Abstract
Background
There has been concern that exposure to lithium early in pregnancy may be associated with a marked increase in the risk of Ebstein’s anomaly (a right ventricular outflow tract obstruction defect) in infants and overall congenital cardiac defects, but data are conflicting and limited.
Methods
We conducted a cohort study involving 1,325,563 pregnancies in women who were enrolled in Medicaid and who delivered a live-born infant between 2000 and 2010. We examined the risk of cardiac malformations among infants exposed to lithium during the first trimester as compared with unexposed infants and, in secondary analyses, with infants exposed to another commonly used mood stabilizer, lamotrigine. Risk ratios and 95% confidence intervals were estimated with control for psychiatric and medical conditions, medications, and other potential confounders.
Results
Cardiac malformations were present in 16 of the 663 infants exposed to lithium (2.41%), 15,251 of the 1,322,955 nonexposed infants (1.15%), and 27 of the 1945 infants exposed to lamotrigine (1.39%). The adjusted risk ratio for cardiac malformations among infants exposed to lithium as compared with unexposed infants was 1.65 (95% confidence interval [CI], 1.02 to 2.68). The risk ratio was 1.11 (95% CI, 0.46 to 2.64) for a daily dose of 600 mg or less, 1.60 (95% CI, 0.67 to 3.80) for 601 to 900 mg, and 3.22 (95% CI, 1.47 to 7.02) for more than 900 mg. The prevalence of right ventricular outflow tract obstruction defects was 0.60% among lithium-exposed infants versus 0.18% among unexposed infants (adjusted risk ratio, 2.66; 95% CI, 1.00 to 7.06). Results were similar when lamotrigine-exposed infants were used as the reference group.
Conclusions
Maternal use of lithium during the first trimester was associated with an increased risk of cardiac malformations, including Ebstein’s anomaly; the magnitude of this effect was smaller than had been previously postulated. (Funded by the National Institute of Mental Health.)
In the early 1970s, results from the International Register of Lithium Babies evaluating infants born to mothers who were treated with lithium early in pregnancy1,2 suggested a risk of Ebstein’s anomaly, a right ventricular outflow tract obstruction defect, that was increased by a factor of 400 (on the basis of two cases associated with lithium exposure) and a risk of overall cardiac defects that was increased by a factor of 5.3 By 1979, the final report included data on 225 infants born to lithium-exposed women; 18 infants had congenital cardiac defects (8%), including 6 with Ebstein’s anomaly (3%).4 On the basis of these data, regulatory agencies concluded that there was evidence of human fetal risk but that potential benefits may warrant use in pregnant women.5
Despite the warnings, lithium remains a first-line treatment for the 1% of women of reproductive age with bipolar disorder in the U.S. population.6 This persistent use has been justified by the existence of more evidence on effectiveness than with other drugs, including data showing that lithium continuation is associated with a reduced risk of mood-episode recurrence during pregnancy and the postpartum period.7 Furthermore, a large body of evidence has shown teratogenicity for some other mood stabilizers, such as valproate.8,9
Other than the findings among lithium-exposed infants in the International Register, most of the information on the safety of lithium during pregnancy that has accumulated in the past 40 years is based on case reports10–14 and small studies (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Several small case–control studies failed to show an association between lithium and Ebstein’s anomaly,15–19 overall cardiac defects,20 or any congenital malformations.21 In two uncontrolled cohort studies of lithium-exposed pregnancies, no cardiac malformations were detected in the 82 newborns examined22,23; another uncontrolled study showed four mild cardiac defects in 79 exposed infants.24 Four controlled cohort studies (involving between 59 and 138 lithium-exposed pregnancies)25–28 showed conflicting results.
Therefore, women with bipolar disorder who are of childbearing age and are planning to become pregnant have to balance the risks and benefits of treatment continuation on the basis of limited and conflicting evidence regarding the safety of lithium for the developing fetus.6,29 Some women discontinue lithium therapy or terminate their pregnancy to avoid the potential teratogenicity of the drug.19,26,27 We designed a large retrospective cohort study involving pregnant women nested in the U.S. Medicaid Analytic eXtract (MAX) to provide evidence on the risk of cardiac malformations in the offspring that is associated with maternal use of lithium in the first trimester.
Methods
Data Source and Study Cohort
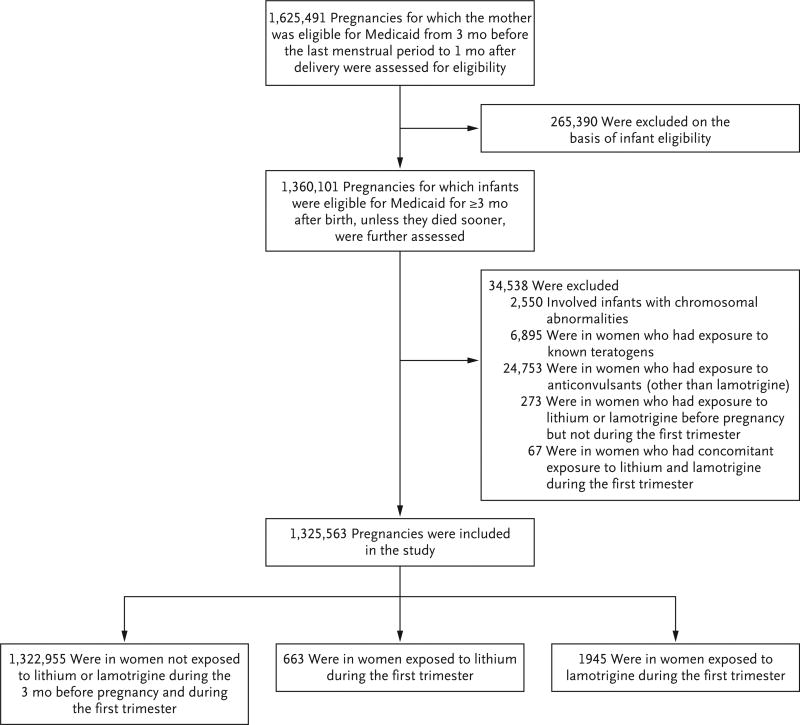
Data were collected from the MAX for 46 U.S. states and the District of Columbia for the years 2000 through 2010. The cohort included all pregnancies in women 12 to 55 years of age that resulted in live births for which Medicaid covered the health care expenses. We excluded women who had private insurance or restricted medical benefits and those who did not have an appropriate enrollment type (i.e., capitated managed care, fee-for-service primary care case management, or no managed care, depending on the state). The approach that was used for the development of the study cohort has been described previously30,31 and is summarized in Figure 1 and the Supplementary Appendix.
Figure 1. Study Cohort.
Women included in the study population could have had more than one pregnancy.
Study Conduct
The study was approved by the institutional review board at Brigham and Women’s Hospital, and the need for informed consent was waived. The study funder (the National Institute of Mental Health) had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Definition of Exposure
Exposure was defined as at least one filled prescription for lithium during the first trimester (first 90 days after the date of the last menstrual period). The primary reference group included women with no lithium or lamotrigine dispensings during the 3 months before the start of pregnancy or during the first trimester. The criterion of no dispensing during the 3 months before the start of pregnancy was imposed to avoid misclassifying as unexposed women who still had medications from an earlier filling available at the start of pregnancy. We also identified a secondary reference group of women who had at least one filled prescription for lamotrigine during the first trimester. Patients who were exposed to both lithium and lamotrigine during the first trimester (67 patients) were excluded. Lamotrigine was chosen for comparison because it is an effective treatment for bipolar disorder,32 often in combination with other psychotropic medications, and has not been associated with an increased risk of congenital malformations.9,33 Women who were exposed to lamotrigine were compared with the reference group of unexposed women and were used as the active reference group for women who were exposed to lithium. The former contrast (exposure to lamotrigine vs. no exposure) allows an indirect and stable comparison of lithium and lamotrigine by using a common reference. The latter contrast can further limit residual confounding by indication.
Outcomes
The primary outcome was the presence of a cardiac malformation in the infant. The positive predictive value for this outcome definition has been previously estimated at 77.6%.34 Secondary outcomes were major congenital malformations overall, defined as the presence of any major malformation, and noncardiac congenital malformations, defined as the presence of a major mal-formation in the absence of a cardiac defect. Detailed information on outcome definitions is provided in the Supplementary Appendix.
Because the use of lithium in early pregnancy has been previously associated with an increased risk of Ebstein’s anomaly, we assessed right ventricular outflow tract obstruction defects as a secondary outcome. We focused on overall right ventricular outflow tract obstruction defects rather than on Ebstein’s anomaly for two reasons: first, Ebstein’s anomaly has a specific code in the International Classification of Diseases, 9th Revision (ICD-9), but it may be classified under the general group of right ventricular outflow tract obstruction malformations; second, clinicians may be more likely to label a right ventricular outflow tract obstruction defect as Ebstein’s anomaly in infants known to have been exposed to lithium than in unexposed infants, and thus Ebstein’s anomaly may be more prone to differential misclassification than right ventricular outflow tract obstruction defects more generally.
Covariates
We considered the following covariates as potential confounders: maternal age at delivery, race or ethnic group, year of delivery, smoking status, maternal psychiatric disorders and medical conditions, concomitant medication use, and general markers of the burden of disease, including the Obstetric Comorbidity Index35 and measures of the intensity of health care use. (For a complete list of covariates, see Tables S2, S3, and S4 in the Supplementary Appendix.) Maternal coexisting conditions and medication use were measured during the 3 months before pregnancy through the end of the first trimester. Measures of health care use (e.g., number of physician visits) were captured only during the 3 months before pregnancy to avoid their being influenced by early awareness of possible pregnancy complications.
Statistical Analysis
For the primary analysis, standardized differences were used to assess covariate balance between the exposed group and the reference group; meaningful imbalances were defined by an absolute standardized difference of more than 0.1.36 Absolute risks of primary and secondary outcomes, and unadjusted risk ratios and risk differences with 95% confidence intervals, were calculated. Exposure propensity scores were estimated as the predicted probability of receiving the treatment of interest (e.g., lithium vs. no treatment), conditional on the covariates described above, with the use of logistic-regression models. For each estimated propensity score, the population in the nonoverlapping areas of the propensity-score distributions was trimmed, and 50 strata were created on the basis of the distribution of the treated women.37 Weights for the reference group were calculated according to the distribution of the treated women among propensity-score strata and were used to estimate adjusted baseline characteristics and adjusted risk ratios and risk differences and 95% confidence intervals in generalized linear models (PROC GENMOD procedure with weight statement, binomial distribution, and log or identity link function). Use of the robust variance estimator to account for correlations within women with multiple pregnancies did not appreciably change confidence intervals; thus, correlation structures were not included in the analyses.
We also performed secondary analyses as above for the outcome right ventricular outflow tract obstruction defects. In addition, we examined the risk of cardiac malformations according to thirds of the first and the highest prescribed daily dose filled during the first trimester to evaluate the presence of a dose–response relation for lithium.
We performed several sensitivity analyses to test the robustness of the primary findings. First, to assess the potential effect of exposure misclassification, we redefined the exposure as having filled at least two prescriptions during the first trimester. Second, to evaluate the potential effect of outcome misclassification, we required the outcome to be based on ICD-9 diagnoses or procedure codes in the infant records only and extended infant follow-up to 1 year for infants who were continuously eligible for Medicaid for at least 1 year. Third, to mitigate possible residual bias due to confounding by indication and associated differences in health care use, we included only women who had at least one recorded diagnosis of bipolar disorder during the baseline period. Fourth, because the cohort included live births only, we examined the potential effect of different proportions of terminations among women treated with lithium versus those untreated within levels of covariates used in the adjustment.38
Results
Study Cohort and Patient Characteristics
The study cohort included 1,325,563 pregnancies that met the inclusion criteria. Of these, 663 (0.05%) were in women who were exposed to lithium and 1945 (0.15%) were in women who were exposed to lamotrigine during the first trimester (Fig. 1).
As compared with unexposed women, women who were exposed to lithium were older, more likely to be white, and had a higher prevalence of psychiatric disorders (particularly bipolar disorder and depression) and pain conditions. They were more likely to use psychotropic and pain medications and had a higher overall burden of disease. (For more on baseline characteristics of lithium-exposed and unexposed women, see Table 1, and Table S2 in the Supplementary Appendix.) After propensity-score adjustment, baseline characteristics were well balanced between the two groups. Characteristics of the women who were exposed to lamotrigine, before and after propensity-score adjustment, are reported in Tables S3 and S4 in the Supplementary Appendix.
Table 1.
Unadjusted and Propensity-Score–Adjusted Baseline Characteristics of Lithium-Exposed and Unexposed Pregnant Women.*
| Characteristic | Unadjusted | Propensity-Score–Adjusted† | ||
|---|---|---|---|---|
| No Exposure |
Exposure to Lithium |
No Exposure |
Exposure to Lithium |
|
| No. of pregnancies | 1,322,955 | 663 | 928,767 | 663 |
|
| ||||
| Age — yr | 24.0±5.8 | 25.6±6.1‡ | 25.6±6.2 | 25.6±6.1 |
|
| ||||
| Race or ethnic group — no. (%)§ | ||||
|
| ||||
| White | 526,603 (39.8) | 490 (73.9)‡ | 708,409 (76.3) | 490 (73.9) |
|
| ||||
| Black | 445,675 (33.7) | 89 (13.4)‡ | 113,987 (12.3) | 89 (13.4) |
|
| ||||
| Hispanic | 195,174 (14.8) | 36 (5.4)‡ | 41,876 (4.5) | 36 (5.4) |
|
| ||||
| Other | 155,503 (11.8) | 48 (7.2)‡ | 64,495 (6.9) | 48 (7.2) |
|
| ||||
| Psychiatric and neurologic conditions — no. (%) | ||||
|
| ||||
| Bipolar disorder | 9,485 (0.7) | 436 (65.8)‡ | 608,904 (65.6) | 436 (65.8) |
|
| ||||
| Depression | 63,110 (4.8) | 167 (25.2)‡ | 257,475 (27.7) | 167 (25.2) |
|
| ||||
| Anxiety | 41,759 (3.2) | 117 (17.6)‡ | 179,054 (19.3) | 117 (17.6) |
|
| ||||
| Pain conditions¶ | 183,219 (13.8) | 184 (27.8)‡ | 272,867 (29.4) | 184 (27.8) |
|
| ||||
| Chronic maternal illness — no. (%) | ||||
|
| ||||
| Hypertension | 24,529 (1.9) | 22 (3.3) | 30,197 (3.3) | 22 (3.3) |
|
| ||||
| Diabetes | 23,287 (1.8) | 18 (2.7) | 26,222 (2.8) | 18 (2.7) |
|
| ||||
| Psychotropic and other medications — no. (%) | ||||
|
| ||||
| Antidepressants | 106,412 (8.0) | 431 (65.0)‡ | 641,793 (69.1) | 431 (65.0) |
|
| ||||
| Antipsychotic agents | 12,366 (0.9) | 308 (46.5)‡ | 394,767 (42.5) | 308 (46.5) |
|
| ||||
| Benzodiazepines | 34,932 (2.6) | 207 (31.2)‡ | 283,907 (30.6) | 207 (31.2) |
|
| ||||
| Other anxiolytic agents | 4,288 (0.3) | 31 (4.7)‡ | 41,285 (4.4) | 31 (4.7) |
|
| ||||
| Other hypnotic agents | 43,150 (3.3) | 114 (17.2)‡ | 154,213 (16.6) | 114 (17.2) |
|
| ||||
| Opioids | 249,821 (18.9) | 253 (38.2)‡ | 370,867 (39.9) | 253 (38.2) |
|
| ||||
| Markers of the burden of disease | ||||
|
| ||||
| Obstetric Comorbidity Index║ | 0.9±1.4 | 1.6±1.8‡ | 1.6±1.8 | 1.6±1.8 |
|
| ||||
| No. of distinct prescriptions | 1.6±2.3 | 4.5±3.7‡ | 4.6±3.7 | 4.5±3.7 |
|
| ||||
| No. of outpatient physician visits | 2.8±4.0 | 8.5±9.8‡ | 8.0±8.5 | 8.5±9.8 |
|
| ||||
| Patients hospitalized — no. (%) | 48,294 (3.7) | 60 (9.0)‡ | 81,082 (8.7) | 60 (9.0) |
Plus–minus values are means ±SD. Maternal coexisting conditions and medication use were measured during the 3 months before pregnancy through the end of the first trimester. Measures of health care use (e.g., number of physician visits) were captured only during the 3 months before pregnancy to avoid their being influenced by early awareness of possible pregnancy complications. Percentages may not sum to 100 because of rounding.
Exposure propensity scores were estimated as the predicted probability of receiving lithium versus no treatment, conditional on the covariates reported in Table S2 in the Supplementary Appendix. For each estimated propensity score, the population in the nonoverlapping areas of the propensity-score distributions was trimmed, and 50 strata were created on the basis of the distribution of the treated women. Weights for the reference group were calculated according to the distribution of the exposed women among propensity-score strata and were used to estimate adjusted baseline characteristics.
There was a meaningful between-group imbalance, as assessed by an absolute standardized difference of more than 0.1.36
Race or ethnic group was identified from Medicaid enrollment files. Other race includes Asian, Native American, other, and unknown.
Pain conditions include neuropathic and nonneuropathic conditions.
The Obstetric Comorbidity Index predicts severe maternal illness. The range is 0 to 45, with higher values associated with a higher burden of maternal illness.35
Absolute and Relative Risks of Cardiac Malformations
The prevalence of cardiac malformations was 2.41 per 100 live births among infants exposed to lithium, 1.15 per 100 among unexposed infants, and 1.39 per 100 among infants exposed to lamotrigine (Table 2). The adjusted risk ratio for cardiac malformations among infants exposed to lithium was 1.65 (95% confidence interval [CI], 1.02 to 2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17 to 4.34) as compared with lamotrigine-exposed infants. The adjusted differences were 0.95 cases per 100 births (95% CI, −0.22 to 2.12) and 1.43 cases per 100 births (95% CI, 0.09 to 2.77), respectively (Table S5 in the Supplementary Appendix). The adjusted risk ratio for cardiac malformations among infants exposed to lamotrigine as compared with unexposed infants was 0.89 (95% CI, 0.61 to 1.30) (Table 2). The adjusted risk ratio for noncardiac defects among infants exposed to lithium as compared with unexposed infants was 1.22 (95% CI, 0.81 to 1.84).
Table 2.
Absolute and Relative Risk of Cardiac, Noncardiac, and Overall Malformations among Infants Exposed to Lithium during the First Trimester as Compared with Lamotrigine-Exposed or Unexposed Infants.*
| Variable | No Exposure to Lithium or Lamotrigine |
Exposure to Lamotrigine |
Exposure to Lithium |
|---|---|---|---|
| No. of pregnancies | 1,322,955 | 1945 | 663 |
| Cardiac malformations | |||
| Events | 15,251 | 27 | 16 |
| Prevalence per 100 births | 1.15 | 1.39 | 2.41 |
| Unadjusted risk ratio (95% CI) | Reference | 1.20 (0.83–1.75) | 2.09 (1.29–3.40) |
| Propensity-score–adjusted risk ratio (95% CI) | Reference | 0.89 (0.61–1.30) | 1.65 (1.02–2.68) |
| Unadjusted risk ratio (95% CI) | — | Reference | 1.74 (0.94–3.21) |
| Propensity-score–adjusted risk ratio (95% CI) | — | Reference | 2.25 (1.17–4.34) |
| Noncardiac malformations | |||
| Events | 27,816 | 49 | 22 |
| Prevalence per 100 births | 2.10 | 2.52 | 3.32 |
| Unadjusted risk ratio (95% CI) | Reference | 1.20 (0.91–1.58) | 1.58 (1.05–2.38) |
| Propensity-score–adjusted risk ratio (95% CI) | Reference | 0.90 (0.68–1.18) | 1.22 (0.81–1.84) |
| Unadjusted risk ratio (95% CI) | — | Reference | 1.32 (0.80–2.16) |
| Propensity-score–adjusted risk ratio (95% CI) | — | Reference | 1.63 (0.96–2.78) |
| Overall malformations | |||
| Events | 43,067 | 76 | 38 |
| Prevalence per 100 births | 3.26 | 3.91 | 5.73 |
| Unadjusted risk ratio (95% CI) | Reference | 1.20 (0.96–1.50) | 1.76 (1.29–2.40) |
| Propensity-score–adjusted risk ratio (95% CI) | Reference | 0.90 (0.72–1.12) | 1.37 (1.01–1.87) |
| Unadjusted risk ratio (95% CI) | — | Reference | 1.47 (1.00–2.14) |
| Propensity-score–adjusted risk ratio (95% CI) | — | Reference | 1.85 (1.23–2.78) |
CI denotes confidence interval.
Secondary and Sensitivity Analyses
The prevalence of right ventricular outflow tract obstruction defects was 0.60 per 100 live births among infants exposed to lithium and 0.18 per 100 among unexposed infants (Table 3). The adjusted risk ratio for specific cardiac malformations that were associated with lithium was 2.66 (95% CI, 1.00 to 7.06) for right ventricular outflow tract obstruction defects and 1.46 (95% CI, 0.84 to 2.57) for other cardiac defects. Although none of the identified right ventricular outflow tract obstruction defects in the lithium-exposed infants were specifically coded in claims as Ebstein’s anomaly, most were consistent with cardiac defects that frequently co-occur with Ebstein’s anomaly (e.g., pulmonary atresia and stenosis). The observed prevalence of Ebstein’s anomaly among unexposed infants was approximately 7 cases per 100,000 births.
Table 3.
Sensitivity and Secondary Analyses of the Relative Risk of Cardiac Malformations Associated with Exposure to Lithium during the First Trimester.*
| Analysis | Reference†
|
Exposure to Lithium
|
Propensity-Score Adjusted Risk Ratio (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| No. of Pregnancies |
No. of Events |
Prevalence per 100 Births |
No. of Pregnancies |
No. of Events |
Prevalence per 100 Births |
||
| Main propensity-score-adjusted analysis | 1,322,955 | 15,251 | 1.15 | 663 | 16 | 2.41 | 1.65 (1.02–2.68) |
|
| |||||||
| Exposure defined by 2 filled prescriptions | 1,322,955 | 15,251 | 1.15 | 177 | <11‡ | 2.82 | 1.61 (0.68–3.83) |
|
| |||||||
| Outcome based on infant records only | 1,322,955 | 13,053 | 0.99 | 663 | 16 | 2.41 | 1.82 (1.12–2.95) |
|
| |||||||
| 1-yr Follow-up§ | 1,095,112 | 16,648 | 1.52 | 550 | 15 | 2.73 | 1.43 (0.87–2.36) |
|
| |||||||
| ≥1 Diagnosis of bipolar disorder¶ | 9,485 | 145 | 1.53 | 436 | 12 | 2.75 | 1.70 (0.95–3.03) |
|
| |||||||
| ≥1 Diagnosis of bipolar disorder, with reference as exposure to lamotrigine¶ | 798 | 13 | 1.63 | 436 | 12 | 2.75 | 2.62 (1.12–6.10) |
|
| |||||||
| Specific cardiac malformations | |||||||
|
| |||||||
| RVOTO defect | 1,322,955 | 2,447 | 0.18 | 663 | <11‡ | 0.60 | 2.66 (1.00–7.06) |
|
| |||||||
| Non-RVOTO defect | 1,322,955 | 12,804 | 0.97 | 663 | 12 | 1.81 | 1.46 (0.84–2.57) |
RVOTO denotes right ventricular outflow tract obstruction.
The reference was no exposure to lithium or lamotrigine, unless otherwise specified.
In accordance with the data-use agreement, we do not report information for frequency cells with less than 11 cases.
The analysis was restricted to infants continuously eligible for at least 1 year.
The analysis was restricted to women with at least one baseline diagnosis of bipolar disorder.
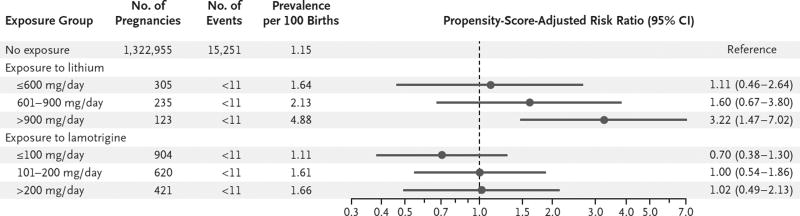
In dose–response analyses based on the first lithium prescription in pregnancy, after propensity-score adjustment (Tables S6 through S13 in the Supplementary Appendix), the risk ratio was 1.11 (95% CI, 0.46 to 2.64) for a daily dose of 600 mg or less, 1.60 (95% CI, 0.67 to 3.80) for 601 to 900 mg, and 3.22 (95% CI, 1.47 to 7.02) for more than 900 mg (Fig. 2). Results were consistent in analyses that used thirds of the highest prescribed dose during the first trimester (Fig. S1 in the Supplementary Appendix). All right ventricular outflow tract obstruction defects that were identified in lithium-exposed infants occurred with a daily dose of more than 600 mg. There was no evidence of a dose–response relationship for lamotrigine (Fig. 2, and Fig. S1 in the Supplementary Appendix).
Figure 2. Absolute and Relative Risk of Cardiac Malformations among Lithium-Exposed and Lamotrigine-Exposed Infants as Compared with Unexposed Infants, Stratified According to the Mother’s Dose of the Drug.
Stratification was according to thirds of the first prescribed daily dose that was filled during the first trimester. A separate exposure propensity score was estimated in each dose stratum as the predicted probability of receiving the treatment-dose range of interest versus no treatment, conditional on the covariates reported in Tables S6 through S9 in the Supplementary Appendix. For each estimated propensity score, the population in the nonoverlapping areas of the propensity-score distributions was trimmed, and 50 strata were created on the basis of the distribution of the treated women. Weights for the reference group were calculated according to the distribution of the exposed women among propensity-score strata and were used to estimate adjusted risk ratios and 95% confidence intervals.
The risk of cardiac malformations among infants exposed to lithium as compared with unexposed infants remained consistently elevated in sensitivity analyses (Table 3). After we accounted for potential differences in the probability of termination of malformed fetuses among exposed and unexposed women, the range of plausible adjusted risk ratios for cardiac malformations among lithium-exposed infants was estimated to be 1.67 to 1.80. (See the Supplementary Appendix.)
Discussion
Findings from this observational study support a modest increase in the risk of cardiac malformations in infants that are associated with lithium use in early pregnancy. On the basis of the 95% confidence interval around the effect estimates, results were consistent with an increased risk of cardiac malformations ranging from 2 to 270%, or up to 2 additional cases per 100 births among pregnancies in women who were exposed to lithium during the first trimester as compared with pregnancies in unexposed women with similar characteristics. The relative risk appeared to be higher for right ventricular outflow tract obstruction defects than for other cardiac defects. Lithium was not significantly associated with noncardiac malformations, although the 95% confidence interval around the risk estimate indicates that some increase in risk cannot be ruled out.
Our results support previous findings of an association between lithium use in pregnant women and cardiac defects in infants, although the magnitude of increased risk appeared considerably lower than originally suggested by the International Register of Lithium Babies.1 Results from this registry-based study were interpreted as supporting a risk of Ebstein’s anomaly that was increased by a factor of 400 among infants exposed in utero to lithium,3 despite substantial methodologic limitations, including the lack of a control group and a possible over-representation of cases owing to retrospective enrollment.4 Since then, anecdotal reports10–14 and small studies have been published with inconclusive results (Table S1 in the Supplementary Appendix). Our cohort of 663 women who were exposed to lithium during the first trimester is more than 4 times larger than that in the largest previously published controlled study.27
Our study also suggests that the association of lithium and cardiac malformations in humans is dose-dependent, with a risk that is increased by a factor of approximately 3 beyond doses of 900 mg per day. Dose-dependent teratogenicity of lithium was previously reported in rodent models,39,40 although it has not been well studied in humans.
Limitations of our study warrant consideration. First, although the availability of detailed maternal information through the MAX data set and our propensity-score adjustment allowed for control for a large number of confounders, we cannot rule out the possibility of residual confounding by characteristics that are not captured or are incompletely captured in our database. For example, suggested risk factors for cardiac malformations, such as obesity, smoking, or alcohol use disorders, appeared to be underrecorded in our data and to be more prevalent among women exposed to lithium (Table S2 in the Supplementary Appendix). Residual confounding by such factors would probably bias relative-risk estimates upward. Our finding of similar results in analyses that were intended to mitigate possible residual confounding (i.e., directly comparing lithium with lamotrigine and restricting analyses to women with at least one diagnosis of bipolar disorder) argues against substantive residual confounding by indication. Second, databases of health care use provide information on filled prescriptions but not on the actual consumption of medications and thus can be prone to the misclassification of drug exposure, which would attenuate observed associations. However, results were similar in sensitivity analyses limited to women who filled at least two prescriptions, under the assumption that this would increase the likelihood that the medication was taken as prescribed and at critical times in organogenesis. Third, databases of health care use may also be prone to outcome misclassification. To address this possibility, we used a previously validated definition for cardiac defects34 and highly specific definitions for overall and noncardiac malformations. Moreover, the results of analyses that required the outcome to be based on ICD-9 diagnoses or procedure codes in the infant records only were consistent with the main results.
Fourth, because of the previously postulated association with lithium, cardiac malformations may have been preferentially investigated among infants exposed to lithium during pregnancy, with the result that our study may have overestimated the true effect of lithium on cardiac defects. However, our outcome definition focused on major cardiac defects that are likely to be clinically consequential for the infant and was restricted to malformations that were recorded several times or had required surgery. Fifth, because the cohort was restricted to live births, spontaneous abortions or planned terminations due to congenital malformations that were diagnosed early in pregnancy were missed. It has been previously documented that therapeutic abortions may be 5 to 10% higher among lithium users than among nonusers.26,27 The quantitative bias analysis, which assessed the potential effect of such missed terminations, suggested that our study may have underestimated the true association between lithium and cardiac malformations if termination frequencies (within levels of the measured covariates) were highly differential; under the most extreme scenario considered, the estimate of the corrected adjusted risk ratio would be closer to 2. Finally, our study focused only on the association of lithium with congenital malformations, without consideration of other potential outcomes that may be relevant for treatment decisions during pregnancy.
Our results suggest that maternal use of lithium during the first trimester is associated with an increased risk of cardiac malformations on the order of 1 additional case per 100 live births when there was exposure early in pregnancy and that this association is dose-dependent. The magnitude of the association was substantially smaller than originally proposed.
Supplementary Material
Acknowledgments
Supported by a grant (R01 MH100216) from the National Institute of Mental Health. Dr. Huybrechts was supported by a career development grant (K01MH099141) from the National Institute of Mental Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Schou M, Goldfield MD, Weinstein MR, Villeneuve A. Lithium, pregnancy. I. Report from the Register of Lithium Babies. Br Med J. 1973;2:135–6. doi: 10.1136/bmj.2.5859.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein MR, Goldfield M. Cardiovascular malformations with lithium use during pregnancy. Am J Psychiatry. 1975;132:529–31. doi: 10.1176/ajp.132.5.529. [DOI] [PubMed] [Google Scholar]
- 3.Nora JJ, Nora AH, Toews WH. Letter: Lithium, Ebstein’s anomaly, and other congenital heart defects. Lancet. 1974;2:594–5. doi: 10.1016/s0140-6736(74)91918-7. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein MR. Lithium treatment of women during pregnancy and in the post-delivery period. Lancaster, United Kingdom: MTP Press; 1980. [Google Scholar]
- 5.Physicians’ desk reference. 53. Montrale, NJ: Medical Economics; 2002. [Google Scholar]
- 6.Yonkers KA, Wisner KL, Stowe Z, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry. 2004;161:608–20. doi: 10.1176/appi.ajp.161.4.608. [DOI] [PubMed] [Google Scholar]
- 7.Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164:1817–24. doi: 10.1176/appi.ajp.2007.06101639. [DOI] [PubMed] [Google Scholar]
- 8.Jentink J, Loane MA, Dolk H, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362:2185–93. doi: 10.1056/NEJMoa0907328. [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–9. doi: 10.1212/WNL.0b013e3182574f39. [DOI] [PubMed] [Google Scholar]
- 10.Rane A, Tomson G, Bjarke B. Effects of maternal lithium therapy in a newborn infant. J Pediatr. 1978;93:296–7. doi: 10.1016/s0022-3476(78)80527-7. [DOI] [PubMed] [Google Scholar]
- 11.Park JM, Sridaromont S, Ledbetter EO, Terry WM. Ebstein’s anomaly of the tricuspid valve associated with prenatal exposure to lithium carbonate. Am J Dis Child. 1980;134:703–4. doi: 10.1001/archpedi.1980.02130190069018. [DOI] [PubMed] [Google Scholar]
- 12.Arnon RG, Marin-Garcia J, Peeden JN. Tricuspid valve regurgitation and lithium carbonate toxicity in a newborn infant. Am J Dis Child. 1981;135:941–3. doi: 10.1001/archpedi.1981.02130340047016. [DOI] [PubMed] [Google Scholar]
- 13.Allan LD, Desai G, Tynan MJ. Prenatal echocardiographic screening for Ebstein’s anomaly for mothers on lithium therapy. Lancet. 1982;2:875–6. doi: 10.1016/s0140-6736(82)90836-4. [DOI] [PubMed] [Google Scholar]
- 14.Long WA, Willis PWIV. Maternal lithium and neonatal Ebstein’s anomaly: evaluation with cross-sectional echocardiography. Am J Perinatol. 1984;1:182–4. doi: 10.1055/s-2007-999999. [DOI] [PubMed] [Google Scholar]
- 15.Correa-Villaseñor A, Ferencz C, Neill CA, Wilson PD, Boughman JA. Ebstein’s malformation of the tricuspid valve: genetic and environmental factors. Teratology. 1994;50:137–47. doi: 10.1002/tera.1420500208. [DOI] [PubMed] [Google Scholar]
- 16.Edmonds LD, Oakley GP. Ebstein’s anomaly and maternal lithium exposure during pregnancy. Teratology. 1990;41:551–2. [Google Scholar]
- 17.Kallen B. Comments on teratogen update: lithium. Teratology. 1988;38:598. doi: 10.1002/tera.1420380607. [DOI] [PubMed] [Google Scholar]
- 18.Sípek A. Lithium and Ebstein’s anomaly. Cor Vasa. 1989;31:149–56. [PubMed] [Google Scholar]
- 19.Zalzstein E, Koren G, Einarson T, Freedom RM. A case-control study on the association between first trimester exposure to lithium and Ebstein’s anomaly. Am J Cardiol. 1990;65:817–8. doi: 10.1016/0002-9149(90)91398-p. [DOI] [PubMed] [Google Scholar]
- 20.Kallen B. Lithium therapy and congenital malformations. In: Schrauser G, Klippel K, editors. Lithium in biology and medicine: new applications and developments. Weinheim, Germany: VCH Verlagsgesellschaft; 1991. pp. 121–30. [Google Scholar]
- 21.Czeizel A, Rácz J. Evaluation of drug intake during pregnancy in the Hungarian Case-Control Surveillance of Congenital Anomalies. Teratology. 1990;42:505–12. doi: 10.1002/tera.1420420507. [DOI] [PubMed] [Google Scholar]
- 22.Kramer A, Knoppert-Van der Klein EAM, Van Kamp IL, Walter FJ, Van Vliet IM, Zitman FG. Bipolar and pregnant? No problem! A clinical evaluation of lithium use during pregnancy. European Neuropsychopharmacology. 2003;13(Suppl 4):S246. [Google Scholar]
- 23.Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 7. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 24.Reis M, Källén B. Maternal use of anti-psychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28:279–88. doi: 10.1097/JCP.0b013e318172b8d5. [DOI] [PubMed] [Google Scholar]
- 25.Källén B, Tandberg A. Lithium and pregnancy: a cohort study on manic-depressive women. Acta Psychiatr Scand. 1983;68:134–9. doi: 10.1111/j.1600-0447.1983.tb06991.x. [DOI] [PubMed] [Google Scholar]
- 26.Diav-Citrin O, Shechtman S, Tahover E, et al. Pregnancy outcome following in utero exposure to lithium: a prospective, comparative, observational study. Am J Psychiatry. 2014;171:785–94. doi: 10.1176/appi.ajp.2014.12111402. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson SJ, Jones K, Johnson K, et al. Prospective multicentre study of pregnancy outcome after lithium exposure during first trimester. Lancet. 1992;339:530–3. doi: 10.1016/0140-6736(92)90346-5. [DOI] [PubMed] [Google Scholar]
- 28.Bodén R, Lundgren M, Brandt L, Reutfors J, Andersen M, Kieler H. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi: 10.1136/bmj.e7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994;271:146–50. [PubMed] [Google Scholar]
- 30.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22:16–24. doi: 10.1002/pds.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licht RW, Nielsen JN, Gram LF, Vestergaard P, Bendz H. Lamotrigine versus lithium as maintenance treatment in bipolar I disorder: an open, randomized effectiveness study mimicking clinical practice — the 6th trial of the Danish University Antidepressant Group (DUAG-6) Bipolar Disord. 2010;12:483–93. doi: 10.1111/j.1399-5618.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 33.Yacobi S, Ornoy A. Is lithium a real teratogen? What can we conclude from the prospective versus retrospective studies? A review. Isr J Psychiatry Relat Sci. 2008;45:95–106. [PubMed] [Google Scholar]
- 34.Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernández-Díaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiol Drug Saf. 2014;23:646–55. doi: 10.1002/pds.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013;122:957–65. doi: 10.1097/AOG.0b013e3182a603bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28:249–57. doi: 10.1097/EDE.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397–407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo KT. Teratogenic effect of lithium carbonate in the foetal mouse. Nature. 1970;225:73–5. doi: 10.1038/225073a0. [DOI] [PubMed] [Google Scholar]
- 40.Smithberg M, Dixit PK. Teratogenic effects of lithium in mice. Teratology. 1982;26:239–46. doi: 10.1002/tera.1420260304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.