Abstract
A convergent and efficient strategy for the synthesis of high-mannose oligosaccharides is described wherein regioselective glycosylations between trichloroacetimidate donors and partially protected acceptors are employed to reduce the number of protection–deprotection steps. Two representative branched mannose oligosaccharides, a mannose heptasaccharide (Man7) and a mannose nonasaccharide (Man9) were constructed via (4+3) and (5+4) glycosylations, respectively. These mannose-containing oligosaccharides were obtained in nine steps in ~25% overall yield and >98% purity on 60–70 mg scales to demonstrate the effectiveness of the strategy.
1. Introduction
High-mannose N-glycans appended to N-linked glycoproteins play important roles in many normal and aberrant biological processes.1 For example, the high-mannose N-glycans displayed on the outer surfaces of many pathogenic microorganisms are recognized by macrophages and dendritic cells, leading to the triggering of immune responses.2 In general, the biological properties and functions of N-glycans are modulated by post-translational modification, that is, by glycosylation and hydrolysis events that occur in the Golgi and lead to structurally diverse N-glycans in vivo.3 In many diseases including cancer, aberrant glycosylation patterns and/or levels of high-mannose N-glycan expression are often observed. For example, in the blood sera of breast cancer patients, concentrations of a nonamannoside (Man9) glycan increase while the sera of normal individuals contain mostly complex- and hybrid-type N-glycans.4 In another example, mice implanted with head and neck tumors display high concentrations of Man5–Man7 N-glycans in their sera compared to the sera of healthy mice.5 Access to structurally diverse and chemically pure samples of high-mannose N-glycans is critical for immunological studies and for the development of vaccines.
Several synthetic strategies have been reported to prepare high-mannose oligosaccharides,6 including tri- to octa-mannosides, but many are situation-specific. Generalized synthetic protocols are still needed. Chemical syntheses of building blocks, including donors and acceptors, often require elaborate design and preparation to allow regio- and stereoselective control of each O-glycosidic linkage formed during assembly. Since there is little theoretical guidance in the design of building blocks, this work is often highly empirical and challenging. One way to simplify these syntheses is to reduce the number of protection–deprotection steps by partially protecting the hydroxyl groups in the building blocks, especially in acceptors, and applying them in regioselective glycosylation. Successful application of lightly protected acceptors exploits intrinsic differences in the regioselectivities and/or relative reactivities of the free hydroxyl groups in the acceptor. Fortunately, significant advances in this understanding have been made over the past few decades.7
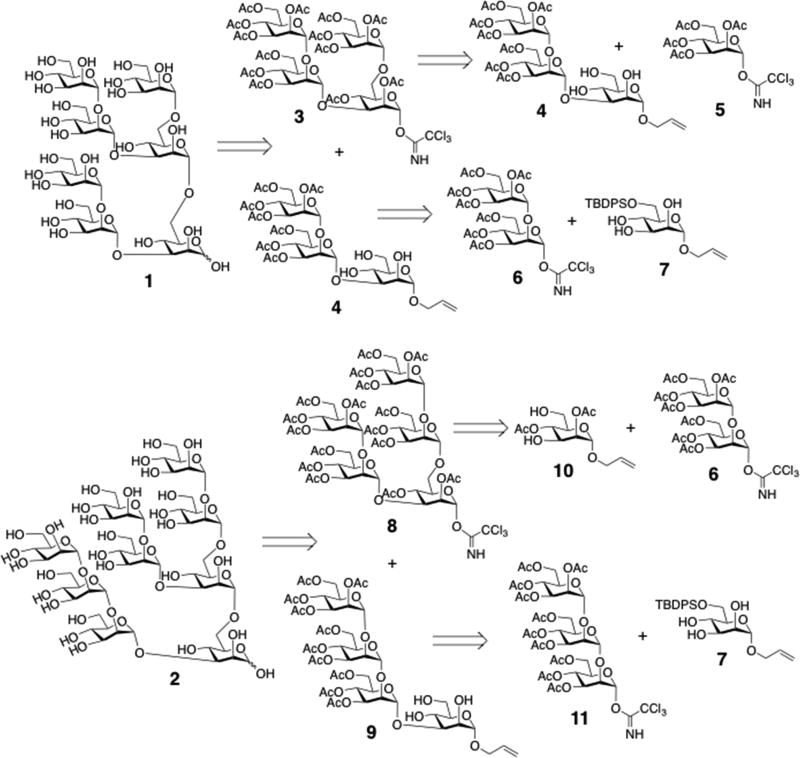
In this report, we describe a convergent and efficient chemical strategy for the rapid assembly of high-mannose oligosaccharides, illustrated with the syntheses of two representative oligomannoses, heptasaccharide 1 and nonasaccharide 2 (Scheme 1). The strategy takes advantage of regioselective glycosylation and the repetitive use of several building blocks with common protecting groups. For example, heptasaccharide 1 was assembled from tetrasaccharide donor 3 and partially protected trisaccharide acceptor 4 through regioselective glycosylation. Donor 3 was prepared from monosaccharide donor 5 and partially protected acceptor 4. Acceptor 4 was constructed from disaccharide donor 6 and lightly protected monosaccharide acceptor 7. In a similar fashion, nonasaccharide 2 was prepared by a straightforward route enabled by the common αMan1→2αMan motifs present on the 1→3 and 1→6 arms of donor 8. This strategy streamlined the syntheses of 1 and 2 by significantly reducing the number of synthetic steps and improving overall yield. Over 50 branched high mannose oligosaccharides have been prepared in high purity and in 20–100 mg quantities using this efficient strategy.
Scheme 1.
Structures of heptasaccharide 1 and nonasaccharide 2, and their retrosynthetic analyses.
2. Results and Discussion
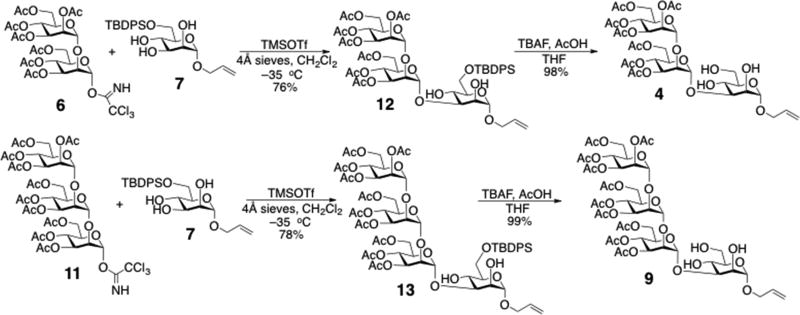
The synthetic strategy started with the preparation of basic building blocks. Elementary mannosyl donors, including mannose monosaccharide donor 5,8 and the α-1,2-linked di- and trisaccharide donors 6 and 11,9,10 were synthesized using methods reported previously. The partially protected triol acceptor 7 was obtained by silylation of allyl α-d-mannopyranoside with tert-butyl(chloride)diphenylsilane (TBDPSCl).11
Previous investigations of the regioselective mannosylation of a 2,3,4-triol mannoside12 showed that the 3-OH exhibits much greater reactivity than 2-OH and 4-OH. Glycosylation of allyl 6-O-TBDPS-α-d-mannopyranoside (7) with Schmidt’s trichloroacetimidate donors in dichloromethane occurred nearly exclusively at the 3-OH position and in good yield with either TMSOTf or BF3·OEt2 as the catalyst. Subsequent regioselective glycosylations were performed between acceptor 7 with donors 6 and 11 upon activation with TMSOTf at −35 °C, affording compounds 12 and 13, respectively, in good yields (Scheme 2). This strategy simplified the need for protective group manipulations, and avoided the use of other specific catalysts.13
Scheme 2.
Regioselective syntheses of acceptors 4 and 9.
The newly formed linkages in compounds 12 and 13 were confirmed from analyses of 1D 1H and 2D 1H-1H-gCOSY spectra. For 12, two cross-peaks observed at 3.01 ppm / 3.94 ppm and 2.33 ppm / 3.98 ppm in the 2D 1H–1H gCOSY spectrum (see Supplementary Data) indicated that 1H signals associated with two free (non-glycosylated) hydroxyl hydrogens with chemical shifts at 3.01 and 2.33 ppm correlated with 1H signals at 3.94 and 3.98 ppm, respectively. Inspection of the J-coupling patterns in the latter signals (see Supplementary Data) led to their assignments to H4 and H2, respectively, since the former appears as a pseudo-triplet (the H4 signal is split by large 3JH3,H4 and 3JH4,H5 values) and the latter appears as a broadened signal containing two small splittings (the H2 signal is split by small 3JH1,H2 and 3JH2,H3 values). These signal multiplicities differ from that expected for H3, which would appear as a doublet of doublets containing a small 3JH2,H3 and a large 3JH3,H4. In the same manner, cross-peaks at 3.29 ppm / 3.93 ppm and 2.45 ppm / 3.99 ppm in the 2D 1H–1H gCOSY spectrum of 13 indicated that H2 and H4 correlate with the free hydroxyl hydrogens, thus confirming glycosylation at OH3. The anomeric configurations of the (1→3)-glycosidic linkages in 12 and 13 were confirmed using the chemical shift of H5, which is typically observed downfield (>3.8 ppm) in α-Man residues relative to that in β-Man residues (~3.4 ppm).14 Having confirmed the presence of α-(1→3) linkages, compounds 12 and 13 were desilylated with tert-butyl ammonium fluoride (TBAF) to give acceptors 4 and 9 in almost quantitative yields (Scheme 2).
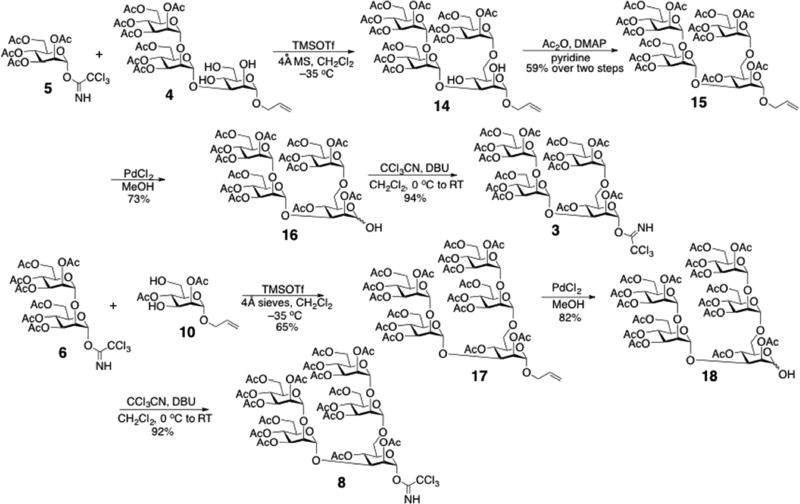
Acceptor 4 was used directly in the following donor constructions. Donors 3 and 8 contain two branches at C3 and C6 of the activated α-Man residue. Donor 3 contains an α-(1→2)-linked Man disaccharide at C3 and an α-Man monosaccharide at C6, while donor 8 contains α-(1→2)-linked Man disaccharides at both sites. Although donors 3 and 8 could be prepared by regioselective glycosylation of acceptor 4 with either monosaccharide donor 5 or disaccharide donor 6, respectively, two strategies were applied for their preparation as shown in Scheme 3. A regioselective glycosylation was performed between donor 5 and acceptor 4, and the product 14 was acetylated to give tetrasaccharide 15 as the donor precursor. Formation of the 1→6 linkage was confirmed from the 13C{1H} NMR spectrum of compound 15, in which only three signals at ~62 ppm were observed, indicating that one of the four C6 carbons of 15 was glycosylated (glycosylation shifts the C6 signal downfield). Synthesis of donor 8 involved a one-step glycosylation of acceptor 10 with 2 equivalents of disaccharide donor 615 to give pentasaccharide 17 in acceptable yield. Compounds 15 and 17 were treated with palladium chloride (PdCl2) to remove the allyl group, and the products were activated with trichloroacetonitrile to furnish trichloroacetimidate donors 3 and 8, respectively.
Scheme 3.
Syntheses of trichloroacetimidate donors 3 and 8.
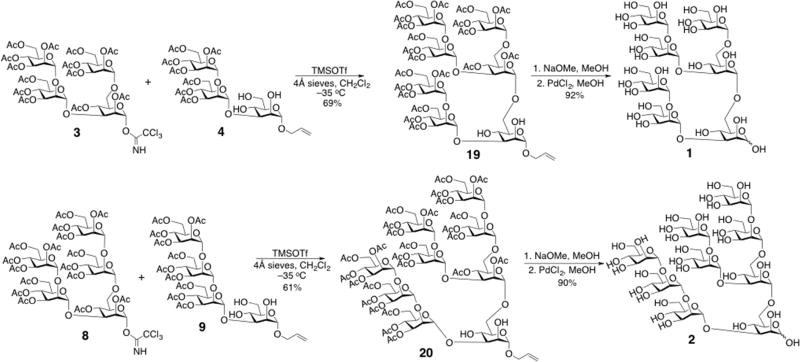
With the required donors and acceptors in hand, the target oligosaccharides 1 and 2 were assembled (Scheme 4). Regioselective glycosylations at C6 were performed between donor 3 and acceptor 4, and between donor 8 and acceptor 9, with activation afforded by TMSOTf at −35 °C to give compounds 19 and 20, respectively. The latter products were characterized by NMR; confirmation of the newly introduced αMan-1,6-αMan linkage was confirmed by observing the downfield shift of the C6 signals upon glycosylation. Compounds 19 and 20 were then deacetylated with sodium methoxide in methanolic solution, deallylated with palladium chloride (PdCl2), and purified by chromatography on a Bio-Gel P-2 column to give 60–70 mg of oligosaccharides 1 and 2 in >98% purity (Scheme 4).
Scheme 4.
Assembly of mannose oligosaccharides 1 and 2.
3. Conclusion
A convergent and efficient chemical strategy has been developed to prepare tri-antennary mannose oligosaccharides in >20% overall yields. Regioselective glycosylation with partially protected acceptors was employed to reduce the number of protection–deprotection steps, thus improving the overall efficiency of the syntheses. The strategy significantly improves access to these important oligosaccharides on milligram–gram scales, which will promote their use in chemical, biochemical, structural, and biomedical studies.
4. Experimental Section
4.1. General Methods
All chemicals were purchased as anhydrous reagent grade and were used without further purification unless otherwise noted. All reactions were performed under anhydrous conditions. Reactions were monitored by thin-layer chromatography (TLC) on silica gel precoated aluminum plates. Zones were detected by heat/charring with a p-anisaldehyde–sulfuric acid visualization reagent.16 Flash column chromatography on silica gel (preparative scale) was performed on the Reveleris® X2 flash chromatography system. 1H and 13C{1H} NMR spectra were recorded at 22 °C on a Bruker Avance III HD 500-MHz or Varian DirectDrive 600-MHz spectrometer. Chemical shifts are reported in δ-units (ppm) relative to the 1H signal of residual CHCl3 at δ 7.24 ppm and the 13C signal at δ 77.23 ppm. Abbreviations for multiplicities and descriptors are: s = singlet; d = doublet; t = triplet; dd = doublet of doublets; dt = doublet of triplets; td = triplet of doublets; q = quartet; m = multiplet. Two-dimensional 1H-1H gCOSY and 1H-13C gHSQC NMR spectra were recorded on the same instruments using Bruker or Varian software. Mass spectrometric analyses were performed on a Bruker microTOF-Q II quadrupole time-of-flight (QTOF) mass spectrometer with an ESI source.
4.2. General Experimental Procedures
4.2.1. Glycosylation
Donor and acceptor were dissolved in anhydrous CH2Cl2, and 4Å molecular sieves were added to the solution. The solution was cooled to −35 °C (dry ice/acetone bath) and was treated with TMSOTf under N2. The reaction mixture was stirred for 2 h and then quenched with the addition of triethylamine. The mixture was filtered through Celite®, the solution was concentrated in vacuo, and the residue was purified by flash chromatography on a silica gel column.
4.2.2. Desilylation
The compound was dissolved in anhydrous THF, and AcOH and TBAF were added to the solution. The solution was stirred at room temperature for 2 h, concentrated in vacuo, and the residue was purified by flash chromatography on a silica gel column.
4.2.3. Acetylation
The compound was dissolved in anhydrous pyridine, and 4-(dimethylamino)pyridine (DMAP) and Ac2O were added to the solution. The solution was stirred for 1 h at room temperature, and then concentrated under high vacuum. The residue was partitioned between EtOAc and water, and the organic layer was washed with saturated aqueous NaHCO3 and brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by flash chromatography on silica gel column.
4.2.4. Deallylation
The compound was dissolved in anhydrous methanol, and PdCl2 was added to the solution. The reaction mixture was stirred at room temperature for 7 h in the dark. The PdCl2 was then removed by filtration and the filtrate was concentrated in vacuo. The residue was purified by either flash chromatography on a silica gel column (compounds 16 and 18), or by chromatography on a Bio-Gel P-2 column (compounds 1 and 2).
4.2.5. Trichloroacetimidation
The compound was dissolved in anhydrous CH2Cl2, the solution was cooled to 0 °C, and trichloroacetonitrile and DBU were added to the solution. The reaction mixture was stirred for 3 h at room temperature and then concentrated in vacuo. The residue was purified by flash chromatography on a silica gel column.
4.2.6. Deacetylation
The compound was dissolved in anhydrous methanol and a small amount of NaOMe was added to the solution until the pH reached 9 (pH was determined using pH paper). The reaction solution was stirred at room temperature overnight, and then neutralized with batch addition of Dowex® HCR W2 ion-exchange resin in the H+ form (16–40 mesh). The resin was removed by filtration, and the filtrate was concentrated in vacuo.
4.3 Experimental Data
4.3.1. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)-6-O-(tert-butyldiphenylsilyl)-α-d-mannopyranoside 12
Compound 12 was prepared using the general glycosylation method above with donor 6 (6.38 g, 8.17 mmol), acceptor 7 (5.61 g, 12.25 mmol), CH2Cl2 (300 mL), 4Å molecular sieves (7 g), and TMSOTf (300 µL, 1.63 mmol). The product was obtained as a white amorphous foam (6.68 g, 6.21 mmol, 76%). [α]D20 +53.2 (c 0.47, MeOH); 1H NMR (600 MHz, Chloroform-d) δ 7.66 (m, 4H), 7.42 – 7.35 (m, 6H), 5.81 (dddd, J = 16.9, 10.4, 6.3, 5.3 Hz, 1H), 5.37 (dd, J = 10.0, 3.4 Hz, 1H), 5.31 (m, 2H), 5.25 – 5.20 (m, 4H), 5.16 (dd, J = 10.4, 1.4 Hz, 1H), 4.93 (d, J = 1.9 Hz, 1H), 4.79 (d, J = 1.8 Hz, 1H), 4.23 (dd, J = 12.2, 5.3 Hz, 1H), 4.17 – 4.05 (m, 7H), 3.98 – 3.84 (m, 6H), 3.62 (dt, J = 9.7, 5.0 Hz, 1H), 3.01 (s, 1H), 2.33 (s, 1H), 2.12, 2.08, 2.06, 2.04, 2.01, 1.99, 1.98 (7s, 7×3H), 1.03 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 171.0 (×2), 170.6, 170.0, 169.9, 169.7 (×2), 135.8 (×2), 133.7, 133.1, 132.9, 130.1 (×2), 128.0 (×2), 118.1, 100.1, 99.0, 98.7, 79.9, 76.4, 71.6, 70.5, 70.3, 69.9, 69.2 (×2), 69.0, 68.6, 68.2, 66.9, 66.6, 65.2, 62.8, 62.7, 27.0, 21.1, 21.0, 20.9 (×3), 20.8, 19.3. HRESIMS: (m/z) calcd for C51H68O23SiNa+ (M+Na)+ 1099.3823; found 1099.3828.
4.3.2. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)-α-d-mannopyranoside 4
Compound 4 was synthesized using the general desilylation method with 12 (6.68 g, 6.21 mmol), THF (100 mL), AcOH (1.07 mL, 18.63 mmol) and TBAF (12.42 mL, 12.42 mmol). The product was obtained as a white amorphous foam (5.10 g, 6.09 mmol, 98%). [α]D20 +55.6 (c 0.42, MeOH); 1H NMR (500 MHz, Chloroform-d) δ 5.82 (dddd, J = 17.2, 10.4, 6.2, 5.3 Hz, 1H), 5.37 (m, 1H), 5.35 – 5.32 (m, 2H), 5.28 (m, 1H), 5.24 (m, 1H), 5.22 – 5.19 (m, 2H), 5.16 (dq, J = 10.4, 1.3 Hz, 1H), 4.92 (d, J = 1.9 Hz, 1H), 4.81 (d, J = 1.6 Hz, 1H), 4.21 (m, 2H), 4.15 – 4.08 (m, 9H), 3.95 – 3.90 (m, 3H), 3.88 (m, 2H), 3.77 (dd, J = 12.0, 2.6 Hz, 1H), 3.54 (dt, J = 9.9, 2.6 Hz, 1H), 2.11, 2.08, 2.06, 2.05, 2.01, 2.01, 1.97 (7s, 7×3H). 13C NMR (126 MHz, CDCl3) δ 171.7, 171.2, 171.1, 170.1, 170.0, 169.7, 169.6, 133.7, 118.0, 100.4, 99.3, 99.1, 80.3, 77.1, 72.5, 71.0, 70.8, 70.0, 69.4, 69.0, 68.6, 68.3, 66.6, 66.5, 65.8, 62.8 (×2), 61.3, 21.1 (×3), 20.9 (×3). HRESIMS: (m/z) calcd for C35H50O23Na+ (M+Na)+ 861.2645; found 861.2650.
4.3.3. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl (1→3)-6-O-(tert-butyldiphenylsilyl)-α-d-mannopyranoside 13
Compound 13 was prepared using the general glycosylation method with donor 11 (1.07 g, 1.00 mmol), acceptor 7 (690 mg, 1.51 mmol), 4Å molecular sieves (1.5 g), and TMSOTf (36 µL, 0.20 mmol). The product was obtained as a white amorphous foam (1.06 g, 0.78 mmol, 78%). [α]D20 +37.1 (c 0.44, MeOH); 1H NMR (600 MHz, Chloroform-d) δ 7.67 – 7.65 (m, 4H), 7.40 – 7.34 (m, 6H), 5.83 (dddd, J = 16.9, 10.4, 6.3, 5.3 Hz, 1H), 5.36 (dd, J = 10.0, 3.4 Hz, 1H), 5.32 – 5.29 (m, 2H), 5.27 – 5.24 (m, 4H), 5.21 – 5.13 (m, 3H), 5.10 (d, J = 2.1 Hz, 1H), 4.92 (d, J = 1.8 Hz, 1H), 4.81 (d, J = 1.7 Hz, 1H), 4.22 (d, J = 4.4 Hz, 1H), 4.18 – 4.06 (m, 10H), 4.05 (t, J = 2.3 Hz, 1H), 3.99 (t, J = 2.2 Hz, 1H), 3.95 – 3.89 (m, 4H), 3.86 (dd, J = 10.8, 5.1 Hz, 1H), 3.65 (dt, J = 9.1, 4.8 Hz, 1H), 3.29 (s, 1H), 2.45 (s, 1H), 2.12, 2.09, 2.08, 2.05, 2.04, 2.03, 2.02, 2.01, 1.98, 1.94 (10s, 10×3H), 1.03 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 171.2, 171.0 (×2), 170.3 (×2), 170.0, 169.9, 169.7 (×2), 169.6, 135.8 (×2), 133.7, 133.3, 133.1, 130.0 (×2), 127.9 (×2), 118.1, 99.9, 99.4, 99.0, 98.7, 80.0, 75.6, 72.1, 70.6, 70.0 (×2), 69.8, 69.4, 69.3, 68.6 (×2), 68.1, 67.3, 66.7, 66.5, 65.0, 62.8, 62.7, 62.5, 27.0, 21.1, 20.9 (×5), 20.8 (×2), 19.4. HRESIMS: (m/z) calcd for C63H84O31SiNa+ (M+Na)+ 1387.4662; found 1387.4658.
4.3.4. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl (1→3)-α-d-mannopyranoside 9
Compound 9 was synthesized using the general desilylation method with compound 13 (1.06 g, 0.78 mmol), THF (50 mL), AcOH (134 µL, 2.34 mmol) and TBAF (1.56 mL, 1.56 mmol). The product was obtained as a white amorphous foam (0.87 g, 0.77 mmol, 99%). [α]D20 +46.4 (c 0.50, MeOH); 1H NMR (500 MHz, Chloroform-d) δ 5.82 (dddd, J = 17.3, 10.4, 6.2, 5.3 Hz, 1H), 5.34 – 5.30 (m, 3H), 5.29 – 5.18 (m, 6H), 5.16 (dq, J = 10.4, 1.3 Hz, 1H), 5.06 (d, J = 2.1 Hz, 1H), 4.91 (d, J = 1.8 Hz, 1H), 4.81 (d, J = 1.7 Hz, 1H), 4.21 – 4.03 (m, 13H), 3.94 (m, 1H), 3.90 – 3.86 (m, 2H), 3.79 (m, 1H), 3.67 (s, 1H), 3.56 (dt, J = 9.8, 2.9 Hz, 1H), 3.13 (s, 1H), 2.47 (s, 2H), 2.11, 2.09, 2.06, 2.04, 2.04, 2.04, 2.00, 2.00, 2.00, 1.96 (10s, 10×3H). 13C NMR (126 MHz, CDCl3) δ 171.4, 171.2, 171.0 (×2), 170.4, 170.0, 169.9, 169.7, 169.6 (×2), 133.7, 117.9, 100.1, 99.3, 99.1 (×2), 80.1, 76.9, 75.8, 72.6, 71.0, 70.4, 69.9, 69.8, 69.3 (×2), 69.2, 68.6, 68.3, 66.9, 66.6, 66.5, 66.0, 62.7, 62.6, 62.6, 61.6, 21.0 (×2), 20.9 (×4), 20.8 (×2). HRESIMS: (m/z) calcd for C47H66O31Na+ (M+Na)+ 1149.3446; found 1149.3440.
4.3.5. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→6)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-2,4-di-O-acetyl-α-d-mannopyranoside 15
Compound 14 was prepared according using the general glycosylation method with donor 5 (1.48 g, 3.00 mmol), acceptor 4 (2.51 g, 3.00 mmol), CH2Cl2 (100 ml), 4Å molecular sieves (2 g), and TMSOTf (108 µL, 0.60 mmol). The afforded product 14 was acetylated with pyridine (100 mL), DMAP (catalytic amt.), and Ac2O (1.1 mL, 12 mmol). Compound 15 was obtained as a white amorphous foam (2.22 g, 1.77 mmol, 59% over two steps). 1H NMR (600 MHz, Chloroform-d) δ 5.83 (ddt, J = 16.6, 10.5, 5.9 Hz, 1H), 5.32 (dd, J = 10.0, 3.4 Hz, 1H), 5.29 – 5.17 (m, 9H), 5.12 (t, J = 10.0 Hz, 1H), 5.09 (d, J = 2.0 Hz, 1H), 5.06 (dd, J = 9.9, 3.1 Hz, 1H), 4.79 (d, J = 1.7 Hz, 1H), 4.76 (2d, J = 2.2 Hz, 2H), 4.19 (dd, J = 12.3, 5.4 Hz, 1H), 4.16 – 4.01 (m, 9H), 3.99 – 3.93 (m, 2H), 3.84 (dd, J = 3.1, 2.0 Hz, 1H), 3.80 (ddd, J = 9.7, 6.5, 2.5 Hz, 1H), 3.70 (dd, J = 10.7, 6.6 Hz, 1H), 3.45 (dd, J = 10.7, 2.5 Hz, 1H), 2.13, 2.09, 2.09, 2.08, 2.06, 2.06, 2.04, 2.00, 1.99, 1.98 (×2), 1.94, 1.92 (13s, 13×3H). 13C NMR (151 MHz, CDCl3) δ 170.9, 170.7, 170.5 (×2), 170.3, 170.1, 170.0, 169.9 (×2), 169.8 (×2), 169.5 (×2), 133.0, 118.9, 99.9, 99.8, 97.3, 96.2, 78.3, 74.8, 70.9, 69.7 (×3), 69.6, 69.5 (×2), 69.0, 68.7, 68.6, 68.6, 68.5, 66.8, 66.3, 66.1, 65.7, 62.7, 62.5, 62.2, 21.0 (×3), 20.9 (×3), 20.8 (×6), 20.7. HRESIMS: (m/z) calcd for C53H72O34Na+ (M+Na)+ 1275.3744; found 1275.3740.
4.3.6. 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→6)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-2,4-di-O-acetyl-α-d-mannopyranosyl trichloroacetimidate 3
Compound 16 was prepared using the general deallylation method with compound 15 (2.22 g, 1.77 mmol), methanol (100 mL), and PdCl2 (20 mg). The afforded product (1.57 g, 1.29 mmol, 73%) was activated using the general acetimidation method with CH2Cl2 (50 mL), trichloroacetonitrile (0.52 mL, 5.16 mmol) and DBU (29 µL, 0.19 mmol). Compound 3 was afforded as a white amorphous foam (1.64 g, 1.21 mmol, 94%). 1H NMR (600 MHz, Chloroform-d) δ 8.84 (s, 1H), 6.17 (d, J = 1.9 Hz, 1H), 5.36 (dd, J = 3.5, 1.9 Hz, 1H), 5.32 – 5.28 (m, 2H), 5.27 – 5.20 (m, 3H), 5.18 (m, 2H), 5.14 (m, 2H), 5.07 (dd, J = 9.9, 3.0 Hz, 1H), 4.78 (d, J = 1.9 Hz, 1H), 4.73 (d, J = 1.7 Hz, 1H), 4.22 – 4.19 (m, 2H), 4.14 (dd, J = 12.4, 3.7 Hz, 1H), 4.10 (m, 3H), 4.05 – 4.02 (m, 2H), 4.01 – 3.97 (m, 2H), 3.94 (m, 1H), 3.86 (t, J = 2.5 Hz, 1H), 3.70 (dd, J = 11.0, 6.1 Hz, 1H), 3.52 (dd, J = 11.0, 2.5 Hz, 1H), 2.18, 2.08, 2.07, 2.07, 2.06, 2.05, 2.03, 2.00, 1.98, 1.97, 1.97, 1.93, 1.90 (13s, 13×3H). 13C NMR (151 MHz, CDCl3) δ 170.9, 170.7, 170.4, 170.3, 170.2, 170.1, 169.9, 169.8 (×3), 169.7, 169.5 (×2), 159.5, 99.7, 97.4, 94.1, 90.6, 78.1, 77.0, 73.8, 71.9, 69.8, 69.7, 69.6, 69.5, 69.4, 69.3, 69.0, 68.6, 68.4, 68.1, 66.4, 66.3, 66.0, 65.5, 62.6, 62.3, 61.9, 60.5, 21.1, 21.0 (×3), 20.9, 20.8 (×4), 20.74 (×3). HRESIMS: (m/z) calcd for C52H68O34NCl3Na+ (M+Na)+ 1378.2590; found 1378.2594.
4.3.7. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→6)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-2,4-di-O-acetyl-α-d-mannopyranoside 17
Compound 17 was synthesized using the general glycosylation method with donor 6 (3.2 g, 4.10 mmol), acceptor 10 (500 mg, 1.64 mmol), 4Å molecular sieves (4.0 g), CH2Cl2 (50 mL), and TMSOTf (148 µL, 0.82 mmol). The product was afforded as a white amorphous foam (1.64 g, 1.06 mmol, 65%). 1H NMR (600 MHz, Chloroform-d) δ 5.83 (dddd, J = 17.3, 10.4, 6.3, 5.4 Hz, 1H), 5.35 (dd, J = 10.0, 3.4 Hz, 1H), 5.32 (dd, J = 10.0, 3.4 Hz, 1H), 5.29 (m, 1H), 5.27 – 5.23 (m, 3H), 5.22 – 5.18 (m, 6H), 5.12 (t, J = 10.0 Hz, 1H), 5.09 (d, J = 2.0 Hz, 1H), 5.06 (dd, J = 10.0, 3.0 Hz, 1H), 4.91 (d, J = 1.9 Hz, 1H), 4.85 (d, J = 1.8 Hz, 1H), 4.78 (d, J = 1.7 Hz, 1H), 4.77 (d, J = 1.9 Hz, 1H), 4.18 – 4.03 (m, 12H), 4.00 – 3.93 (m, 4H), 3.84 (dd, J = 3.1, 2.0 Hz, 1H), 3.81 (ddd, J = 10.6, 6.5, 2.5 Hz, 1H), 3.72 (dd, J = 10.5, 6.6 Hz, 1H), 3.45 (dd, J = 10.5, 2.6 Hz, 1H), 2.14, 2.10, 2.10, 2.09, 2.09, 2.06, 2.05, 2.03, 2.01, 2.01, 1.99, 1.99, 1.99, 1.97, 1.96, 1.94 (16s, 16×3H). HRESIMS: (m/z) calcd for C65H88O42Na+ (M+Na)+ 1563.4632; found 1563.4630.
4.3.8. 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→6)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-2,4-di-O-acetyl-α-d-mannopyranosyl trichloroacetimidate 8
Compound 18 was synthesized using the general deallylation method with compound 17 (1.64 g, 1.06 mmol), methanol (50 mL), and PdCl2 (20 mg). The afforded product (1.30 g, 0.87 mmol, 82%) was activated to the trichloroacetimidate using the general acetimidation method with trichloroacetonitrile (0.35 mL, 3.48 mmol), DBU (19 µL, 0.13 mmol) and CH2Cl2 (50 mL) to afford product 8 (1.32 g, 0.80 mmol, 92%) as a white amorphous foam. 1H NMR (600 MHz, Chloroform-d) δ 8.87 (s, 1H), 6.21 (d, J = 1.9 Hz, 1H), 5.41 (dd, J = 3.6, 2.0 Hz, 1H), 5.39 – 5.31 (m, 4H), 5.29 – 5.25 (m, 2H), 5.23 (m, 3H), 5.18 (d, J = 2.1 Hz, 1H), 5.15 (dd, J = 10.0, 3.2 Hz, 1H), 5.12 (dd, J = 9.9, 3.1 Hz, 1H), 4.91 (d, J = 1.9 Hz, 1H), 4.86 (d, J = 1.9 Hz, 1H), 4.82 (d, J = 1.9 Hz, 1H), 4.24 (dd, J = 9.6, 3.5 Hz, 1H), 4.21 – 4.17 (m, 3H), 4.14 – 4.09 (m, 6H), 4.03 – 3.96 (m, 5H), 3.90 (dd, J = 3.0, 2.1 Hz, 1H), 3.75 (dd, J = 10.9, 5.9 Hz, 1H), 3.56 (dd, J = 10.9, 2.6 Hz, 1H), 2.23, 2.13, 2.13, 2.12, 2.11, 2.10, 2.09, 2.05, 2.05, 2.04, 2.02, 2.02 (×2), 2.01, 1.98, 1.98 (16s, 16×3H). HRESIMS: (m/z) calcd for C64H84O42NCl3Na+ (M+Na)+ 1666.3456; found 1666.3453.
4.3.9. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→6)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-2,4-di-O-acetyl-α-d-mannopyranosyl-(1→6)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-α-d-mannopyranoside 19
Compound 19 was synthesized using the general glycosylation method with donor 3 (110 mg, 0.08 mmol), acceptor 4 (90 mg, 0.11 mmol), CH2Cl2 (50 mL), 4Å molecular sieves (200 mg) and TMSOTf (4.3 µL, 0.024 mmol). The product was obtained as a white amorphous foam (114 mg, 0.056 mmol, 69%). 1H NMR (600 MHz, Chloroform-d) δ 5.83 (ddt, J = 16.5, 10.7, 5.8 Hz, 1H), 5.36 – 5.15 (m, 16H), 5.10 (m, 2H), 5.07 (dd, J = 9.8, 3.0 Hz, 1H), 4.89 (d, J = 1.9 Hz, 1H), 4.88 (d, J = 1.7 Hz, 1H), 4.79 (d, J = 1.8 Hz, 1H), 4.78 (d, J = 1.9 Hz, 1H), 4.78 (d, J = 2.0 Hz, 1H), 4.24 (td, J = 5.0, 2.6 Hz, 1H), 4.19 – 4.01 (m, 17H), 3.96 – 3.91 (m, 4H), 3.86 – 3.80 (m, 3H), 3.73 – 3.67 (m, 3H), 3.46 (dd, J = 10.9, 2.2 Hz, 1H), 3.17 (d, J = 4.8 Hz, 1H), 2.99 (d, J = 6.6 Hz, 1H), 2.14 (s, 3H), 2.10 (s, 6H), 2.09 (s, 9H), 2.08 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H), 1.99 (s, 9H), 1.98 (s, 3H), 1.96 (s, 3H), 1.95 (s, 3H), 1.94 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 171.3, 171.0 (×2), 170.9 (×2), 170.8, 170.5 (×2), 170.4 (×2), 170.1, 170.0 (×2), 169.9 (×3), 169.6 (×2), 169.5 (×2), 133.7, 118.1, 100.8, 99.7, 99.6, 99.3, 99.2, 97.0 (×2), 82.0, 78.0, 77.0, 74.5, 71.6, 71.0, 70.6, 70.4, 69.9, 69.8, 69.7, 69.6 (×2), 69.5, 69.3 (×2), 69.1, 68.8 (×2), 68.6, 68.5 (×2), 68.4, 66.8, 66.7, 66.5, 66.4, 66.3, 66.1, 65.8, 65.7, 63.0, 62.7, 62.6, 62.5, 61.9, 21.2 – 20.7.
4.3.10. Allyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→6) [2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-2,4-di-O-acetyl-α-d-mannopyranosyl-(1→6)-[2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→2)-3,4,6-tri-O-acetyl-α-d-mannopyranosyl-(1→3)]-α-d-mannopyranoside 20
Compound 20 was synthesized using the general glycosylation method with donor 8 (150 mg, 0.091 mmol), acceptor 9 (120 mg, 0.11 mmol), CH2Cl2 (50 mL), 4Å molecular sieves (200 mg), and TMSOTf (5.0 µL, 0.027). The product was afforded as a white amorphous foam (144 mg, 0.055 mmol, 61%). 1H NMR (600 MHz, CD3OD) δ 5.96 (m, 1H), 5.43 – 5.16 (m, 25H), 5.13 (d, J = 1.9 Hz, 1H), 5.10 (d, J = 1.9 Hz, 1H), 5.02 (d, J = 1.8 Hz, 1H), 4.99 (d, J = 1.8 Hz, 1H), 4.91 (d, J = 1.6 Hz, 1H), 4.81 (d, J = 1.7 Hz, 1H), 4.34 (dd, J = 3.1, 1.8 Hz, 1H), 4.31 – 4.13 (m, 22H), 4.07 – 4.01 (m, 5H), 3.99 (ddd, J = 6.4, 3.1, 1.9 Hz, 2H), 3.91 (d, J = 9.6 Hz, 1H), 3.88 (dd, J = 9.6, 3.1 Hz, 1H), 3.81 (dd, J = 11.1, 5.4 Hz, 1H), 3.73 (m, 1H), 3.70 (m, 1H), 3.65 (m, 1H), 2.19 (s, 3H), 2.15 (s, 3H), 2.15 (s, 3H), 2.15 (s, 3H), 2.14 (s, 3H), 2.14 (s, 3H), 2.13 (s, 3H), 2.12 (s, 3H), 2.10 (s, 3H), 2.08 (2s, 6H), 2.07 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H), 2.05 (s, 3H), 2.05 (s, 3H), 2.05 (s, 3H), 2.04 (s, 3H), 2.04 (s, 3H), 2.03 (s, 3H), 2.02 (s, 6H), 1.99 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H). 13C NMR (151 MHz, CD3OD) δ 173.0, 172.8, 172.7, 172.6 (×2), 172.5, 172.3 (×2), 172.2, 171.9, 171.8 (×3), 171.7, 171.6, 171.5 (×6), 171.4 (×2), 171.3 (×2), 171.2, 135.3, 118.1, 102.0, 101.1, 100.9 (×2), 100.6 (×2), 100.5, 99.2, 98.7, 81.1, 79.8, 78.8, 78.6, 77.7, 75.2, 73.2, 72.1, 72.0, 71.6, 71.0, 70.9, 70.8, 70.7, 70.6, 70.5, 70.2, 70.1 (×2), 69.9, 69.2, 67.7, 67.5 (×2), 67.4, 67.3 (×2), 67.2, 66.8, 64.0, 63.7, 63.4, 63.3, 63.2, 63.0 (×2), 21.6 – 20.5.
4.3.11. α-d-Mannopyranosyl-(1→6)-[α-d-mannopyranosyl-(1→2)-α-d-mannopyranosyl-(1→3)]-α-d-mannopyranosyl-(1→6)-[α-d-mannopyranosyl-(1→2)-α-d-mannopyranosyl-(1→3)]-α/β-d-mannopyranose 1
Compound 1 was obtained using the global deprotection and general deacetylation methods with compound 19 (114 mg, 0.056 mmol), methanol (20 mL) and a small amount of NaOMe, followed by use of the general deallylation method with PdCl2 (10 mg). The product was obtained as a white solid (60 mg, 0.052 mmol, 92%) after purification by chromatography on a Bio-Gel P2 column (2.7 cm × 100 cm). 1H NMR (600 MHz, D2O) δ 5.42 – 5.41 (d, J = 1.8; d, J = 1.7 Hz, 1H), 5.37 (d, J = 1.7 Hz, 1H), 5.14 (d, J = 1.8 Hz, 0.64H (α-H)), 5.06 (m, 1H), 5.05 (d, J = 1.8 Hz, 1H), 4.92 (d, J = 1.7 Hz, 1H), 4.90 (d, J = 1.7 Hz, 0.26H (β-H)), 4.87 – 4.86 (d, J = 1.8 Hz; d, J = 1.8 Hz, 1H), 4.17 – 3.54 (m, 42H). 13C NMR (151 MHz, D2O) δ 102.2 (×2), 100.7, 100.6, 100.5, 99.3, 99.2, 94.1, 78.8, 78.6 (×2), 78.4, 78.3, 73.2 (×2), 73.1 (×2), 72.6, 70.7, 70.6, 70.5, 70.2 (×2), 70.0, 69.9 (×2), 69.4, 66.9, 66.8, 66.7, 66.6 (×2), 65.7, 65.6, 65.2, 61.0, 60.8. HRESIMS: (m/z) calcd for C42H72O36Na+ (M+Na)+ 1175.2825; found 1175.2829.
4.3.12. α-d-Mannopyranosyl-(1→2)-α-d-mannopyranosyl-(1→6)-[α-d-mannopyranosyl-(1→2)-α-d-mannopyranosyl-(1→3)]-α-d-mannopyranosyl-(1→6)-[α-d-mannopyranosyl-(1→2)-α-d-mannopyranosyl-(1→2)-α-d-mannopyranosyl-(1→3)]-α/β-d-mannopyranose 2
Compound 2 was obtained using the global deprotection and general deacetylation methods with compound 20 (144 mg, 0.055 mmol), methanol (20 mL), and a small amount of NaOMe, followed by use of the general deallylation method with PdCl2 (10 mg). The product was obtained as a white amorphous solid (74 mg, 0.05 mmol, 90%) after purification by chromatography on a Bio-Gel P2 column (2.7 × 100 cm). 1H NMR (600 MHz, D2O) δ 5.41 (d, J = 1.9 Hz, 1H), 5.36 (d, J = 1.7 Hz, 1H), 5.31 (d, J = 1.8 Hz, 1H), 5.15 (d, J = 1.8 Hz, 1H), 5.14 (d, J = 1.8 Hz, 0.67H (α-H)), 5.05 (m, 3H), 4.90 (d, J = 1.9 Hz, 0.33H (β-H)), 4.87 – 4.86 (d, J = 1.9 Hz; d, J = 1.9 Hz, 1H), 4.15 – 3.54 (m, 54H). 13C NMR (151 MHz, D2O) δ 102.2, 102.1, 102.0, 100.6 (×2), 100.5, 99.3, 97.8, 94.0, 93.6, 78.8, 78.6, 78.5 (×2), 78.4, 78.3, 73.1 (×3), 73.0 (×2), 72.5, 71.0, 70.5, 70.1 (×2), 69.9 (×2), 69.8, 69.4, 66.8, 66.7 (×2), 66.6 (×2), 65.5 (×2), 65.4, 65.3, 65.2 (×2), 61.0, 60.9 (×2), 60.8 (×2). HRESIMS: (m/z) calcd for C54H92O46Na+ (M+Na)+ 1499.4219; found 1499.4216.
Supplementary Material
Acknowledgments
A. S. thanks the National Science Foundation (CHE 1402744), and Q. P. thanks the National Institutes of Health (SBIR Contract HHSN261201500020C) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary Data
NMR data for compounds 1–4, 8–9, 12–13, 15, 17 and 19–20 are provided in the supplementary data.
References
- 1.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. [PubMed] [Google Scholar]
- 2.van Vliet SJ, Grun C, van Kooyk Y. In: Protein-Carbohydrate Interactions in Infectious Diseases. Bewley CA, editor. RSC Publishing; Oxford, UK: 2006. [Google Scholar]
- 3.(a) Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, Miyoshi E, Gu J. FEBS J. 2008;275:1939–1948. doi: 10.1111/j.1742-4658.2008.06346.x. [DOI] [PubMed] [Google Scholar]; (b) An HJ, Gip P, Kim J, Wu S, Park KW, McVaugh CT, Schaffer DV, Bertozzi CR, Lebrilla CB. Mol. Cell. Proteomics. 11(4):M111.010660. doi: 10.1074/mcp.M111.010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Leoz MLA, Young LJT, An HJ, Kronewiter SR, Kim J, Miyamoto S, Borowsky AD, Chew HK, Lebrilla CB. Mol. Cell. Proteomics. 10(1):M110. 002717. doi: 10.1074/mcp.M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lattova E, Varman S, Bezabeh T, Petrus L, Perrault H. J. Am. Soc. Mass Spectr. 2008;19:671–685. doi: 10.1016/j.jasms.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 6.(a) Du Y, Zhang M, Kong F. Tetrahedron. 2001;57:1757–1763. [Google Scholar]; (b) Sarkar S, Dutta S, Das G, Sen AK. Tetrahedron. 2011;67:4118–4122. [Google Scholar]; (c) Jiang R, Liang X-M, Jin S-H, Lu H-Z, Dong Y-H, Wang D-Q, Zhang JJ. Synthesis. 2016;48:213–222. [Google Scholar]; (d) Uriel C, Gomez AM, Lopez JC, Fraser-Reid B. Org. Biomol. Chem. 2012;10:8361–8370. doi: 10.1039/c2ob26432c. [DOI] [PubMed] [Google Scholar]; (e) Zhang Y, Chen C, Jin L, Tan H, Wang F, Cao H. Carbohydr. Res. 2015;401:109–114. doi: 10.1016/j.carres.2014.09.010. [DOI] [PubMed] [Google Scholar]; (f) Zhang J, Kong F. Tetrahedron: Asymmetry. 2002;13:243–252. [Google Scholar]; (g) Matsuo I, Miyazaka T, Isomura M, Sakakibara T, Ajisaka K. J. Carbohydr. Chem. 1998;17:1249–1258. [Google Scholar]; (h) Bailey JJ, Bundle DR. Org. Biomol. Chem. 2014;12:2193–2213. doi: 10.1039/c3ob42194e. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lee D, Taylor MS. Synthesis. 2012;44:3421–3431. [Google Scholar]; (b) Saloranta T, Leino R. Synlett. 2015;26:421–425. [Google Scholar]; (c) Jager M, Minnaard AJ. Chem. Commun. 2016;52:656–664. doi: 10.1039/c5cc08199h. [DOI] [PubMed] [Google Scholar]; (d) Das R, Mukhopadhyay B. ChemistryOpen. 2016;5:401–433. doi: 10.1002/open.201600043. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Matwiejuk M, Thiem J. Eur. J. Org. Chem. 2011;29:5860–5875. [Google Scholar]; (f) Smiljanic N, Sami Halila, Moreau V, Djedaïni-Pilard F. Tetrahedron Letters. 2003;44:8999–9002. [Google Scholar]
- 8.Wu L, Sampson NS. ACS Chem. Biol. 2014;9:468–475. doi: 10.1021/cb400550j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Gorska K, Huang K-T, Chaloin O, Winssinger N. Angew. Chem. Int. Ed. 2009;48:7695–7700. doi: 10.1002/anie.200903328. [DOI] [PubMed] [Google Scholar]; (b) Daines AM, Greatrex BW, Hayman CM, Hook SM, Mcburney WT, Rades T, Rendle PM, Sims IM. Bioorg. Med. Chem. 2009;17:5207–5218. doi: 10.1016/j.bmc.2009.05.043. [DOI] [PubMed] [Google Scholar]; (c) Upreti M, Ruhela D, Vishwakarma RA. Tetrahedron. 2000;56:6577–6584. [Google Scholar]
- 10.Matsuo I, Isomura M, Miyazaki T, Sakakibara T, Ajisaka K. Carbohydr. Res. 1998;305:401–413. doi: 10.1016/s0008-6215(97)10001-5. [DOI] [PubMed] [Google Scholar]
- 11.MSelma A-P, MJesus S. Tetrahedron. 1996;52:10785–10798. [Google Scholar]
- 12.Cmoch P, Pakulski Z. Tetrahedron: Asymmetry. 2008;19:1494–1503. [Google Scholar]
- 13.(a) Maggi A, Madsen R. Eur. J. Org. Chem. 2013:2683–2691. [Google Scholar]; (b) Gouliaras C, Lee D, Chan L, Taylor MS. J. Am. Chem. Soc. 2011;133:13926–13929. doi: 10.1021/ja2062715. [DOI] [PubMed] [Google Scholar]; (c) Muramatsu W, Yoshimatsu H. Adv. Synth. Catal. 2013;355:2518–2524. [Google Scholar]; (d) Oshima K, Yamauchi T, Shimomura M, Miyauchi S, Aoyama Y. Bull. Chem. Soc. Jpn. 2002;75:1319–1324. [Google Scholar]
- 14.(a) Crich D, Li W, Li H. J. Am. Chem. Soc. 2004;126:15081–15086. doi: 10.1021/ja0471931. [DOI] [PubMed] [Google Scholar]; (b) Pistorio SG, Yasomanee JP, Demchenko AV. Org. Lett. 2014;16:716–719. doi: 10.1021/ol403396j. [DOI] [PubMed] [Google Scholar]
- 15.(a) Zong GH, Yan SQ, Liang XM, Zhang JJ, Wang DQ, Kong FZ. Chin. Chem. Lett. 2009;20:127–130. [Google Scholar]; (b) Nishiyama K, Takakusagi Y, Kusayanagi T, Matsumoto Y, Habu S, Kuramochi K, Sugawara F, Sakaguchi K, Takahashi H, Natsugari H, Kobayashi S. Bioorg. Med. Chem. 2009;17:195–202. doi: 10.1016/j.bmc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Schaumberg JP, Hokanson GC, French JC, Smal E, Baker DC. J. Org. Chem. 1985;50:1651–1656. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.