Abstract
Background
Continuous flow left ventricular assist device (CF-LVAD) patients have a high prevalence of gastrointestinal bleeding from the small bowel. Video capsule endoscopy (VCE) is often used for diagnosis in these patients, but efficacy has yet to be determined. In this study, we evaluated the efficacy of VCE in the management of CF-LVAD patients with suspected small bowel bleeding by comparing to a non-VCE CF-LVAD control group.
Methods
We retrospectively reviewed the charts of all patients with CF-LVADs implanted at Stanford Hospital from January 2010 to October 2015. Patients were included in the study if there was a clinical suspicion of small bowel bleeding and either a negative upper endoscopy or colonoscopy.
Results
A total of 26 patients met inclusion criteria for a total of 15 encounters where VCE was done, and 25 where VCE was not done. There were no statistical differences when comparing these groups in terms of medical therapy use (thalidomide or octreotide), enteroscopy use (double-balloon or push), intervention on lesions, or any 30-day outcomes. There was no advantage to VCE with regard to the composite endpoint time to re-bleed or death related to re-bleeding (median 114 vs. 161 days, P = 0.15) after removing patients who did not get a VCE due to death or critical illness.
Conclusions
We did not find VCE changed management or outcomes in CF-LVAD patients with suspected small bowel bleeding at our institution when compared to a non-VCE control group. Our experience is small and single center, and larger, multi-center studies could further elucidate the utility of VCE in this patient population.
Keywords: Left ventricular assist device, Capsule endoscopy, Small bowel bleeding, Obscure gastrointestinal bleeding, Arteriovenous malformation
Introduction
Gastrointestinal (GI) bleeding occurs in 18-40% of patients with continuous flow left ventricular assist devices (CF-LVAD) or around 0.17 bleeds/patient-year [1]. Potential causes of GI bleeding in this population include anti-coagulant use [2, 3], acquired von Willebrand syndrome from shear stress [4-6], and arteriovenous malformations possibly caused by reduced pulse pressure and hypoperfusion [3].
Prior case series have found that the source of GI bleeding in CF-LVAD patients is most commonly the upper GI tract (48%), followed by the lower GI tract (22%), then small bowel (15%), with 22% of unknown location. Most GI bleeding is from arteriovenous malformations (29%), followed by gastritis (22%), ulcer (13%), diverticular (6%), with 22% of unknown etiology [7].
The current treatment for suspected small bowel bleeding is similar to non-LVAD patients [8]. It is recommended that patients first undergo an upper endoscopy and colonoscopy, with consideration for a second look endoscopy. If negative, one possible next step is video capsule endoscopy (VCE) [9]. However, the efficacy of VCE in CF-LVAD patients is not well established.
The primary purpose of this study was to evaluate the ability of capsule endoscopy to improve outcomes including reduction of recurrent bleeding in patients with CF-LVADs and suspected small bowel bleeding. We retrospectively compared a cohort of CF-LVAD patients who had VCE to a similar cohort that did not undergo VCE.
Methods
Patient selection
We retrospectively reviewed the charts of all CF-LVAD patients who were implanted at Stanford University Hospital between January 2010 and October 2015 and had a procedure performed for GI bleeding. GI bleeding was defined as melena, hematochezia, hematemesis, and/or heme occult positive stool with a drop in hemoglobin. All patients were admitted to the hospital for the workup of their GI bleeding. Patients were included in our study if they had a clinical suspicion for small bowel GI bleeding, and either an esophagogastroduodenoscopy (EGD) and/or colonoscopy with no clear source of bleeding. The study protocol was reviewed and approved for human subject research by the Stanford University Institutional Review Board.
Data collection
Patient electronic charts were reviewed retrospectively, and baseline data prior to endoscopy, details regarding endoscopy, outcomes, and follow-up were collected. Data were grouped by individual episodes of GI bleeding, with some patients having multiple encounters for GI bleeding. These encounters were then divided into two groups: those in which VCE was performed, and those in which it was not performed.
Procedures
All VCE was performed with a PillCam® device (Given® Imaging) and read by a trained gastroenterologist. Prior to VCE all patients received at least 2 L of Golytely® for bowel preparation. Interference, image quality, prep quality, complications from capsule endoscopy, and last location in the GI tract were recorded if available. For all endoscopy, findings were documented, including type of lesion, location, source of bleeding, and intervention. The way in which VCE affected future management including enteroscopy, radiologic imaging, interventional radiology, and use of thalidomide and octreotide was also recorded.
Outcomes
The primary outcome of the study was time to re-bleeding or death related to GI bleeding. Data were censored at the time of transplant, unrelated death, or last contact in the chart. Additionally, several short-term (30 day) outcomes were assessed including re-bleeding, death, re-admission, length of stay, and units of red cells transfused after the first procedure. Other 30-day adverse events including deep vein thrombosis, pulmonary embolism, suspected or confirmed pump thrombus, myocardial infarction, stroke, and death were also recorded. Due to rarity, these events were analyzed together under the category “total adverse events.”
Data analysis
All binary data were compared using Fisher’s exact test and displayed as percentages. For binary data 95% confidence intervals were calculated using the binomial distribution. All non-parametric data were compared using the Wilcoxon rank sum test and displayed as medians with interquartile ranges (IQR) where appropriate. All parametric data were compared using a two-sample t-test and displayed as means. Categorical data were compared using the Chi-square test of significance. Analysis of our primary outcome, time to death or re-bleed, was performed using the Kaplan-Meier method with the log-rank test of significance. All statistical analysis was conducted using Stata version 13.1 (StataCorp LP).
Results
Baseline characteristics
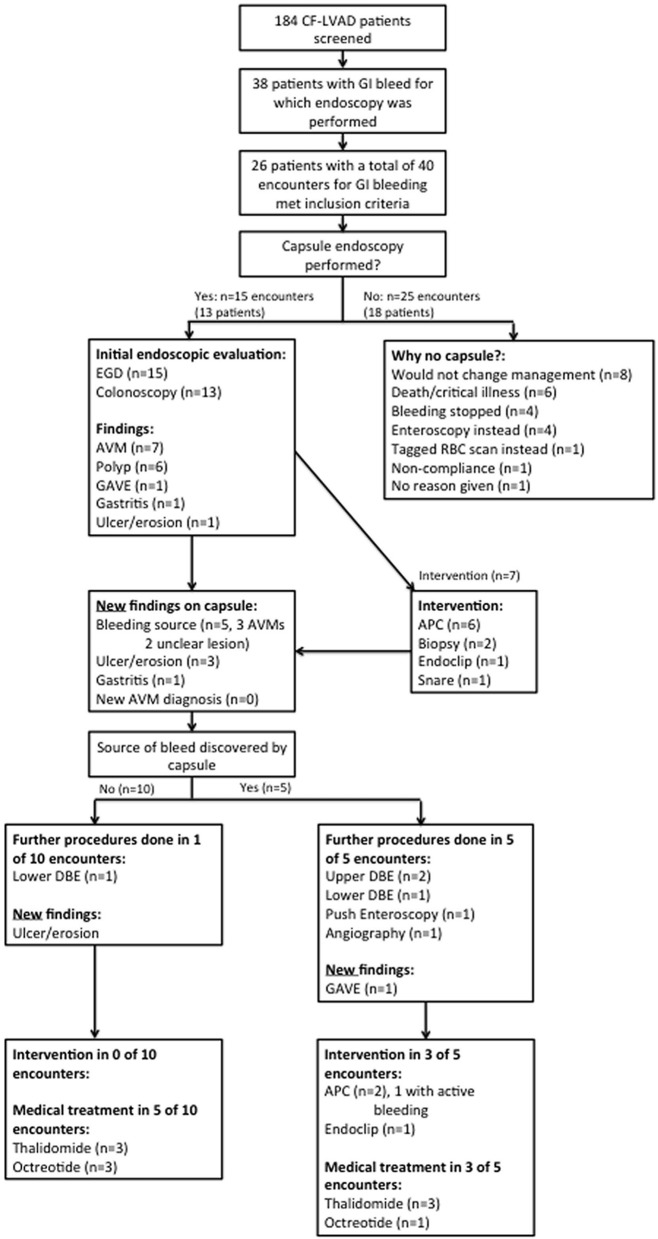
A total of 184 CF-LVAD patients were screened and of these 38 patients (20.7%) had a GI bleed for which endoscopy was performed. Of these 38 patients, 26 met the inclusion criteria for our study with a total of 40 encounters for GI bleeding (Fig. 1). There were 15 encounters in which VCE was performed and 25 in which VCE was not performed.
Figure 1.
Flow chart for inclusion in the study and subsequent management of CF-LVAD patients with suspected small bowel bleed.
In comparing baseline characteristics (Table 1), the biggest difference noted was that the VCE group was more likely to have undergone both an EGD and colonoscopy (86.6 vs. 36.0%, P = 0.003, Fisher’s exact test). The reasons patients did not undergo colonoscopy in the VCE group include a low clinical suspicion for lower GI bleed (n = 1, 6.7%) and a prior negative colonoscopy (n = 1, 6.7%). The reasons patients did not undergo colonoscopy in the non-VCE group include low clinical suspicion for lower GI bleed (n = 8, 32%), a prior negative colonoscopy (n = 4, 16%), and death/critical illness (n = 4, 16%). The VCE group also had higher serum platelet levels, though both groups had values that were within normal limits. The non-VCE group demonstrated a trend towards greater need for mechanical ventilation pre-endoscopy, and a trend to greater aspirin use prior to endoscopy. The remainder of the baseline characteristics showed no statistical differences.
Table 1. Baseline Characteristics of VCE and non-VCE Encounters.
| Capsule (n = 15) | No capsule (n = 25) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (mean) | 63.1 (57.0 - 69.3) | 63.7 (59.1 - 68.3) | 0.884 |
| Sex (% male) | 93.3 (68.1 - 99.8) | 68.0 (46.5 - 85.1) | 0.117 |
| Ethnicity (% White/Black/Hispanic/Asian) | 46.6/33.3/6.7/13.3 | 44.0/36.0/20.0/0 | 0.224 |
| Co-morbidities | |||
| VAD as destination therapy (%) | 66.7 (38.4 - 88.2) | 48.0 (27.8 - 68.7) | 0.332 |
| Ischemic cardiomyopathy (%) | 53.3 (26.6 - 78.7) | 72.0 (50.6 - 87.9) | 0.310 |
| Diabetes (%) | 53.3 (26.9 - 78.7) | 36.0 (18.0 - 57.5) | 0.336 |
| COPD or asthma (%) | 40.0 (16.3 - 67.7) | 24.0 (9.4 - 45.1) | 0.311 |
| Prior GI bleed (%) | 40.0 (16.3 - 67.7) | 48.0 (28.0 - 68.7) | 0.747 |
| ASA status (mean) | 3.33 (2.88 - 3.79) | 3.20 (2.96 - 3.44) | 0.549 |
| Clinical presentation | |||
| Melena (%) | 60.0 (32.3 - 83.7) | 84.0 (63.9 - 95.5) | 0.135 |
| Hematochezia (%) | 33.3 (11.8 - 61.6) | 16.0 (4.5 - 36.1) | 0.225 |
| Hematemesis (%) | 0 (0 - 21.8) | 12.0 (2.5 - 31.2) | 0.279 |
| Occult bleed (%) | 20.0 (4.3 - 48.1) | 8.0 (0.1 - 26.0) | 0.345 |
| Anticoagulation | |||
| Aspirin (%) | 73.3 (44.9 - 92.2) | 96.1 (79.6 - 99.9) | 0.056 |
| Warfarin (%) | 80.0 (51.9 - 95.7) | 80.0 (59.3 - 93.2) | 1.000 |
| Heparin (%) | 13.3 (1.7 - 40.5) | 16.0 (4.5 - 36.1) | 1.000 |
| Argatroban (%) | 0 (0 - 21.8) | 4.0 (0.1 - 20.4) | 1.000 |
| Plavix (%) | 0 (0 - 21.8) | 4.0 (0.1 - 20.4) | 1.000 |
| Eptifatide (%) | 0 (0 . 21.8) | 0 (0 - 13.7) | 1.000 |
| Laboratory values | |||
| RBC transfusion (median units 72 h prior to scope) | 3 (0 - 4) | 2 (1 - 3) | 0.591 |
| Platelet transfusion (median units 72 h prior to scope) | 0 (0 - 0) | 0 (0 - 0) | 0.267 |
| FFP transfusion (median units 72 h prior to scope) | 0 (0 - 0) | 0 (0 - 1) | 0.276 |
| Hemoglobin (mean) | 7.97 (7.38 - 8.56) | 8.55 (8.05 - 9.04) | 0.135 |
| Platelets (mean) | 245 (198 - 290) | 188 (154 - 222) | 0.041 |
| Creatinine (mean) | 1.25 (1.19 - 1.62) | 1.40 (1.19 - 1.62) | 0.421 |
| Dialysis (%) | 0 (0 - 21.8) | 12.0 (2.5 - 31.2) | 0.279 |
| INR (mean) | 1.83 (1.52 - 2.14) | 2.06 (1.79 - 2.32) | 0.269 |
| Vital signs before endoscopy | |||
| Intubated prior (%), not including for procedure | 0 (0 - 21.8) | 24.0 (9.4 - 45.1) | 0.067 |
| Inspired O2 (median %) | 21.0 (21.0 - 21.0) | 21.0 (21.0 - 40.0) | 0.378 |
| Vasopressors (median) | 0 (0 - 0) | 0 (0 - 0) | 0.140 |
| Initial workup | |||
| EGD and Colonoscopy (%) | 86.6 (59.5 - 98.3) | 36.0 (18.0 - 57.5) | 0.003 |
| EGD only (%) | 13.3 (1.7 - 40.5) | 64.0 (42.5 - 82.0) | 0.003 |
VAD: ventricular assist device; COPD: chronic obstructive pulmonary disease; GI: gastrointestinal; ASA: American Society of Anesthesiology; RBC: red blood cell; INR: international normalized ratio; EGD: esophagogastroduodenoscopy.
Management
In the 25 encounters in which VCE was not used, the most common reason cited was the belief that VCE would not change management (n = 8, 32%) (Fig. 1). Other common reasons included critical illness or death (n = 6, 24%), cessation of bleeding (n = 4, 16%), or performance of either push enteroscopy or double balloon enteroscopy (DBE) instead (n = 4, 16%). In the 15 encounters in which VCE was used, it was because there was either no source of bleeding seen on endoscopy, or lesions were discovered by endoscopy but felt not to be the source of bleeding.
VCE was helpful in localizing the source of bleeding in five additional cases (33%) where bleeding was not identified on endoscopy, and was also helpful in diagnosing other lesions not seen on endoscopy (Fig. 1). A new diagnosis of arteriovenous malformations (AVMs) was never made by VCE in our cohort, as such lesions were always seen on preceding endoscopy.
A positive VCE was more likely to lead to additional endoscopy. In the five cases where a bleeding source was discovered by VCE, three DBEs, one push enteroscopy, and one angiography were performed afterwards, and additional argon plasma coagulation (APC) was performed in two cases, and endoclipping in one case (Fig. 1). There was only one instance when an actively bleeding lesion was intervened upon. In the 10 cases when a bleeding source was not discovered by VCE, only one DBE was performed without endoscopic intervention (Fig. 1). A VCE positive for bleeding however did not influence medical management (treatment with thalidomide or octreotide). In patients with a positive VCE medical treatments were initiated in 60% of cases, compared to 50% with a negative VCE.
The VCE and non-VCE cohort overall were managed in similar ways (Table 2). There were no differences in endoscopic intervention, performance of push enteroscopy and DBE, or initiation of medical treatment with thalidomide or octreotide (Table 2).
Table 2. Endoscopic Findings and Management of VCE and Non-VCE Encounters.
| Capsule (n = 15) | No capsule (n = 25) | P value | |
|---|---|---|---|
| Findings | |||
| AVM (%) | 46.7 (21.3 - 73.4) | 32.0 (14.9 - 53.5) | 0.502 |
| Ulcer or erosion (%) | 33.3 (11.8 - 61.6) | 20.0 (6.8 - 40.7) | 0.457 |
| Gastritis (%) | 13.3 (1.7 - 40.5) | 12.0 (2.5 - 31.2) | 1.000 |
| Polyp (%) | 40.0 (16.3 - 67.7) | 8.0 (0.9 - 26.0) | 0.036 |
| GAVE (%) | 13.3 (1.7 - 40.5) | 4.0 (0.1 - 20.4) | 0.545 |
| Endoscopic intervention | |||
| APC (%) | 40.0 (16.3 - 67.7) | 24.0 (9.4 - 45.1) | 0.311 |
| Clip (%) | 13.3 (1.7 - 40.5) | 12.0 (2.5 - 31.2) | 1.000 |
| Injection (%) | 0 (0 - 21.8) | 0 (0 - 13.7) | 1.000 |
| Snare (%) | 6.7 (0.1 - 31.9) | 0 (0 - 13.7) | 0.375 |
| Management | |||
| IR intervention (%) | 6.7 (0.2 - 31.9) | 0 (0 - 13.7) | 0.375 |
| Tagged RBC scan (%) | 0 (0 - 21.8) | 8.0 (0.9 - 26.0) | 0.519 |
| Start octreotide or thalidomide (%) | 46.7 (21.3 - 73.4) | 52.0 (31.3- 72.2) | 1.000 |
| Double balloon enteroscopy (%) | 20.0 (4.3 - 48.1) | 12.0 (2.5 - 31.2) | 0.654 |
| Push enteroscopy (%) | 6.7 (0.2 - 31.9) | 12.0 (2.5 - 31.2) | 1.000 |
| Any enteroscopy (%) | 26.7 (7.8 - 55.1) | 20.0 (6.8 - 40.7) | 0.705 |
AVM: arteriovenous malformation; GAVE: gastric antral vascular ectasia; APC: argon plasma coagulation; IR: interventional radiology; RBC: red blood cell.
Outcomes
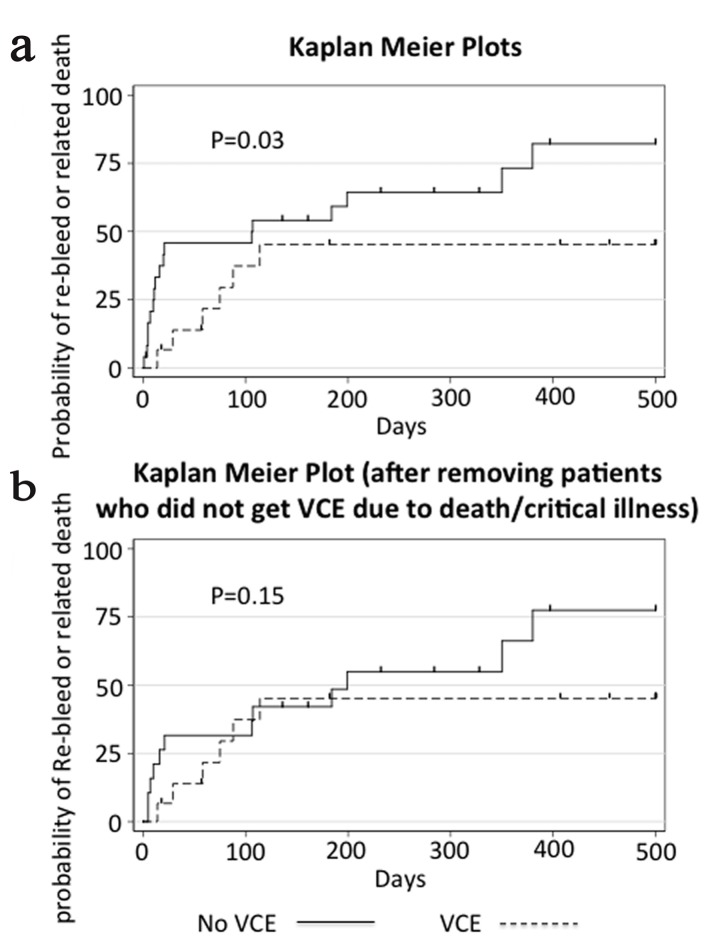
Time to re-bleed or death related to GI bleed, our primary outcome, was longer in the group that received VCE (median 114 days (IQR 57 - 587)) compared to the group that did not (median 106 days (IQR 10 - 232)) (P = 0.03 log-rank test) (Fig. 2). Our sample size was not large enough to adjust our analysis for baseline characteristics. After excluding the patients who did not receive a capsule endoscopy because of death or critical illness, there was no difference between the VCE and non-VCE groups (median 114 (IQR 57 - 587) vs. 161 (IQR 16 - 328), P = 0.15, log-rank test).
Figure 2.
Kaplan-Meier plot showing time to re-bleed or related death. Panel a shows the cumulative failure function for our primary endpoint with all patient encounters included. Panel b shows the same data but after removal of patients in the control group who did not get VCE due to death or critical illness. Data were censored at the time of transplant, death unrelated to GI bleeding, or last contact in the chart, and is indicated by a vertical dash in the figure. P values displayed are calculated by the log-rank test.
There were no statistical differences in 30-day outcomes between the VCE and non-VCE groups (Table 3). There was a trend towards higher readmissions in the VCE group, and a trend towards higher total adverse events in the non-VCE group. There were no deaths in the VCE group at 30 days, while the death rate was 20% in the non-VCE group (only 12% related to GI bleeding), but these differences were not statically significant. In a subgroup analysis removing deaths within 30 days, there were still no significant differences seen in 30-day outcomes.
Table 3. Thirty Day Outcomes for VCE and Non-VCE Encounters.
| Events within 30 days | Capsule (n = 15) | No capsule (n = 25) | P value |
|---|---|---|---|
| Readmission (%) | 46.7 (21.3 - 73.4) | 16.0 (4.5 - 36.1) | 0.065 |
| Related readmission (%) | 20.0 (4.3 - 48.1) | 8.0 (1.0 - 26.0) | 0.345 |
| Length of stay (median days) | 12 (5 - 30) | 6 (3 - 15) | 0.117 |
| Any adverse event (%) | 6.7 (0.2 - 31.9) | 36.0 (18.0 - 57.5) | 0.060 |
| Any adverse event or re-bleeding (%) | 33.3 (11.8 - 61.6) | 56.0 (34.9 - 75.6) | 0.204 |
| PRBC transfusion (median units) | 3 (0 - 7) | 1 (0 - 10) | 0.864 |
| Death (%) | 0 (0 - 21.8) | 20.0 (6.8 - 40.7) | 0.137 |
| Death related to GI bleed (%) | 0 (0 - 21.8) | 12.0 (2.5 - 31.2) | 0.279 |
PRBC: packed red blood cell: GI: gastrointestinal.
There were no complications associated with VCE itself, including no episodes of capsule retention. There were no issues reported with signal capture or interference. Prep quality was rated as excellent in 13.3% of capsules, good in 6.7%, fair in 13.3%, poor in 33.3%, and was not rated in 33.3% of capsules. The capsule was last noted in the stomach in 6.67% of cases, the small intestine in 26.7%, the colon in 53.3%, and location was not recorded in 13.3% of cases.
Discussion
Our study is the first to compare the use of VCE in CF-LVAD patients to a control group that did not undergo VCE. This is a valuable comparison because the utility of VCE has not been definitively established in this patient population. However, it is generally accepted as a part of the workup in suspected small bowel bleeding based on extrapolation from other patients groups [9, 10].
Our study demonstrated that VCE was helpful in making new diagnoses of GI bleeding not visible on prior endoscopic workup. The diagnostic yield for identifying the source of bleeding was 33.3%, which was similar to other studies that report yields of 31-40% [11, 12]. VCE was also helpful in guiding recommendations for further procedures such as DBE enteroscopy (Fig. 1), which is a finding similar to another study involving CF-LVAD patients [12]. A large study in non-VAD patients demonstrated the importance of capsule endoscopy guidance for subsequent DBE, with lesions found during the first 60% of the capsule study better suited for upper DBE, and lesions during the last 40% better suited for lower DBE [13]. Additionally, we found capsule endoscopy to be safe in LVAD patients, with no complications related to VCE, no capsule retention, and no device interference, which is consistent with prior studies [12, 14, 15].
However, in examining our data more closely, only one additional intervention on an actively bleeding lesion was performed as a result of 15 total VCEs (6.7%). Additionally, VCE never resulted in a new diagnosis of AVMs, so thus may not be particularly helpful when deciding if medical treatment with octreotide or thalidomide is needed. When comparing the group that got VCE to the control group that did not, we found there were no statistical differences in management (Table 2).
When evaluating our primary endpoint, time to re-bleed or death related to GI bleeding, the group that received VCE fared better. However, the baseline characteristics trended towards the VCE group being healthier, and in 6/25 cases the non-VCE group did not get a capsule because of either death or critical illness. After excluding these six cases from our analysis, there was no longer a significant difference between the VCE and non-VCE groups (Table 3).
Our outcomes are similar to a recent retrospective study evaluating 30 patients undergoing VCE. They found no differences in patients with positive vs. negative VCEs in terms of re-bleeding or mortality, and no difference in bleeding rates among those who had endoscopy after VCE vs. those who did not [12]. Another study evaluated a total of nine encounters for GI bleeding using capsule endoscopy. They found bleeding cessation after 100% of studies, but it seems unlikely that capsule endoscopy led to this outcome in many cases [14]. Neither of these studies had a control group that did not receive VCE for comparison.
If the findings of our study are supported by future studies, there may be reason to proceed directly to either upper or lower DBE, or trial empiric pharmacotherapy in patients with known AVMs [16] depending on the clinical situation. In LVAD patients, both thalidomide and octreotide have efficacy in treatment of arteriovenous malformations, which are thought to be the etiology of the majority of small bowel bleeds [16, 17].
Doing DBE first potentially allows faster intervention on bleeding lesions. In one retrospective study of suspected small bowel bleeding in VAD patients, enteroscopy done within 24 h of presentation resulted in faster resolution of GI bleeding, fewer units of blood, and less procedures [18]. In another study involving obscure bleeding in non-LVAD patients, urgent DBE had suprior yield, and resulted in more intervention on target lesions compared to urgent capsule endoscopy [19]. A 2007 meta-analysis suggested that complete upper and lower DBE had better diagnostic utility than capsule endoscopy alone [20]. DBE, when performed first, may also be more cost effective [21]. However, a 2013 meta-analysis suggested that capsule endoscopy was better overall for detection of occult GI bleeding, and argued for a complementary role, with the two studies detecting different lesions [22]. If DBE is going to be done before capsule endoscopy in LVAD patients, upper DBE is likely to be of higher yeild based on prior studies which have shown that all bleeding small bowel lesions in VAD patients were found in either the duodenum or jejunum [7]. Although it is hard to localize lesions precisely on capsule endoscopy, most of the lesions in our study were proximal and better accessed by upper DBE.
In conclusion, our institutional experience does not support the routine use of VCE in CF-LVAD patients with suspected small bowel bleeding. Consideration may instead be given to empiric treatment with thalidomide or octreotide in patients with known AVMs, or early use of DBE.
The limitations of this study include its retrospective design, small sample size, and non-randomized data. The sample size was too small to make meaningful corrections for differences in baseline characteristics. Future studies are needed with larger sample sizes and multicenter data.
References
- 1.Harvey L, Holley CT, John R. Gastrointestinal bleed after left ventricular assist device implantation: incidence, management, and prevention. Ann Cardiothorac Surg. 2014;3(5):475–479. doi: 10.3978/j.issn.2225-319X.2014.08.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, Rogers JG. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail. 2011;4(6):779–784. doi: 10.1161/CIRCHEARTFAILURE.111.962613. [DOI] [PubMed] [Google Scholar]
- 3.Crow S, John R, Boyle A, Shumway S, Liao K, Colvin-Adams M, Toninato C. et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137(1):208–215. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Meyer AL, Malehsa D, Budde U, Bara C, Haverich A, Strueber M. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC Heart Fail. 2014;2(2):141–145. doi: 10.1016/j.jchf.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Crow SS, Joyce DD. Are centrifugal ventricular assist devices the answer to reducing post-implantation gastrointestinal bleeding? JACC Heart Fail. 2014;2(2):146–147. doi: 10.1016/j.jchf.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Geisen U, Heilmann C, Beyersdorf F, Benk C, Berchtold-Herz M, Schlensak C, Budde U. et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33(4):679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 7.Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. Gastrointest Endosc. 2014;80(3):435–446. doi: 10.1016/j.gie.2014.03.040. e431. [DOI] [PubMed] [Google Scholar]
- 8.Guha A, Eshelbrenner CL, Richards DM, Monsour HP Jr. Gastrointestinal bleeding after continuous-flow left ventricular device implantation: review of pathophysiology and management. Methodist Debakey Cardiovasc J. 2015;11(1):24–27. doi: 10.14797/mdcj-11-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG clinical guideline: diagnosis and management of small bowel bleeding. Am J Gastroenterol. 2015;110(9):1265–1287. doi: 10.1038/ajg.2015.246. quiz 1288. [DOI] [PubMed] [Google Scholar]
- 10.Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E. et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47(4):352–376. doi: 10.1055/s-0034-1391855. [DOI] [PubMed] [Google Scholar]
- 11.Elmunzer BJ, Padhya KT, Lewis JJ, Rangnekar AS, Saini SD, Eswaran SL, Scheiman JM. et al. Endoscopic findings and clinical outcomes in ventricular assist device recipients with gastrointestinal bleeding. Dig Dis Sci. 2011;56(11):3241–3246. doi: 10.1007/s10620-011-1828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amornsawadwattana S, Nassif M, Raymer D, LaRue S, Chen CH. Video capsule endoscopy in left ventricular assist device recipients with obscure gastrointestinal bleeding. World J Gastroenterol. 2016;22(18):4559–4566. doi: 10.3748/wjg.v22.i18.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Chen H, Dai J, Gao Y, Ge Z. Predictive role of capsule endoscopy on the insertion route of double-balloon enteroscopy. Endoscopy. 2009;41(9):762–766. doi: 10.1055/s-0029-1215009. [DOI] [PubMed] [Google Scholar]
- 14.Truss WD, Weber F, Pamboukian SV, Tripathi A, Peter S. early implementation of video capsule enteroscopy in patients with left ventricular assist devices and obscure gastrointestinal bleeding. ASAIO J. 2016;62(1):40–45. doi: 10.1097/MAT.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 15.Bandorski D, Holtgen R, Stunder D, Keuchel M. Capsule endoscopy in patients with cardiac pacemakers, implantable cardioverter defibrillators and left heart assist devices. Ann Gastroenterol. 2014;27(1):3–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Draper K, Kale P, Martin B, Cordero K, Ha R, Banerjee D. Thalidomide for treatment of gastrointestinal angiodysplasia in patients with left ventricular assist devices: case series and treatment protocol. J Heart Lung Transplant. 2015;34(1):132–134. doi: 10.1016/j.healun.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Loyaga-Rendon RY, Hashim T, Tallaj JA, Acharya D, Holman W, Kirklin J, Pamboukian SV. Octreotide in the management of recurrent gastrointestinal bleed in patients supported by continuous flow left ventricular assist devices. ASAIO J. 2015;61(1):107–109. doi: 10.1097/MAT.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 18.Sarosiek K, Bogar L, Conn MI, O'Hare B, Hirose H, Cavarocchi NC. An old problem with a new therapy: gastrointestinal bleeding in ventricular assist device patients and deep overtube-assisted enteroscopy. ASAIO J. 2013;59(4):384–389. doi: 10.1097/MAT.0b013e318299fcd3. [DOI] [PubMed] [Google Scholar]
- 19.Aniwan S, Viriyautsahakul V, Angsuwatcharakon P, Kongkam P, Treeprasertsuk S, Rerknimitr R, Kullavanijaya P. Comparison of urgent video capsule endoscopy and urgent double-balloon endoscopy in massive obscure gastrointestinal bleeding. Hepatogastroenterology. 2014;61(135):1990–1994. [PubMed] [Google Scholar]
- 20.Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol. 2007;13(32):4372–4378. doi: 10.3748/wjg.v13.i32.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008;68(5):920–936. doi: 10.1016/j.gie.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, He Q, Liu J, Ma F, Zhi F, Bai Y. Combined use of capsule endoscopy and double-balloon enteroscopy in the diagnosis of obscure gastrointestinal bleeding: meta-analysis and pooled analysis. Hepatogastroenterology. 2013;60(128):1885–1891. [PubMed] [Google Scholar]