD4+ T cells become the main producers of these two cytokines at later time points (peak response around day 8–12 post infection).
Time considerations
In vivo experiments should always be carefully planned. Time needed to perform some of the procedures described in this unit depends directly on the skills of the experimenter. Times given here are for experimenter with practice.
Mice should be received in the animal facility at least 1 week prior to infection to allow their acclimatization to their new environment. Culture of C. rodentium should be started 2 days prior to infection of the mice. Preparation and enumeration of the bacterial inoculum from the 3–3.5 hour long culture used to infect animals takes about 30 min. The time required to inject mice will depend on the number of mice and the efficiency of the injections. Light anesthesia, restrain and intragastric administration of the inoculum by oral gavage take about 5 min per mouse. Mice should be given only water (starvation) for 8–12 hours before infection. Monitoring and full recovery of mice after anesthesia take about 1 hour. After inoculation, the course of the infection is typically analyzed for up to 2–3 weeks in wild-type mice, when the bacteria are completely cleared.
Stool sample collection takes between 5 and 15 min per mouse. Homogenization of stools, dilution and plating take about 25 min for each mouse. For organ recovery and bacterial enumeration in different organs, euthanasia takes 5 min, dissection and organ collection takes 10 min for each animal, preparation of tissue homogenates takes 10 min per animal, and dilution and plating take 10 min per animal. Colony forming units are counted on plates after 18–24 hours of incubation for measurement of bacterial loads in feces or after 18–48 hours of incubation for measurement of bacterial loads in infected organs.
For histological assessment of intestinal inflammation induced by C. rodentium, euthanasia takes 5 min, dissection and harvesting of the large intestine takes 10 min for each animal. Tissue samples used for histopathological analysis should be fixed for at least 24 hours. Paraffin embedding, histological processing and staining take up to 48 hours for each sample. Scoring of the extent of intestinal inflammation takes about 5–10 min per sample.
The preparation of frozen bacterial stocks from an overnight culture takes about 20 min. The generation of a growth curve for liquid culture of C. rodentium can take up to 3 days. Ex vivo organ cultures last 16–24 hours. The entire procedure to isolate immune cells from the large intestine takes about 21/2–3 hours for 6–8 mice. Staining of cell surface and intracellular antigens for the analysis of immune cell responses by flow cytometry takes about 5 hours with the re-stimulation of cells (21/2 hours without).
Acknowledgments
This project was supported by the National Institute of Allergy and Infectious Diseases Intramural Research Program. O.J.H is supported by an Early Career Grant from National Psoriasis Foundation. The content of this publication does not necessarily reflect the views and policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. We thank Jacqueline Kehr for critical reading of this manuscript.
Literature cited
- Barthold SW, Coleman GL, Jacoby RO, Livestone EM, Jonas AM. Transmissible murine colonic hyperplasia. Vet Pathol. 1978;15:223–236. doi: 10.1177/030098587801500209. [DOI] [PubMed] [Google Scholar]
- Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhinder G, Sham HP, Chan JM, Morampudi V, Jacobson K, Vallance BA. The Citrobacter rodentium mouse model: studying pathogen and host contributions to infectious colitis. J Vis Exp. 2013:e50222. doi: 10.3791/50222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenshtein D, McBee ME, Schauer DB. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol. 2008;24:32–37. doi: 10.1097/MOG.0b013e3282f2b0fb. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12:612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- Collins JW, Meganck JA, Kuo C, Francis KP, Frankel G. 4D multimodality imaging of Citrobacter rodentium infections in mice. J Vis Exp. 2013:e50450. doi: 10.3791/50450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepin VF, Collins JW, Habibzay M, Frankel G. Citrobacter rodentium mouse model of bacterial infection. Nat Protoc. 2016;11:1851–1876. doi: 10.1038/nprot.2016.100. [DOI] [PubMed] [Google Scholar]
- Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann N Y Acad Sci. 2006;1072:28–38. doi: 10.1196/annals.1326.008. [DOI] [PubMed] [Google Scholar]
- Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, Larijani M, Gosselin J, Ivanov II, Martin A, Casellas R, Philpott DJ, Girardin SE, McCoy KD, Macpherson AJ, Paige CJ, Gommerman JL. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2011;481:199–203. doi: 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Hill DA, Minkah N, Kirn T, Troy A, Artis D, Bushman F. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect Immun. 2009;77:4668–4678. doi: 10.1128/IAI.00493-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio A, Morales CI, Camara NO, Almeida RR. Innate Sensing of the Gut Microbiota: Modulation of Inflammatory and Autoimmune Diseases. Front Immunol. 2016;7:54. doi: 10.3389/fimmu.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Koroleva EP, Halperin S, Gubernatorova EO, Macho-Fernandez E, Spencer CM, Tumanov AV. Citrobacter rodentium-induced colitis: A robust model to study mucosal immune responses in the gut. J Immunol Methods. 2015;421:61–72. doi: 10.1016/j.jim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Luperchio SA, Newman JV, Dangler CA, Schrenzel MD, Brenner DJ, Steigerwalt AG, Schauer DB. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of C. rodentium and mouse-pathogenic Escherichia coli. J Clin Microbiol. 2000;38:4343–4350. doi: 10.1128/jcm.38.12.4343-4350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luperchio SA, Schauer DB. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 2001;3:333–340. doi: 10.1016/s1286-4579(01)01387-9. [DOI] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Mallick EM, McBee ME, Vanguri VK, Melton-Celsa AR, Schlieper K, Karalius BJ, O’Brien AD, Butterton JR, Leong JM, Schauer DB. A novel murine infection model for Shiga toxin-producing Escherichia coli. J Clin Invest. 2012;122:4012–4024. doi: 10.1172/JCI62746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy R, Girard F, FitzGerald AJ, Frankel G. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol Lett. 2006;265:126–132. doi: 10.1111/j.1574-6968.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7:1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–577. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- Schauer DB, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer DB, Zabel BA, Pedraza IF, O’Hara CM, Steigerwalt AG, Brenner DJ. Genetic and biochemical characterization of Citrobacter rodentium sp. nov. J Clin Microbiol. 1995;33:2064–2068. doi: 10.1128/jcm.33.8.2064-2068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FA, Rodrigues BL, Ayrizono ML, Leal RF. The Immunological Basis of Inflammatory Bowel Disease. Gastroenterol Res Pract. 2016;2016:2097274. doi: 10.1155/2016/2097274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, Frankel G, Dougan G. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2003;71:5077–5086. doi: 10.1128/IAI.71.9.5077-5086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, Fu YX. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 2011;10:44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance BA, Deng W, Knodler LA, Finlay BB. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect Immun. 2002;70:2070–2081. doi: 10.1128/IAI.70.4.2070-2081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales AD, Woodward MJ, Pearson GR. Attaching-effacing bacteria in animals. J Comp Pathol. 2005;132:1–26. doi: 10.1016/j.jcpa.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles S, Clare S, Harker J, Huett A, Young D, Dougan G, Frankel G. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell Microbiol. 2004;6:963–972. doi: 10.1111/j.1462-5822.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- Willing BP, Vacharaksa A, Croxen M, Thanachayanont T, Finlay BB. Altering host resistance to infections through microbial transplantation. PLoS One. 2011;6:e26988. doi: 10.1371/journal.pone.0026988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]


