Abstract
Purpose
Findings from RTOG 0617 suggested that collateral radiation to the heart may contribute to early death in patients receiving chemoradiation therapy for non-small cell lung cancer (NSCLC); however, reports of cardiac toxicity after thoracic radiation therapy (RT) remain limited. Because pericardial disease is the most common cardiac complication of thoracic RT, we investigated the incidence of and risk factors for pericardial effusion (PCE) in patients enrolled in a phase II, prospective randomized study of intensity modulated RT (IMRT) versus proton therapy for locally advanced NSCLC.
Methods and Materials
From July 2009 through April 2014, 201 patients were prospectively treated with proton beam therapy or IMRT to 60-74 Gy with concurrent chemotherapy. The primary endpoint (grade ≥2 PCE) was diagnosed upon review of follow-up images. Clinical characteristics and cardiac dose-volume parameters associated with PCE were identified via Cox proportional hazards modeling and recursive partitioning analysis of null Martingale residuals. Reproducibility was evaluated in a separate retrospective cohort of 301 patients.
Results
Cumulative incidence rates of PCE among patients in the trial were 31.4% at 1 year and 45.4% at 2 years, with median time to PCE of 8.9 months. Several cardiac dose-volume parameters (e.g., V20–V65) predicted PCE, but heart V35 (HV35) was the most strongly associated, with cut-off volume of 10%. On multivariable analysis, HV35 >10% independently predicted PCE (hazard ratio [HR] 2.14, p=0.002), a finding that maintained reproducibility in the retrospective validation cohort. Other factors associated with PCE included receipt of adjuvant chemotherapy (HR 2.82, p<0.001) and prior cardiac disease (HR 1.68, p=0.020).
Conclusions
PCE was common after RT for NSCLC, occurring in nearly half of patients even after moderate radiation doses to the heart. Adjuvant chemotherapy may increase the risk of PCE, and HV35 >10% may identify patients at risk for development of this cardiac toxicity.
Keywords: pericardial effusion, cardiac toxicity, non-small cell lung cancer, radiotherapy, chemotherapy
Introduction
The standard of care for locally advanced, inoperable non-small cell lung cancer (NSCLC) is radiation therapy (RT) and concurrent chemotherapy (1), with lung and esophageal toxicity traditionally considered the major treatment-related sequelae (2). The effects of cardiac irradiation in such patients have been largely deprioritized, because survival time was considered too short for the patients to manifest RT-related cardiac toxicity. This notion has been questioned, however, after a phase III trial (Radiation Therapy Oncology Group [RTOG] 0617) demonstrated an association between the cardiac dose-volume variables V5 and V30 with poor survival, suggesting that collateral radiation to the heart could contribute to early mortality in this population (3). As disease-specific survival rates continue to improve for patients with locally advanced NSCLC, factors that may influence overall survival warrant increased consideration.
The most common cardiac complication of thoracic RT is pericardial disease, which may be accompanied by pericardial effusion (PCE) and in some cases cardiac tamponade (3, 4). In one autopsy series, 70% of patients with a history of thoracic RT had evidence of pericardial damage (5). However, clinical reports of cardiac toxicity after definitive chemoradiation for NSCLC are limited, and details regarding treatment-related PCE in NSCLC are sparse.
Thus, we sought to investigate the incidence of and potential risk factors for PCE in the context of a large prospective randomized trial of patients treated with chemoradiation for locally advanced NSCLC. In addition to clinical risk factors, we sought to determine reproducible dose-volume parameters associated with PCE that could potentially serve as clinical predictors of this cardiac toxicity.
Methods and Materials
Patients
Patients in the prospective cohort were enrolled in an NCI-sponsored randomized controlled trial comparing intensity-modulated radiation therapy (IMRT) versus passive scattering proton beam therapy for locally advanced, inoperable NSCLC (6). A total of 201 patients treated between June 2009 and April 2014 at a single institution were included in the analysis.
The retrospective validation dataset included patients treated with either definitive IMRT or proton therapy for inoperable NSCLC. Patients with a history of chest irradiation or PCE before treatment were excluded. A total of 301 patients treated from April 2004 through May 2010 at the same institution as the prospective-trial patients were included in the validation analysis.
This study was approved by the institutional review board, and patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act.
Treatment
All patients were treated with image-guided IMRT or proton therapy to a prescribed dose of ≥60 Gy in conventional daily fractionation using treatment techniques described elsewhere (7, 8). For the prospective patient group, the choice of radiation modality was designated in a randomized fashion, and in the retrospective group the modality was chosen at the discretion of the treating physician.
Cardiac Contours and DVH Parameters
All patients underwent standardized heart contouring as defined in the RTOG Atlas (9), and all contours were reviewed by a board-certified radiation oncologist. The following cardiac dosimetric parameters were extracted from the treatment planning system for each patient: mean heart dose, maximum heart dose, and the proportion of cardiac volume (%V) receiving more than a certain dose of radiation, ranging from 20 Gy (V20) to 65 Gy (V65) in increments of 5 Gy.
Follow-up and Primary Endpoint
Post-treatment follow-up evaluations included surveillance radiographic studies of the chest, in the form of either computed tomography (CT) scans with contrast or positron emission tomography (PET)/CT scans. Standard follow-up imaging took place 6 weeks after RT completion, followed by every 3-6 months for the next 2 years, and subsequently every 6-12 months thereafter. Each scan was evaluated for PCE, with findings documented in diagnostic radiology reports and confirmed independently by a board-certified radiation oncologist. CT imaging is considered a sensitive test for the detection of PCE (10, 11). PCE is readily apparent on CT imaging, in which the thickness of normal pericardium is generally accepted as 2 mm (12).
PCE was graded according to the Common Terminology Criteria for Adverse Events v4.0 as follows: grade 2, asymptomatic effusion; grade 3, effusion with physiologic consequences (warranting management or close follow-up); grade 4, life-threatening consequences, urgent intervention indicated; and grade 5, death. All patients with grade ≥3 toxicity underwent echocardiography for additional evaluation. Clinical records were reviewed for additional evidence of cardiac complications.
Statistical Analyses
The primary endpoint was PCE development, with time to development computed from the start of RT to the date of first documented PCE. Patients who did not develop PCE were censored at time of last follow-up or death. Actuarial rates of freedom from PCE development and overall survival (OS) were calculated via the Kaplan-Meier method, with log-rank tests used to evaluate potential differences between groups.
Various clinical factors (age, sex, clinical disease stage, tumor location and histology, radiation modality, receipt of chemotherapy, history of cardiac disease, smoking status [current/former vs. never], alcohol use, diabetes) and cardiac dose-volume parameters were assessed for potential association with PCE development. Cardiac disease was defined as any history of ischemic (e.g. coronary artery disease), structural (e.g. aortic stenosis, aortic valve replacement), mechanical (e.g. heart failure), or conductive (e.g. atrial fibrillation) abnormality involving the heart itself. Cardiac risk factors such as hypertension and hyperlipidemia were excluded from this definition and documented separately. Patients with preexisting pericardial effusion were excluded from the analysis.
Univariate and multivariable analyses were conducted via Cox proportional hazards modeling, with hazard ratios (HRs) calculated for PCE development. Patient demographic and clinical characteristics in both the prospective and retrospective patient groups were compared by using t-tests for continuous variables and χ2 tests for categorical variables.
Evaluated dose thresholds ranged from V20 to V65 in increments of 5 Gy, with %V ranging from 5% to 95%. Rates of PCE development were compared between subsets of patients with cardiac V(dose)≤%V versus V(dose)>%V (e.g. V10≤15% versus V10>15%) via Cox proportional hazards analysis. Dose-volume constraints (e.g., V10>15%) found to be statistically significant (p<0.05) were considered candidate dosimetric factors influencing the risk of PCE development.
Optimal cut-off values (%V) for dose thresholds (V20-V65) were identified by using recursive partitioning analysis of null Martingale residuals (NMRs), derived from fit of the null (constant-only) Cox proportional hazards model of the DVH parameter data (13). For each dose threshold (V20-V65), the volume parameter (5%-95%) demonstrating the greatest absolute difference in mean NMRs between corresponding subgroups was selected as the optimal cut-off value.
Statistical analyses were conducted with SAS version 9.4 (SAS Institute Inc.; Cary, NC), Stata version 9 (StataCorp, 2005; Stata Statistical Software: Release 9; College Station, TX: StataCorp LP), and SPSS version 23 (IBM Corp, release 2015; IBM SPSS Statistics for Windows, Version 23.0; Armonk, NY: IBM Corp).
Results
The median follow-up time for the prospective group was 23.9 months (range 2.0–82.4 months), which was comparable to the median follow-up time of the retrospective group (20.3 months [range 1.7–70.8 months]). In the prospective group, the median prescribed RT dose was 74 Gy (range 60–74 Gy) in daily 2-Gy fractions, similar to the median prescribed dose in the retrospective group of 70 Gy (range 48–87.5 Gy) in daily 2-Gy fractions (range 1.71–3 Gy per fraction). The median mean heart dose was 12.2 Gy (range 0.01-41 Gy) in the prospective group and 8.6 Gy (range 0-47 Gy) in the retrospective group, respectively.
Clinical and demographic variables of the randomized prospective and retrospective patient cohorts are shown in Table 1. To establish the retrospective dataset as being appropriate for validation of the results, each variable was evaluated for potential differences between the two groups. No significant differences were found with regard to patient age, sex, smoking status, diabetes, disease stage, tumor histology, radiation modality, receipt of induction chemotherapy, or receipt of adjuvant chemotherapy. Greater proportions of patients in the concurrent group received concurrent chemotherapy (99.0% vs. 84.7%, p<0.001), had right-sided tumors (62.2% vs. 37.8%, p=0.005), and consumed alcohol (48.8% vs. 36.3%, p=0.005) in the relative to the retrospective group. In contrast, a greater proportion of patients in the retrospective group had pre-existing heart disease (60.5% vs. 45.3% in the prospective group, p=0.001).
Table 1. Clinical and demographic variables in the prospective (n=201) and retrospective (n=301) patient groups.
| Variable | No. of Patients (%), Prospective Group | HR (95% CI) | P Value* | No. of Patients (%), Retrospective Group | P Value† |
|---|---|---|---|---|---|
| Age | |||||
| >70 | 62 (30.8) | 0.73 (0.44–1.22) | 0.227 | 106 (35.2) | 0.309 |
| <70 | 139 (69.2) | 195 (64.8) | |||
| Sex | |||||
| Female | 88 (43.8) | 0.84 (0.55–1.29) | 0.428 | 126 (41.9) | 0.670 |
| Male | 113 (56.2) | 175 (58.1) | |||
| Smoker | |||||
| Yes | 183 (91.0) | 0.83 (0.42–1.66) | 0.603 | 272 (90.4) | 0.798 |
| No | 18 (9.0) | 29 (9.6) | |||
| Alcohol Use | |||||
| Yes | 98 (48.8) | 1.17 (0.77–1.79) | 0.462 | 109 (36.3) | 0.005 |
| No | 103 (51.2) | 192 (63.7) | |||
| Diabetes | |||||
| Yes | 36 (17.9) | 0.89 (0.48–1.64) | 0.708 | 39 (13.2) | 0.127 |
| No | 165 (82.1) | 262 (86.8) | |||
| Heart Disease | |||||
| Yes | 91 (45.3) | 1.32 (0.86–2.01) | 0.202 | 182 (60.5) | 0.001 |
| No | 110 (54.7) | 119 (39.5) | |||
| Disease Stage | |||||
| I-II | 15 (7.5) | 33 (11.0) | |||
| III-IV | 174 (86.6) | 0.86 (0.60–1.23) | 0.414 | 248 (82.4) | 0.391 |
| Recurrence | 12 (6.0) | 20 (6.6) | |||
| Tumor Histology | |||||
| Non-Adeno | 95 (47.3) | 0.92 (0.66–1.28) | 0.628 | 184 (61.1) | 0.062 |
| Adenocarcinoma | 106 (52.7) | 117 (38.9) | |||
| Tumor Location | |||||
| Left | 76 (37.8) | 117 (38.9) | |||
| Right | 125 (62.2) | 0.66 (0.43–1.02) | 0.058 | 169 (56.1) | 0.005 |
| Other | 15 (5.0) | ||||
| RT Modality | |||||
| Proton | 75 (37.3) | 0.92 (0.59–1.44) | 0.704 | 128 (42.5) | 0.244 |
| Photon (IMRT) | 126 (62.7) | 173 (57.5) | |||
| Chemotherapy | |||||
| Induction | 66 (32.8) | 0.99 (0.63-1.55) | 0.952 | 95 (31.6) | 0.764 |
| Concurrent | 199 (99.0) | 20 (0.001–7×105) | 0.575 | 255 (84.7) | <0.001 |
| Adjuvant | 36 (17.9) | 2.18 (1.36–3.49) | 0.001 | 63 (20.9) | 0.405 |
Abbreviations: HR, hazard ratio; CI, confidence interval; RT, radiation therapy; IMRT, intensity-modulated radiation therapy.
Cox proportional hazards for univariate analysis of associations with PCE development
Chi-squared test to assess differences in categorical variables between the two patient groups
PCE Incidence
In the prospective group, PCE developed in 86 patients (42.8%; 81 [40.3%] grade 2 and 5 [2.5%] grade 3). Although the median time to PCE development was 8.9 months (range 0.7–40.2 months), the incidence slowly increased over time, with 1-year and 2-year cumulative incidence rates of PCE of 31.4% and 45.4% (Fig. 1). Findings from the retrospective validation group were similar, with PCE developing in 118 patients (39.2%); 116 [38.5%] grade 2 and 2 [0.7%] grade 3). The median time to PCE development was 8.7 months (range 0.3–55.6 months), with 1-year and 2-year cumulative incidence rates of PCE of 28.9% and 40.8% (Fig. 1).
Figure 1.
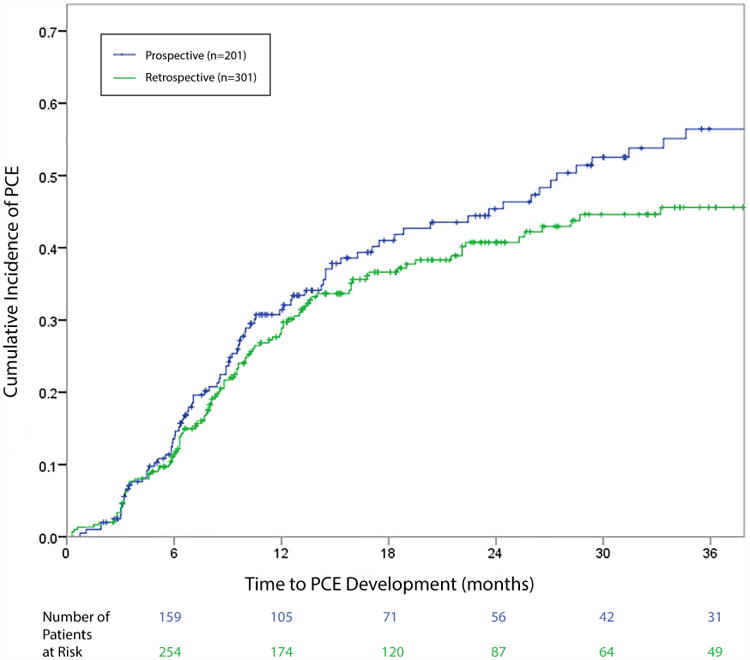
Cumulative incidence of pericardial effusion (PCE) in the prospective (training) and retrospective (validation) patient groups. No significant difference was found in PCE rates between the prospective group (31.4% at 1 year, 45.4% at 2 years) and the retrospective (validation) group (28.9% at 1 year, 40.8% at 2 years) (log-rank p=0.364). Median time to PCE development was 8-9 months in both groups.
In the prospective group, OS rates were higher for patients who developed PCE than for those who did not (69.0% vs. 44.3% at 2 years, p=0.012). This initial gap closed upon longer follow-up, however, with 5-year OS rates of 43.3% versus 25.6%. Findings for the retrospective group were similar, with 2-year OS rates of 60.4% who did develop PCE versus 46.9% for patients who did not (p=0.047). This initial difference also diminished over longer follow-up, with corresponding 5-year OS rates of 28.8% versus 25.7%. Mean heart dose was not predictive of OS in either the prospective (p=0.665) or retrospective (p=0.052) group.
Cardiac Dose-Volume Variables Associated with PCE Development
On univariate analysis, differences in the risk of PCE development were apparent from several cardiac DVH parameters, ranging from V20 to V65 as well as mean heart dose (Table 2). Optimal cut-off values for each dose threshold were subsequently identified via recursive partitioning analysis of the NMRs (Fig. 2). Of the various cardiac dose variables, heart V35 (HV35) was most strongly associated with PCE development, with a cut-off volume of 10% (p=0.004). Indeed, the cumulative incidence of PCE development was significantly higher in patients who received HV35>10% versus HV35≤10% (38.2% vs. 21.1% at 1 year and 56.2% vs. 27.1% at 2 years; log-rank p=0.003) (Fig. 3a). All patients who experienced grade 3 PCE had an HV35 that exceeded 10%.
Table 2. Cardiac dose-volume parameters associated with development of pericardial effusion.
| Cardiac Dose-Volume Parameter | Cutoff Volume (%V)* | HR (95% CI) | P Value† |
|---|---|---|---|
| Heart V20 | 21 | 1.72 (1.17-2.65) | 0.014 |
| Heart V25 | 21.66 | 1.72 (1.13-2.62) | 0.012 |
| Heart V30 | 18.95 | 1.77 (1.16-2.71) | 0.009 |
| Heart V35 | 10.20 | 2.02 (1.26-3.23) | 0.004 |
| Heart V40 | 9.21 | 1.88 (1.19-2.97) | 0.007 |
| Heart V45 | 7.99 | 1.95 (1.23-3.10) | 0.005 |
| Heart V50 | 7.00 | 1.93 (1.23-3.02) | 0.004 |
| Heart V55 | 5.41 | 1.98 (1.25-3.14) | 0.004 |
| Heart V60 | 4.98 | 1.74 (1.13-2.68) | 0.012 |
| Heart V65 | 3.02 | 1.76 (1.14-2.73) | 0.011 |
| Mean Heart Dose | 12.00 Gy | 1.79 (1.16-2.77) | 0.008 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Identified via recursive partitioning analysis of the null Martingale residuals
Cox proportional hazards
Figure 2.
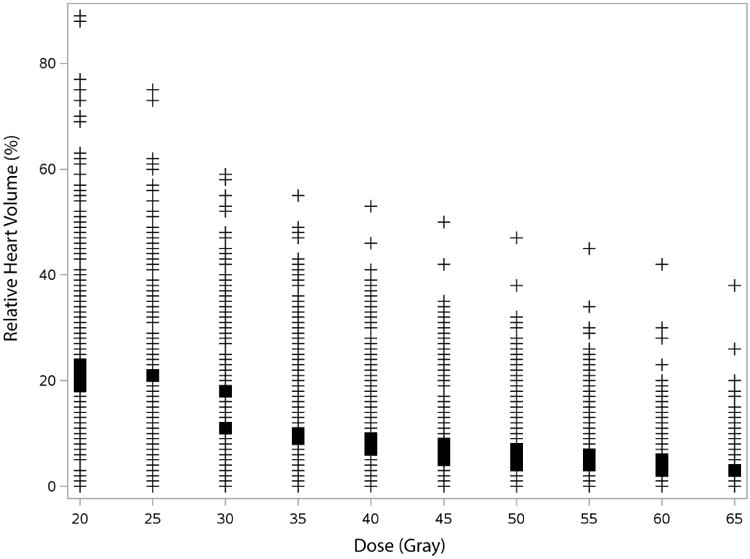
Cardiac dose-volume variables associated with development of pericardial effusion (PCE). Plotted points (non-weighted) represent actual cardiac volume levels (%V) for each dose threshold (V20-V65) witnessed in the prospective patient population (n=201). Bold marks on columns represent cardiac dose-volume histogram (DVH) variables for which a significant difference (p<.05) was found in freedom from PCE development in patients with V(dose) <%V versus V(dose) ≥%V. Optimal cut-off values for each heart dose (within the bold marks) were identified via recursive partitioning analysis of the null Martingale residuals.
Figure 3.
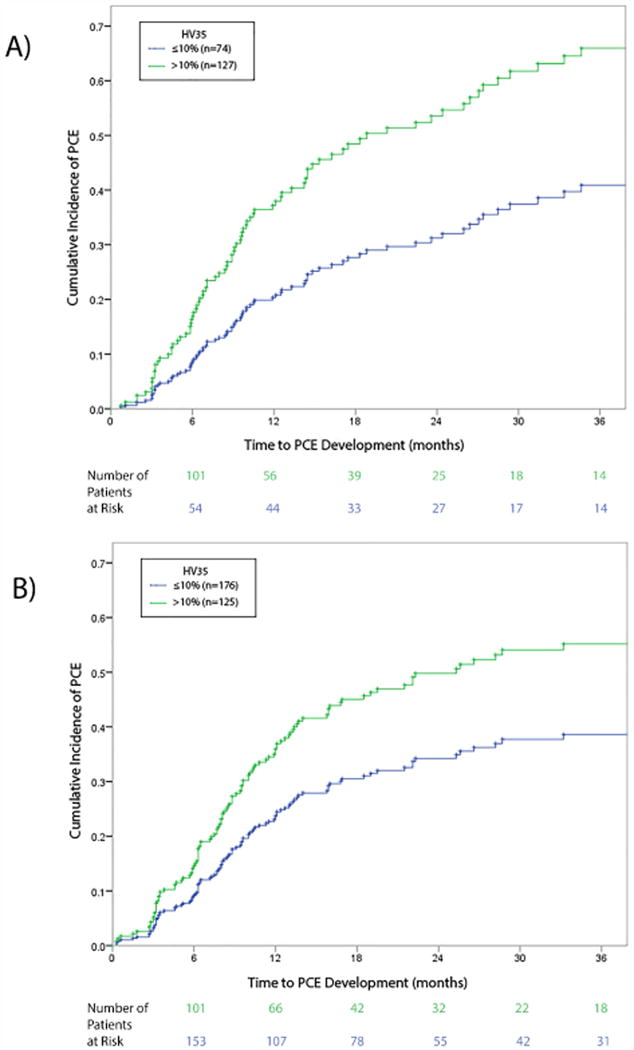
A cardiac dose threshold of heart volume (HV)35 >10% predicts PCE development in both patient groups. (A) In the patients from the prospective trial, the cumulative incidence of PCE was significantly higher for patients with HV35 >10% (38.2% vs. 21.1% for HV35 ≤10% at 1 year, and 56.2% vs. 27.1% HV35 ≤10% at 2 years; log-rank p=0.003). (B) Results were similar findings in the retrospective (validation) patient group: the cumulative incidence of PCE was higher for those with HV35 >10% than for those with HV35 ≤10% (37.7% vs. 23.3% at 1 year, and 49.5% vs. 34.5% at 2 years; log-rank p=0.006).
Clinical Factors Associated with PCE Development
Each of the clinical and demographic variables listed in Table 1 was evaluated for possible association with PCE development. On univariate analysis, the only factor associated with PCE development was receipt of adjuvant chemotherapy (p=0.001). Of note, there was no difference in PCE incidence with regard to proton versus photon radiation modality (p=.704).
From the dosimetric findings described above, we next selected HV35>10% as a cardiac dose-volume parameter to validate in multivariable analysis, along with clinical factors expected to be associated with PCE development (p<0.25). Multivariable analysis (Table 3) revealed the following independent risk factors for PCE development: HV35>10% (HR 2.14, p=0.002), receipt of adjuvant chemotherapy (HR 2.82, p<0.001), and history of cardiac disease (HR 1.68, p=0.020). Having a right-sided tumor was associated with a lower risk of PCE development (HR 0.52, p=0.004). Age (≥70 years vs. <70 years) was not found to be significant (p=0.127). As a trial of another cardiac dose metric, mean heart dose (>12 Gy vs. ≤12 Gy) was incorporated into the multivariate analysis, both in lieu of and in addition to HV35>10%. In either case, tumor laterality maintained statistical significance as an independent predictor for PCE (p=.003).
Table 3. Multivariable analysis of clinical and cardiac dose-volume variables associated with PCE development in prospective and retrospective patient groups.
| Variable | Prospective Group | Retrospective Group | ||
|---|---|---|---|---|
| HR (95% CI) | P Value* | HR (95% CI) | P Value* | |
| HV35 | ||||
| >10% vs. <10≤ | 2.14 (1.31-3.51) | 0.002 | 1.52 (1.05-2.21) | 0.027 |
| Age, years | ||||
| ≥70 vs. <70 | 0.67 (0.40-1.12) | 0.127 | 0.93 (0.61-1.42) | 0.741 |
| Tumor Location | ||||
| Right vs. Left | 0.52 (0.33-0.81) | 0.004 | 1.14 (0.79-1.66) | 0.477 |
| Adjuvant Chemotherapy | ||||
| Yes vs. No | 2.82 (1.72-4.65) | <0.001 | 1.40 (0.93-2.09) | 0.108 |
| Cardiac History | ||||
| Yes vs. No | 1.68 (1.09-2.60) | 0.020 | 0.81 (0.56-1.18) | 0.278 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Cox proportional hazards model
In the validation set (the retrospective group), the cumulative incidence of PCE development was also higher for patients with HV35>10% than for those with HV35≤10% (37.7% vs. 23.3% at 1 year and 49.5% vs. 34.5% at 2 years; log-rank p=0.006) (Fig. 3b). HV35>10% was also validated as being an independent predictor of PCE in the retrospective group on multivariable analysis (HR 1.52, p=0.027), using the same variables as were assessed for the prospective group (Table 3).
Discussion
In this study investigating PCE development after chemo-RT for locally advanced NSCLC, our pertinent findings were as follows: (a) the incidence was high at almost 50%, with median onset of less than 1 year; (b) clinical variables associated with this toxicity included receipt of adjuvant chemotherapy, history of cardiac disease, and a left-sided tumor; and (c) HV35>10% was the most robust cardiac dose-volume variable associated with PCE development.
It has been shown that radiation therapy may be responsible for roughly 16% of pericardial effusions detected in a general population of cancer patients (14). The rates of PCE development in our study were surprisingly high, with 2-year cumulative incidence of 45.4% in the prospective group and 40.8% in the retrospective group. These findings are supported by previous reports of 27.7% to 52.2% for patients with esophageal cancer (15–18), suggesting that PCE may be a common yet underreported toxicity after thoracic RT.
Despite this high incidence, most cases were asymptomatic, as evident from our relatively low rates of grade≥3 toxicity in both groups. Interestingly, developing PCE seemed to confer an initial survival benefit in this study, although this apparent difference diminished with longer follow-up time. Of note, a modest survival benefit has also been demonstrated for patients who develop mild radiation pneumonitis (6), suggesting that the development of some mild inflammatory reaction may be correlated with tumor response. Regardless, although most cases of PCE seem to be self-limiting, a small percentage of patients will ultimately require intervention for symptomatic disease or cardiac compromise (19–21); therefore, PCE should be evaluated, particularly for high-risk patients.
In this study, clinical variables significantly associated with PCE development included receipt of adjuvant chemotherapy, history of cardiac disease, and having a left-sided tumor. Adjuvant chemotherapy was the most strongly associated factor on multivariate analysis. This is not entirely surprising, as cardiac toxicity has been linked to several chemotherapeutic agents used to treat NSCLC, including platinum-based agents (22, 23), taxanes (24), gemcitabine (25), bevacizumab, and vinorelbine (26). Reports are also emerging reports on PCE development after the use of targeted inhibitors (27) and immunotherapeutic agents (28). On the basis of our findings, we suggest applying a stricter cardiac dose threshold with selective cardiac sparing for high-risk patients for whom adjuvant chemotherapy is planned.
Our analysis also revealed several cardiac dose-volume variables associated with PCE development, ranging from V20 to V65. Previous investigations of esophageal cancer have similarly demonstrated relationships between dose-volume variables and PCE (16, 18, 29–31), yet discrepancies are evident regarding the target volume at risk. A few studies have focused on the pericardium itself, although the defined contour volumes vary widely from paper to paper (16, 30, 31). Unfortunately, these variations make interpretation and practical application of the findings difficult, as the pericardium is not routinely considered or contoured at most radiation treatment facilities. We instead focused on associations with whole heart volume, a structure routinely contoured by radiation oncologists and dosimetrists, in an effort to identify relevant variables that are translatable among providers. In our study, the volume of heart was delineated in a standardized fashion for each patient as previously defined (9).
This approach is similar to other studies demonstrating associations with whole heart volume (18, 29, 31), although no consensus has been reached regarding which dose-volume variables are truly significant for PCE development. For example, Hayashi et al. found cardiac V10 to be an influential factor, with a cut-off value of 72.8% (18), whereas another Japanese study found V45, V50, and V55 to be associated with cardiac disease, with corresponding cut-off values as low as 15%, 10%, and 5% (29). Yet another group found cardiac V20, V30, and V40 to correlate with cardiac toxicity (31). Unfortunately, the wide range of previously reported values reflects an overall lack of reproducible variables that can be easily applied in clinical practice.
In our study, the strongest dose-volume predictor of PCE development was HV35>10%, which was confirmed on multivariate analysis. To address the issue of reproducibility, we subsequently validated this threshold value in a separate, larger, retrospective group of analogous patients, and this value maintained its significance as an independent predictor of PCE development. Given the relatively robust nature of the supportive data, HV35>10% could be implemented to reduce the likelihood of PCE development or progression, particularly for high-risk patients with pre-existing heart disease sensitive to further functional cardiac impairment (e.g. heart failure). As the strongest predictor, this cardiac metric can function as a surrogate dose constraint for the likely continuous dose-volume relationship underlying these findings.
Limitations of this study include its single-institution nature, a constraint that we attempted to mitigate by using a validation set. While similar on many levels, the retrospective group consisted of a more heterogeneous population than the randomized prospective group treated on trial. There was a significantly greater proportion of left-sided tumors and patients with history of cardiac disease in the retrospective group. Though not statistically significant, there was also a slightly greater proportion of early stage disease, accompanied by smaller treatment volumes, in the retrospective group versus the prospective group, which consisted of locally advanced cases. Furthermore, while the proportion of patients receiving adjuvant chemotherapy was similar, there was greater variation with respect to regimen and timing within the retrospective group. Finally, clinical documentation of the retrospective group, dating back to 2004, was unavoidably less comprehensive than that of the prospective group treated on trial starting in 2009, roughly 5 years later. These discrepancies could account for the differences in significant findings on multivariate analysis.
The incidence of grade≥3 PCE was admittedly low in both of our patient groups, thereby limiting our ability to evaluate predictors of high-grade toxicity. The follow-up time in our study may be too short to detect symptomatic disease, as reported intervals for symptomatic pericardial disease range from 2 months up to 145 months after RT (5). Given the expected survival times of these patients, however, the initial 1-2 years after RT are arguably the most critical in terms of adverse events. Further, the median timeframe for developing PCE in both of our patient groups was less than 1 year, thereby supporting the adequacy of follow-up in the context of this study. Of note, however, our study was not designed to evaluate for other cardiac toxicities secondary to radiation (e.g. ischemic heart disease), which could have a larger impact on cardiac-related mortality. Such toxicities are likely to have different dose-volume relationships and temporal incidences than pericardial effusion. A truly comprehensive evaluation of all cardiac-related toxicities likely requires a longer follow-up period than that of the current investigation.
Finally, we acknowledge that the suggested dose threshold may be difficult to achieve in some cases, such as those cases in which target volumes encompass a significant portion of the heart (e.g., left lower lobe tumors). We advocate for selective cardiac sparing specifically for high-risk patients with mitigating factors, such as pre-existing heart disease or effusion, with the goal of preventing potential exacerbation of clinical symptoms. Although this dose threshold may seem low in comparison to traditional cardiac dose constraints, with modern RT-planning techniques a goal of HV35 ≤10% can be achieved for many patients and may thus be considered for patients at high risk of cardiac-related morbidity.
In conclusion, this is the first study, to our knowledge, to investigate the incidence of and risk factors for PCE in patients treated with RT for NSCLC. We found that the incidence of PCE was high, at almost 50%, with median onset of less than a year. Several clinical factors were associated with PCE, including receipt of adjuvant chemotherapy, and our findings suggest that even moderate doses of radiation may increase the probability of this adverse event. Therefore, we suggest a cardiac dose constraint of HV35 ≤10% for patients at particular risk of clinical sequelae from the development or progression of this toxicity.
Summary.
We investigated the incidence of and risk factors for pericardial effusion in patients with locally advanced non-small cell lung cancer in a prospective randomized controlled trial. Pericardial effusion occurred in nearly half of all patients even after moderate radiation doses to the heart. Adjuvant chemotherapy may increase the risk of pericardial effusion, and a cardiac V35 >10% may predict for the development of this toxicity
Acknowledgments
We extend gratitude towards Christine (Chris) F. Wogan for her valuable assistance in editing and formatting this manuscript.
Funding: Supported in part by Cancer Center Support (Core) Grant CA016672 from the National Institute of Cancer, National Institutes of Health, to The University of Texas MD Anderson Cancer Center; and by grant PO1-CA 021239 from the National Cancer Institute.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anon Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol Off J Am Soc Clin Oncol. 1997;15:2996–3018. doi: 10.1200/JCO.1997.15.8.2996. [DOI] [PubMed] [Google Scholar]
- 2.Werner-Wasik M, Paulus R, Curran WJ, et al. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non-small-cell lung cancer: analysis of the radiation therapy oncology group (RTOG) database. Clin Lung Cancer. 2011;12:245–251. doi: 10.1016/j.cllc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson RG, Mayfield WR, Normann S, et al. Radiation-associated valvular disease. Chest. 1991;99:538–545. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]
- 5.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 6.Liao ZX, Lee JJ, Komaki R, et al. Bayesian randomized trial comparing intensity modulated radiation therapy versus passively scattered proton therapy for locally advanced non-small cell lung cancer. J Clin Oncol. 2016;34 [Google Scholar]
- 7.Tucker SL, Liu A, Gomez D, et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2016;119:495–500. doi: 10.1016/j.radonc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen QN, Ly NB, Komaki R, et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2015;115:367–372. doi: 10.1016/j.radonc.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong BY, Lee KR, MacArthur RI. Diagnosis of pericardial effusion by computed tomography. Chest. 1982;81:177–181. doi: 10.1378/chest.81.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Tomoda H, Hoshiai M, Furuya H, et al. Evaluation of pericardial effusion with computed tomography. Am Heart J. 1980;99:701–706. doi: 10.1016/0002-8703(80)90618-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZJ, Reddy GP, Gotway MB, et al. CT and MR imaging of pericardial disease. Radiogr Rev Publ Radiol Soc N Am Inc. 2003;23 doi: 10.1148/rg.23si035504. Spec No:S167-180. [DOI] [PubMed] [Google Scholar]
- 13.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. Springer Science & Business Media; 2000. [Google Scholar]
- 14.El Haddad D, Iliescu C, Yusuf SW, et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion. J Am Coll Cardiol. 2015;66:1119–1128. doi: 10.1016/j.jacc.2015.06.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 16.Fukada J, Shigematsu N, Takeuchi H, et al. Symptomatic pericardial effusion after chemoradiation therapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys. 2013;87:487–493. doi: 10.1016/j.ijrobp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Tamari K, Isohashi F, Akino Y, et al. Risk factors for pericardial effusion in patients with stage I esophageal cancer treated with chemoradiotherapy. Anticancer Res. 2014;34:7389–7393. [PubMed] [Google Scholar]
- 18.Hayashi K, Fujiwara Y, Nomura M, et al. Predictive factors for pericardial effusion identified by heart dose-volume histogram analysis in oesophageal cancer patients treated with chemoradiotherapy. Br J Radiol. 2015;88:20140168. doi: 10.1259/bjr.20140168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumekawa Y, Kaneko K, Ito H, et al. Late toxicity in complete response cases after definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Gastroenterol. 2006;41:425–432. doi: 10.1007/s00535-006-1771-8. [DOI] [PubMed] [Google Scholar]
- 20.Kodama K, Takami H, Izumi M, et al. Late cardiac effect of radiation therapy on a young woman with mediastinal Hodgkin's lymphoma. Gen Thorac Cardiovasc Surg. 2016;64:51–54. doi: 10.1007/s11748-014-0423-9. [DOI] [PubMed] [Google Scholar]
- 21.Loire R, Saint-Pierre A. [Radiation-induced pericarditis. Long-term outcome. 45 cases with thoracotomy and biopsy] Presse Medicale Paris Fr 1983. 1990;19:1931–1936. [PubMed] [Google Scholar]
- 22.Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 23.Yano S, Shimada K. Vasospastic angina after chemotherapy by with carboplatin and etoposide in a patient with lung cancer. Jpn Circ J. 1996;60:185–188. doi: 10.1253/jcj.60.185. [DOI] [PubMed] [Google Scholar]
- 24.Fossella FV, Lee JS, Murphy WK, et al. Phase II study of docetaxel for recurrent or metastatic non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 1994;12:1238–1244. doi: 10.1200/JCO.1994.12.6.1238. [DOI] [PubMed] [Google Scholar]
- 25.Kido H, Morizane C, Tamura T, et al. Gemcitabine-induced pleuropericardial effusion in a patient with pancreatic cancer. Jpn J Clin Oncol. 2012;42:845–850. doi: 10.1093/jjco/hys099. [DOI] [PubMed] [Google Scholar]
- 26.Zabernigg A, Gattringer C. Myocardial infarction associated with vinorelbine (Navelbine) Eur J Cancer Oxf Engl 1990. 1996;32A:1618–1619. doi: 10.1016/0959-8049(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 27.Kastoon T, Stump CS, Thomson SP, et al. Erlotinib-associated exacerbation of hypothyroidism with pericardial tamponade. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2012;18:e111–113. doi: 10.4158/EP11182.CR. [DOI] [PubMed] [Google Scholar]
- 28.Kushnir I, Wolf I. Nivolumab-Induced Pericardial Tamponade: A Case Report and Discussion. Cardiology. 2017;136:49–51. doi: 10.1159/000447053. [DOI] [PubMed] [Google Scholar]
- 29.Ogino I, Watanabe S, Iwahashi N, et al. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther. Onkol. Organ Dtsch Rontgengesellschaft Al. 2016;192:359–367. doi: 10.1007/s00066-016-0956-1. [DOI] [PubMed] [Google Scholar]
- 30.Martel MK, Sahijdak WM, Ten Haken RK, et al. Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys. 1998;40:155–161. doi: 10.1016/s0360-3016(97)00584-1. [DOI] [PubMed] [Google Scholar]
- 31.Konski A, Li T, Christensen M, et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2012;104:72–77. doi: 10.1016/j.radonc.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


