Abstract
The human gut has been continuously exposed to a broad spectrum of intestinal organisms, including viruses, bacteria, fungi, and parasites (protozoa and worms), over millions of years of coevolution, and plays a central role in human health. The modern lifestyles of Western countries, such as the adoption of highly hygienic habits, the extensive use of antimicrobial drugs, and increasing globalisation, have dramatically altered the composition of the gut milieu, especially in terms of its eukaryotic “citizens.” In the past few decades, numerous studies have highlighted the composition and role of human intestinal bacteria in physiological and pathological conditions, while few investigations exist on gut parasites and particularly on their coexistence and interaction with the intestinal microbiota. Studies of the gut “parasitome” through “omic” technologies, such as (meta)genomics, transcriptomics, proteomics, and metabolomics, are herein reviewed to better understand their role in the relationships between intestinal parasites, host, and resident prokaryotes, whether pathogens or commensals. Systems biology–based profiles of the gut “parasitome” under physiological and severe disease conditions can indeed contribute to the control of infectious diseases and offer a new perspective of omics-assisted tropical medicine.
Introduction
Every human subject has a specific gut microbiota that may change over their life span due to complex interactions between host genetics, immune response, diet, and environment [1] under physiological and pathological conditions [2]. Indeed, several recent studies have demonstrated the multitude of ways by which the microbiota has influenced human health and physiology [3]. Alterations in the human gut microbiota have been associated with a range of illnesses in the developed world, including inflammatory bowel disease (IBD), obesity, type 2 diabetes, allergies, and even autism, through the gut–brain axis [4, 5].
The definition of microbiota is related to the complex community of microorganisms mainly composed of bacteria but also including viruses, Archaea, eukaryotes such as fungi, and protozoa living in consortia in sites such as the gastrointestinal (GI) tract [3]. The human gut “virome,” composed mainly of bacteriophages [6], and the “mycobiome,” composed of yeasts and other fungi [7], have the potential to modify and regulate bacterial communities and hence modify and regulate human health. Among others, parasitic protozoa [8, 9], worms [10], and even eukaryotic commensals, such as Blastocystis hominis and Dientamoeba fragilis [11], can be of great importance. Particularly, it is still a matter of debate whether Blastocystis is associated or not with gut dysbiosis conditions [12], while interactions between helminths, protozoans, and the host immune system have been demonstrated [13, 14], as in the case of intestinal helminths, whose absence in the gut has been proposed as a risk factor for allergic/autoimmune/inflammatory diseases, including IBD [13, 15, 16]. Moreover, interactions between parasites and bacterial communities in the human gut may have a profound impact on the alteration of parasite virulence, course of both mucosal and systemic parasitic infection, and host immune response to the parasite, possibly explaining the observed variability in disease expression [14, 17, 18].
Based on such considerations, it is foreseeable that the exploration of parasites, protozoa, and worms within microbiota communities by “omic” technologies may provide more fully comprehensive information on gut prokaryote profiles.
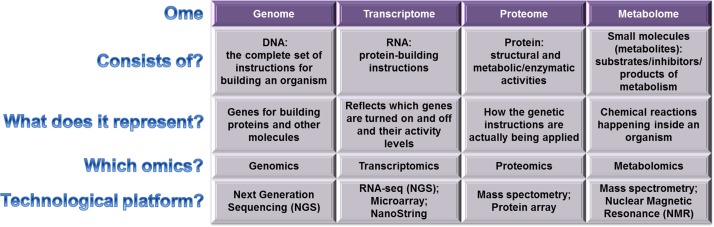
Such “omics”-based approaches are built on a holistic vision of the systems analysed, systems in which “all components are considered in complex ecological networks” in order to provide complete profiles of genes/transcripts/proteins/metabolites (Fig 1) [19, 20].
Fig 1. Summary of current “omic” technologies for “parasitome” investigations.
When applied to the study of prokaryotic consortia/communities, these technologies are denominated metagenomics, metatranscriptomics, metaproteomics, and meta-metabolomics and allow a non-targeted and high-throughput searching of the genetic scaffold and functional reservoir of the microbiota system, providing profiles of different organisms at the same time [21, 22]. Although some findings from metagenomic and metabolomic approaches to neglected tropical diseases are already available [23], meta-omic developments in the study of human gut “parasitome” are still in their infancy.
In this review, “omic” technologies (Table 1), applied to the study of human gut parasites (i.e., gut “parasitome”), are presented with the aim of furthering the understanding of gut parasite impact on intestinal ecology and dysbiosis. Much relevant work has been done on “model” parasites that are not primarily associated with the gut—e.g., liver flukes and blood flukes. As an example, an integrated transcriptomic and proteomic approach allowed researchers to describe the Fasciola hepatica secretory proteome, thus identifying proteins such as cathepsin, peroxiredoxin, glutathione S-transferase (GST), and fatty acid-binding proteins (FABPs) essential for the design of the first-generation anti-fluke vaccines and flukicidal drugs [24]. FABPs were also found to be the most abundant excretion/secretion proteins (ESPs) of Schistosoma japonicum, highlighting the vital importance of these proteins in the evasion process from the host immune system [25]. Based on these pivotal discoveries, Schistosoma FABPs and GSTs were selected by the World Health Organization as anti-Schistosoma vaccine candidates [26].
Table 1. Scheme of reported investigations on a human or animal model gut “parasitome” by exploiting “omic” technologies.
| Parasite |
“Omic” approach |
Sample | Technique | Major results | Reference |
|---|---|---|---|---|---|
| Ancylostoma caninum | Proteomics | Adult worm from small intestines of stray dogs | 1-DE LC-ESI-MS/MSa; protein or peptide OGEb and shotgun LC-ESI-MS/MS | Description of proteins from the excretory/secretory products | [74] |
| Ancylostoma ceylanicum | Genomics | Hookworms culture and infection in golden hamster (Mesocricetus auratus) | Genomic library; Illumina sequencing | Complete sequence of hookworm genome and definition of temporal genes expression | [48] |
| Transcriptomics | RNA-seq library, Illumina sequencing | ||||
| Blastocystis spp. | Metagenomics | Faecal DNA from 2 groups of patients, positive or negative for Blastocystis | NGSc: Ion Torrent sequencing | Blastocystis colonisation is positively associated with increased bacterial diversity and with higher abundance of Clostridia class, Ruminococcaceae, and Prevotellaceae | [37] |
| Faecal DNA from cohorts of healthy individuals and patients with Crohn’s disease, ulcerative colitis | NGS: WGSd Illumina sequencing | Blastocystis colonisation is positively associated with species richness and with species present in lean individuals, such as the Prevotella or Ruminococcus enterotype | [33] | ||
| Blastocystis ST7 | Proteomics | Axenic culture of an isolate obtained from a Singaporean patient with intestinal disorders | 1-DE LC-ESI-MS/MSa | Definition of secreted protease candidates for the effects induced at the parasite–host interface and involved in mucus degradation: legumain peptidase ACY95293.1, and cathepsin B peptidase CBK25506.2. | [72] |
| Cryptosporidium parvum | Proteomics | Excysted sporozoites from lambs | 2-DE MALDI TOFe; 1-DE LC-ESI-MS/MS; Shotgun MudPitf LC-ESI-MS/MS |
Definition of sporozoite proteome | [59] |
| Excysted and non-excysted calf oocysts | Stable isotope labelling iTRAQg LC-ESI-MS/MS; Shotgun LC-ESI-MS/MS | Definition of excysted and non-excysted oocysts proteome; specific proteins augmented in excysted invasive sporozoites: many ribosomal (40S and 60S) and heat shock chaperonin (Hsp70 and Hsp90) | [60] | ||
| Metabolomics | Human faecal samples | Untargeted GC-MSh | Higher abundance of phenylalanine, valine, isoleucine, serine, succinic acid, and threitol, lower levels of xylose in infected sample; faecal metabolite profiles generated are able to identify infected individuals | [86] | |
| Faecal sample from mice infected with S26 isolate (obtained from a naturally infected calf) | Chemical Derivatization GC-MS | Less abundant metabolites and intermediaries involved in energy metabolism (key nutrients scavenged by Cryptosporidium to supplement its metabolic pathway) were detected in infected mice than in uninfected mice | [87] | ||
| Encephalitozoon cuniculi | Proteomics | GB-M1 cultured in Madin-Darby canine kidney or human foreskin fibroblast cells | 2-DE MALDI-TOF/ESI LC-MS/MS; Shotgun LC-ESI-MS/MS |
Identification of a reference map of the major proteins expressed during late sporogony | [62] |
| Entamoeba histolytica | Proteomics | Axenic cultured trophozoites (strain HMI:IMSS) | 2-DE LC-ESI-MS/MS | Identification of specific parasite proteins that promote host invasion | [68] |
| 1-DE LC-ESI-MS/MS | Identification of the cell surface–associated proteome | [67] | |||
| Axenic cultured trophozoites (strains HMI:IMSS and Rahman) | 2-DIGEi MALDI-TOF/ESI LC-MS/MS | Identification of important molecular component defining physiologically relevant virulence phenotype | [70] | ||
| Entamoeba histolytica and Entamoeba dispar | Proteomics | Axenic cultured trophozoites (strain HMI:IMSS of E. hystolytica and SAW760 of E. dispar) | 2-DE MALDI-TOF | Identification of more proteins which are involved in Entamoeba pathogenicity | [69] |
| Entamoeba invadens | Transcriptomics | Axenic cultured trophozoites (IP-1 strain) | Affymetrix platform microarray | First description of transcriptional and metabolic regulatory networks dynamics taking place during E. invadens encystation | [89] |
| Metabolomics | CEj-ESI-TOF-MS/MS | ||||
| Giardia duodenalis | Metabolomics | Stool from patients with confirmed infection and controls with diarrhoea | GC-MS | First report of the Volatile Organic Compounds that could be biomarkers of Giardia infection | [88] |
| Giardia lamblia | Proteomics | Cultured WBC6 (ATCC catalog number 50803) trophozoites | 1-DE LC ESI-MS/MS | Definition of the protein repertoire of peripheral and encystation-specific vesicles that have key roles in proliferation and transmission to a new host | [61] |
| Heligmosomoides polygyrus | Metagenomics | Distal ileum and cecal tip of C57BL/6 healthy and infected mice; L3 larvae and adult worms from duodenum of infected mice | NGS: 16S rRNA gene targeted amplification and Sanger-style sequencing; quantitative PCR clone library analysis | Infection significantly alters the gut microbiota with increased Lactobacillaceae bacterial load | [42] |
| Helminths (Trichuris spp., Ascaris spp., hookworm) | Metagenomics | Faecal sample of helminth-infected or uninfected indigenous Malaysians and New York City residents |
NGS: V4 16S rRNA gene targeted amplification; Illumina sequencing | Significant effect of helminth colonisation on the diversity, bacterial community structure and function of the gut microbiota | [45] |
| Necator americanus | Metagenomics | Faecal samples of experimentally infected human volunteers (affected by celiac disease) on a gluten-free diet | NGS: 16S rRNA gene targeted amplification; 454 pyrosequencing | Hookworm infection did not have a major impact on the community structure of the intestinal microbiota | [41] |
| Prior and post dietary gluten exposure faecal samples of experimentally infected human patients (affected by celiac disease) | Microbial species richness increases during the challenge with escalating doses of dietary gluten, a potential mechanism by which hookworm infection could positively impact gluten-induced inflammation and intestinal immune homeostasis | [40] | |||
| Genomics | L3i and adult worms from intestines of Golden Syrian Hamster infected subcutaneously with the Anhui strain | NGS: WGS and 454 pyrosequencing | Draft genome sequence and postgenomic analyses to unveil the immunobiology of human hookworm disease | [49] | |
| Transcriptomics | Rna-seq: Roche/454 and Illumina cDNA libraries; Genome Sequencer Titanium FLX and Illumina sequencing |
||||
| Proteomics | OGE and shotgun LC-ESI-MS/MS; protein microarray | ||||
| Metabolomics | Urine and blood samples from infected and control Syrian hamsters | 1H NMRk | Unveil the biochemical consequences of infection | [90] | |
| Urine and blood samples from infected and control Syrian hamsters coinfected with Schistosoma japonicum | [91] | ||||
| Strongyloides stercoralis | Transcriptomics | Parasites from faecal samples of infected individual of the endemic area of La Safor (Valencia, Spain) and propagated on axenic culture | cDNA library, 454 pyrosequencing | First comprehensive database of third larval stage transcripts | [65] |
| Proteomics | Shotgun LC-ESI-MS/MS | First study of the S. stercoralis proteome | [64] | ||
| Taenia solium | Proteomics | Gravid proglottids from Peruvian patients' stools | LC fractionation and MALDI TOF MS/MS | Definition of oncosphere proteome | [63] |
| Metacestodes from naturally infected pigs in Zambia and Perú | 1-DE LC-ESI-MS/MS | First report of the metacestode excretion/secretion proteome | [73] | ||
| Trichinella spiralis | Proteomics | Worms (isolated from the small intestine of infected rats) cocultured with different strains of bacteria | iTRAQ bidimensional LC-ESI-MS/MS | Comprehension of microbe-induced alterations in the survival and reproduction of T. spiralis in vitro: Lactobacillus bulgaricus and L. acidophilus were beneficial; Salmonella enterica and Escherichia coli O157:H7 (EHEC) were not | [76] |
| Trichuris suis | Metagenomics | Luminal colon content of control and infected piglets | NGS: 16S rRNA gene targeted amplification and WGS 454 pyrosequencing | Identification of the infection significant impact on the proximal colon microbiota composition at both the phylum and genus levels (key genera: Mucispirillum, Succinivibrio, and Ruminococcus) supported by metabolic and functional data | [43] |
| Metabolomics | GC-MS | ||||
| Trichuris trichiura | Genomics | A clinically isolated adult male | NGS: WGS, Illumina sequencing | High-quality draft genome assembly | [79] |
| Transcriptomics | Stool sample of infected Ecuadorian children | NGS: Ion Torrent sequencing | First transcriptomic analysis of the adult stage of the human whipworm | [78] | |
| Proteomics | Shotgun LC-ESI-MS/MS | Identification of proteins with immunomodulatory effects | [77] | ||
| Unknown parasites | Metagenomics | Wild rat faeces | NGS: V9 18S rRNA gene targeted amplification; Illumina sequencing | Novel method to determine host alimentary tract parasite infections | [50] [51] |
a 1-DE LC-ESI-MS/MS: monoDimensional gel Electrophoresis Liquid Chromatography-ElectropSprayIonisation-tandem Mass spectrometry
b OGE: OFFGEL fractionation by isoelectric focusing
c NGS: Next Generation Sequencing
d WGS: Whole Genome Sequencing
e 2-DE MALDI TOF: biDimensional Matrix-Assisted Laser Desorption/ionization Time-of flight mass spectrometry
f MudPit: Multi-dimensional Protein Identification technology
g iTRAQ: isobaric Tags for Relative and Absolute Quantitation
h GC-MS: gas chromatography-mass spectrometry
i 2-DIGE: bidimensional-DIfference Gel Electrophoresis
j CE: Capillary electrophoresis
k 1H NMR: Proton Nuclear Magnetic Resonance spectroscopy
ESP-induced early changes in host cells highlighted by proteomics were also confirmed for Opisthorchis viverrini [27, 28], and indeed, plasma actin-related protein 3 (ARP3) autoantibody and 14-3-3 eta protein were identified as putative new diagnostic markers of opisthorchiasis [29, 30].
Below, “omics”-based investigations on human gut protozoa and worms will be discussed to update the state of the art on gut “parasitome” citizens by “omic” technologies.
Methods
Literature searches in PubMed until October 31, 2016 were performed using a search strategy designed to identify relevant studies for this review from the following 2 categories: (i) evaluating genes, transcripts, proteins, and metabolites of parasites colonising/invading the human gut and (ii) approaches in “omics”-based research methodologies. Two investigators independently evaluated articles resulting from these searches and any relevant references cited in those articles for inclusion in this study.
DNA-based “omics”: Genomics and metagenomics
Since the beginning of the 21st century, tremendous advances in DNA sequencing technologies have emerged, allowing for the study of genomes in greater depth and therefore better decoding of their structural and functional attributes. In particular, next-generation sequencing (NGS) technologies have displayed high-throughput sequencing power and are composed of a number of different modern methods that sequence nucleotides faster and cheaper than Sanger capillary electrophoresis, including HiSeq/MiSeq Illumina, Roche 454, Ion torrent: Proton/PGM, SOLiD, PacBio, and Oxfordnanopore platforms [31].
Briefly, NGS pipelines are based on (a) sample collection, (b) nucleic acid extraction, (c) library and (d) template preparation, and (e) sequencing reaction completed by (f) genome and read alignments during the data analysis [32]. The application of NGS could be basically divided into the following 2 categories: (a) de novo genomic sequencing by mate-paired and whole genome shotgun (WGS) strategies for determining the complete DNA sequence of an organism’s genome at a single time and (b) targeted sequencing of gene or locus for the analysis of specific mutations and phylogenetic and evolutionary studies. 16S rRNA for bacterial and 18S rRNA for eukaryotic genes are the most investigated targeted sequences because their high degree of sequence conservation across many groups of organisms provides the most suitable method for microorganism identification by so-called targeted metagenomics.
Metagenomics to highlight gut bacterial and eukaryotic relationships
NGS platforms allow metagenomic studies, which have revolutionised microbiology and related fields, to investigate whole prokaryotic communities in terms of the presence and relative abundance of microorganisms. Metagenomics is potentially important in describing the interplay of prokaryotic communities with their eukaryotic counterparts within the whole intestinal ecological system. By using this approach, Blastocystis was described in healthy individuals but not in patients with Crohn’s disease [33]. Particularly, lower Blastocystis colonisation levels were observed in subjects characterised by a Bacteroides-driven enterotype as compared with Prevotella- or Ruminococcus-driven enterotypes and were positively associated with species richness [33]. This aspect is particularly interesting because disease enterophenotypes, such as those related to IBD, obesity, and nonalcoholic fatty liver disease/steatohepatitis (NAFLD), are generally inversely related to bacterial richness [34–36]. Hence, Blastocystis may be considered a richness-dependent marker [33, 37].
Clearly, the biocomplexity of the intestinal lumen suggests that interactions between parasites and the intestinal microbiota would also influence inflammation. Recent studies have investigated the potential therapeutic properties of GI nematodes in modulating regulatory responses in the host gut and thereby promoting immune homeostasis [38]. Epidemiologic studies noted a reduced susceptibility to inflammatory diseases (e.g., asthma) in the presence of hookworm infection [39]. Indeed, it has been suggested that Necator americanus may alleviate chronic inflammation in celiac disease but also maintain prokaryotic species richness, thereby reestablishing the GI tract’s microbial eubiosis and immune homeostasis [38, 40]. Probably, the effects of N. americanus is explicit on microbiota species richness rather than on community structure or relative abundance of individual bacterial species [41].
In an interleukin-10 (IL-10) gene-deficient murine model of IBD, infection by Heligmosomoides polygyrus was evaluated for treatment of colitis, and indeed, the amelioration of colonic inflammation was observed in wild-type C57BL/6 mice [42]. One proposed mechanism was that H. polygyrus infection favours the outgrowth or suppression of certain bacteria, which in turn help modulate host immunity. Indeed, Lactobacillaceae significantly increased in abundance in the ileum of the infected mice, supporting the concept that helminth infection shifts the composition of intestinal bacteria [42]. The alteration of prokaryotic community structure was also observed in an animal model (pig) during a Trichuris suis infection [43]. Interestingly, the meta-taxonomy alterations were detected by both targeted metagenomics and WGS sequencing. Amongst the 15 phyla identified, the abundances of Proteobacteria and Deferribacteres were changed in infected pigs (IPs). Seventeen genera, such as Oscillibacter, Succinivibrio, Sporobacter, Spirochaeta, Paraprevotella, and Mitsuokella, were significantly affected (P ≤ 0.05). The relative abundance of Oscillibacter, the second most abundant genus in the colon microbiota of control pigs (CPs), decreased from 7.8% of CPs to 2.8% of IPs. Similarly, the relative abundance of Succinivibrio decreased from 3.6% of CPs to 0.4% of IPs. On the other hand, an 86-fold expansion in the relative abundance of Mucispirillum to 0.09% was registered in IPs, accounting for all observed changes in the phylum Deferribacteres [43].
Because “parasitome” is strictly related to environmental (e.g., geographic and temporal clusters, etc.) and host determinants of parasite infection (e.g., age, immunological status, travels, community behaviours) [44], socioeconomic and anthropologic factors were also evaluated in a study on helminth colonisation and alterations of gut microbiota in a group of Malaysian indigenous people [45]. An increased ecological diversity and a higher abundance in the classes Alphaproteobacteria and Mollicutes, the order Bacteroidales, and in particular, its family Paraprevotellacae were observed in helminth-infected people. In contrast, helminth-negative people showed an increased abundance in the Bifidobacterium spp. [45]. Although differences in the distribution of bacterial operational taxonomic units (OTUs) between infected and noninfected Malaysian people were smaller than those observed between urban United States and Malaysian residents, higher bacterial diversity (i.e., α- and β-diversity) definitely appeared associated with helminth colonisation, once more suggesting that helminth-driven alterations of microbiota are especially evident in terms of richness [45].
On the other hand, Trichuris-driven infections were observed to ameliorate colitis by restoring mucosal barrier functions acting on mucus production and on diversity of mucosal bacteria in a macaque model [46].
The above diversity in metagenomic reports are clearly due to different helminth species/hosts investigated, dissimilar infectious doses used to inoculate human volunteers or animal models, different technological platforms, and the different sample types studied (e.g., mucosa versus faeces); however, such diversity of conclusions is also linked to the high complexity of gut prokaryotic and eukaryotic communities. Hence, the standardisation of “omic” procedures can represent the first step to making more homogenous report inferences.
Future of DNA-based studies on “parasitome” investigations: The challenge of sequencing parasite genomes
The standardisation of procedures and a comparison of different results from different metagenomic pipelines are completely lacking in the specific field of “parasitome” characterisation. High sequence similarities between related species and/or the absence of parasite sequences in available current databases are still the major weaknesses of metagenomics-driven approaches to obtaining accurate species-level identification of parasites. Enormous efforts have been undertaken during the past few years to increase the availability of extended parasite databases (http://parasite.wormbase.org/, http://www.sanger.ac.uk/science/collaboration/50HGP, http://eupathdb.org/eupathdb/ [47]), as in the case of Ancylostoma ceylanicum [48] and N. americanus [49], for example. Hopefully, in the near future, genomes of more parasites will become available. Nevertheless, 18S rDNA-based metagenomic approaches developed to facilitate the detection of eukaryotic parasites are characterised by a sensitivity at least as high as the conventional microscopy-based method [50, 51].
Epigenomics to highlight gut “parasitome” and host relationships
Among the application of NGS technologies, there is the study of the epigenome, i.e., the study of any potentially stable and heritable changes in gene activity and expression without altering DNA sequences. DNA methylation and histone modifications are examples of tightly regulated mechanisms that produce such DNA changes.
DNA methylation sequencing and chromatin immunoprecipitation followed by sequencing (ChIP -Seq) enable the precise genomic localisation of epigenetic markers to decipher gene activity and expression as well as chromatin state. There is growing interest in epigenetics for its role in the development and reproduction of parasites and host–parasite interactions through potentially mutual modulation of genomes. Epigenetic studies of parasites are mostly linked to malaria and schistosomiasis; only a few are related to gut-related parasitoses. Amongst protozoa, Cryptosporidium parvum encodes candidate methyltransferases, although no proteins were identified; in Entamoeba histolytica and Trichinella spiralis, methylated DNA was identified, as well as the presence of DNA methyltransferases or their coding genes [52]. Also, the modulation of epigenetic host processes was demonstrated in helminth-induced immune suppression [53]. Future epigenetic research on parasites will provide us with better knowledge of both environmental signals and parasite sensor and executor molecules, which determine different parasite development and virulence programs. Moreover, this knowledge will give us potential opportunities for disease intervention.
RNA- and protein-based “omics”
The identification and quantification of transcriptionally active regions of the genome (the transcriptome) and of the ultimate products of the transcription (the proteome) are of fundamental importance to elucidate biological functions. NGS technologies allow us to also examine all the RNA and its differential expression, thanks to an additional step of the operative pipeline: after RNA extraction, researchers synthesise complementary DNA (cDNA) from RNA [54]. Proteomics deals with the determination of proteins of a biological system by biophysical and biochemical methods. It has been revolutionised by the advent of mass spectrometry (MS), a sensitive and rapid analytical method for protein identification and quantification that enables large-scale, fast, and systematic measurements of proteomes in space and time. Basically, an MS proteomic experiment consists of the following: (a) protein extraction and purification from matrices, (b) direct analysis (top-down approach) or enzymatic digestion (bottom-up approach), (c) optional protein/peptide separation based on liquid chromatography, (d) mass-to-charge and intensity detection of protein/peptide and their induced fragments by MS, and (e) protein identification and quantification by de novo or database-driven data analysis [55].
Gut parasites have been analysed by transcriptomics or proteomics to highlight invasive and diagnostic features, while, to our knowledge, metatranscriptomic or metaproteomic studies of gut prokaryotic and eukaryotic communities, including parasites, have not yet been performed.
Elucidation of parasite life cycle stage–specific characteristics
Transcriptomic and proteomic studies may provide evidence of a parasite’s gene expression products in order to have a more comprehensive, functional picture of each parasite’s vital stages and metabolism [22, 56]. This effort has been supported by the introduction of advanced bioinformatic resources for the handling of transcriptomic and proteomic data, which integrate whole genome sequences and annotations with expressed sequence tags [47, 57, 58]. So far, proteomics has described the following parasite cycle features: (i) secretion and excystation proteins of C. parvum oocyst/sporozoite stages [59, 60]; (ii) Giardia trophozoites’ peripheral and encystation-specific vesicles, organelles that have key roles in proliferation and transmission to a new host, respectively [61]; (iii) Encephalitozoon cuniculi spore-rich cell populations, representing the major protein reservoir expressed during late sporogony [62]; (iv) Taenia solium activated oncospheres, involved in gut penetration and immune evasion machineries [63]; and (v) Strongyloides stercoralis infective filariform larvae (L3i) [64]. Moreover, the transcriptome of S. stercoralis L3i has been annotated to provide a comprehensive database for genomic, proteomic, and metabolomic explorations of S. stercoralis [65].
Focus on parasite surface and secreted proteins
Several proteomic investigations have focused on surface proteins because they are thought to be involved in the host–parasite interactions, immune response, and disease processes [66]. In the case of E. histolytica, more proteins than expected have been recently catalogued as surface associated, a phenomenon that may be caused by its high membrane turnover [67]. The surface-associated proteome can indeed elucidate molecular mechanisms that regulate virulence in E. histolytica [68]. A large number of proteins that can potentially act as new virulence factors were highlighted by comparing the proteome of E. histolytica with that of the closely related but nonpathogenic E. dispar [69] and investigating different virulent E. histolytica strains [70].
Gut parasite secretory products have been intensively studied, especially with reference to their proteolytic activities. Several enteric pathogens, in fact, can modulate the protease balance, which regulates the intestinal epithelial cell microenvironment, inducing intestinal pathobiology [71]. Moreover, specific parasite-secreted proteases stimulate gut secretion or inflammation, alter gut integrity, inhibit host immunity, and therefore promote disease, such as in Entamoeba spp. or Giardia spp. infections, in which cysteine proteases are effective virulence factors. Secreted proteases were also characterised in Blastocystis culture supernatants, allowing protease candidates to increase intestinal permeability [72]. The differences observed in terms of virulence between E. histolytica and E. dispar were indeed demonstrated to be due to the secretion of specific proteases and, specifically, to increased protease activity by E. histolytica [71].
In some cases, invasive stage highlights may contribute in providing new diagnostic tools. Taenia solium metacestodes are able to maintain a host infection by developing many protective mechanisms, including the production of ESPs. Such ESPs were investigated by Victor et al. [73] in an effort to unveil more proteins involved in parasite survival strategies as well as to better understand the interaction between metacestodes and their host.
ESP proteomic analysis was also performed in A. caninum [74], a model for human hookworm infections, providing insight into the biology of hookworm and immunomodulatory mechanisms by which these worms establish and maintain chronic infections in their host. The identified ESPs could be useful in the development of both anti-helminthic vaccines/drugs and therapeutic agents for inflammatory or autoimmune diseases. For example, 1 of these identified secreted proteins of A. caninum, the tissue inhibitor of metalloproteinase (TIMP)-like anti-inflammatory protein-2 (AIP-2), has been demonstrated to promote positive regulatory T-cell response and suppress airway inflammation in a mouse model, thereby showing a novel potential therapeutic drug for allergic asthma [75]. Also of interest is a recent proteomic work that investigated the physiological and biological influence of the gut microbiota on the parasite T. spiralis, suggesting that specific gut microbes may be considered as therapeutic agents to prevent trichinellosis [76].
Host–parasite relationships and immune response
The host immune response and, consequently, the development and manifestation of chronic human inflammatory diseases may be modulated by infection with helminthic parasites, as in the case of Trichuris trichiura, which exerts a protective effect against atopy, and allergic and autoimmune diseases [77, 78]. Therefore, the immunomodulatory effect of T. trichiura adult worm extract was investigated to identify proteins acting as drug molecules for allergic and other inflammatory diseases [77]. The same authors characterised the adult stage transcriptome of T. trichiura [78], which contributed to the functional annotation of a recently released genome draft [79]. It is worthwhile to also mention the protein array technique (miniaturisation of thousands of assays on 1 small plate) facilitating the analysis of host immune response to parasite antigens; Tang and colleagues probed the serum of patients infected with N. americanus with an array of 564 recombinant proteins inferred from the genome of the parasite and identified 22 antigens that were significant targets of anti-hookworm immune responses and might form the basis of sensitive and specific serodiagnostic tests [49].
Future direction for protein-based approach: New frontiers of post-translational modifications and protein–protein interactions
Until now, human gut parasite transcriptomics and proteomics have not been analysed in depth; modern technological MS-based platforms are able to perform more sophisticated analyses, such as the study of the intact protein complexes and the detection of direct protein–protein interactions, the application of which in the interpretation of host–parasite interaction networks will be of invaluable help in the battle against infection [80]. Elucidation of protein post-translational modifications (PTMs; i.e., phosphorylation, acetylation, glycosylation, etc.) is another fundamental target in research on parasites for their critical role in protein function and therefore in the progression and outcome of infection. As an example, histone PTMs of E. histolytica and G. lamblia might be involved in host–parasite interactions in terms of virulence and morphological differentiation [81].
Metabolomics
The GI tract is a dynamic metabolic and immunologically active ecosystem, and its complete set of metabolites reflects both the enzymatic pathways of host and gut inhabitants and the complex network that connects them. Metabolomics aims to monitor metabolite components in a system and determine their quantitative dynamic change. Two technologies commonly associated with metabolome analyses are nuclear magnetic resonance (NMR) spectroscopy and gas or liquid MS, well suited for identification and quantitation of small-molecular-weight metabolites in a high-throughput fashion [82]. Recent findings describe the roles of microbial metabolites in regulating host physiology, immunity, and pathology [83–85]. Therefore, metabolomics provides a novel approach to studying the microbiota but also the “parasitome” and its interactions with the host counterpart. Indeed, perturbations of gut metabolite profiles reflect changes in cellular regulation and physiological processes that may result from parasitic infections, and these profiles may provide a pathway for biomarker discovery, drug targets, and improved diagnoses [23].
From metabolomic patterns to diagnostic and therapeutic tools for protozoa
Despite the differences in faecal metabolite profiles in Cryptosporidium-infected humans [86] and mice [87], metabolomics clearly differentiates between infected and uninfected states. Such metabolic differential patterns may be useful for the diagnosis of Cryptosporidium infections and to improve microscopy or PCR-based diagnoses, which are often hampered by sensitivity limits due to low numbers of oocysts in faeces because of intermittent shedding. Giardia lacks mitochondria and depends on fermentative metabolism, showing unique metabolic pathways. Volatile organic compounds (VOCs) may therefore represent specific markers of Giardia infection in stools, hence presenting a potential role for the diagnosis of giardiasis [88].
Because the elucidation of the encystation process could further the improvement of control measures against parasitic infectious diseases, metabolic and transcriptomic changes occurring during the encystation of E. invadens, a relative of E. histolytica that infects reptiles, have been investigated [89]. The encystation process leads to decreased levels of most metabolites involved in glycolysis and of all nucleotides, while the intermediates of chitin biosynthesis, some biogenic amines, and γ-aminobutyric acid increase. Because chitin does not occur in vertebrates, its synthetic pathway represents an excellent parasite-specific target for developing new chemotherapeutics.
Metabolomic patterns to unveil bacteria, parasite, and host interplay
VOC-based analysis, coupled with metagenomic analysis, as previously described, was performed on the luminal contents of pigs infected with T. suis [43]. Twenty-six percent of all identified colonic metabolic pathways were affected by T. suis presence, with a drop out of cofactors for carbohydrate and lysine biosynthesis. Moreover, the observed accumulation of oleic acid in IPs suggested altered fatty acid absorption, hence enhancing local inflammation. Therefore, T. suis exhibited a central role in the microbiota–host axis [43].
Wang and collaborators [90] pioneered the strategy of metabolic profiling of blood and urine to investigate biochemical consequences of N. americanus infection in an animal model to determine the host metabolic response. One of the prominent changes noted was the alteration of host energy-related metabolism, which was reflected in an increased concentration of lipoprotein and lipids and a decreased concentration of glucose in the blood. Additionally, a number of urinary metabolites was found to increase in infected hamsters, including p-cresol-glucuronide and 2-aminoadipate [90]. The same authors performed the same metabolic investigation after coinfection with S. japonicum and N. americanus, noting again a reduced concentration of the gut microbial-related metabolite hippurate in the hamster urine [91]. The decrease in hippurate levels is common to all helminth infections studied to date. However, it is evident that no single metabolite can be a specific marker for parasitosis: the metabolic signature itself, at least in theory, could be a diagnostic reference.
Conclusions and perspectives
The strength of “omic” approaches resides in their ability to provide complete profiles of genes/transcripts/proteins/metabolites, overcoming the classical genetic/biochemical studies based on single or few target molecules, giving a broader perspective of parasite biology and, as a consequence, improving parasite control programs and diagnostics.
After reviewing studies based on “omic” technologies, possible markers of parasite infection have emerged, as well as putative vaccine targets. However, although interesting data have been revealed, considerable work remains to improve current “omics”-based operational pipelines in order to fully understand the 3-way interactions between host, prokaryotic communities, and parasites. Future research should include the extended sequencing of parasite genomes; one of the actual shortcomings of “omic” studies is the poor characterisation of new parasite genes both in their sequences and functions. More deeply curated annotations and affordable metagenomic pipelines are needed for the description of parasites within the gut microbiota environment. Moreover, new metaproteomic procedures (MS differential profiling by multiple reaction monitoring-like acquisition), now available for prokaryotic communities, need to be applied to eukaryotic citizens (the “parasitome”) of the gut ecological system in order to greatly improve the distinction between host and parasite proteins as well as to identify as-yet unknown proteins.
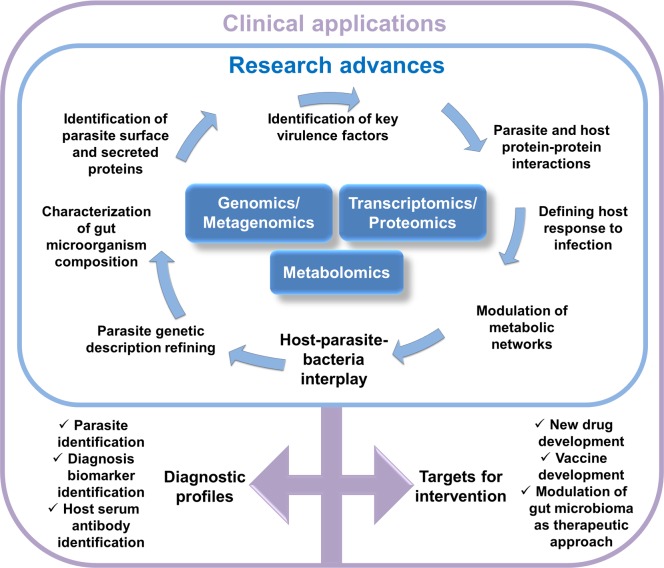
Therefore, “omic” technologies are now promising tools capable of leading to the discovery of new key pathways which may improve diagnostic and therapeutic approaches for parasite-linked GI diseases within the context of microbiota/parasitome/host co-metabolism and response to infections (Fig 2).
Fig 2. Multi-omic basic research will define molecular mechanisms on the basis of host–parasite–bacteria cross-talk on the road to more effective translational research.
Key learning points
The human gut is a complex ecological system composed of host and symbiotic prokaryotic cells and plays a central role in human health. Intestinal eukaryotic parasites (i.e., protozoa and helminths) are the other important components of this coevolved community, being able to modify the composition/activity of the gut prokaryotes through excreted/secreted molecules or evoking a response from the host’s immune system. At the same time, bacteria may exert profound effects on parasite physiology and survival within the intestinal niche.
The “omic” approaches (genomics, transcriptomics, proteomics, and metabolomics), based on the recent development of efficient analytical and data mining methods, allow the overall evaluation of gene/transcripts/protein/metabolite scaffolds of a biological system under specific conditions and time points. This offers non-targeted, high-throughput, and deep systems biology analyses that may be the key to decoding the functional activity of the human gut community.
Metagenomic data pipelines, developed for studying prokaryotes, are valuable tools for the detection of eukaryotic DNA signatures in gut microbiota communities; existing metagenomic data from studies across geographical reservoirs can be used to produce standard profiles of prokaryotes of healthy populations, enabling us to identify dysbiosis. Such reference microbiota can be exploited to assess actual prokaryotic–eukaryotic relationships within the ecology of the human intestinal niche.
A deeper “omic” and new “meta-omic” profiling of both the parasite and parasite–microbiota–host interplay will further assist the discovery of the entire biological machinery of the gut community and will have a valuable impact in unveiling new diagnostic and virulence markers as well as promising targets for vaccination.
Top 5 papers
Lee SC, Tang MS, Lim YA, Choy SH, Kurtz ZD, Cox LM, et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl Trop Dis. 2014;8(5): e2880.
Tang YT, Gao X, Rosa BA, Abubucker S, Hallsworth-Pepin K, Martin J, et al. Genome of the human hookworm Necator americanus. Nat Genet. 2014;46(3):261–9.
Biller L, Matthiesen J, Kuhne V, Lotter H, Handal G, Nozaki T, et al. The cell surface proteome of Entamoeba histolytica. Molecular & cellular proteomics: MCP. 2014;13(1):132–44.
Jiang HY, Zhao N, Zhang QL, Gao JM, Liu LL, Wu TF, et al. Intestinal microbes influence the survival, reproduction and protein profile of Trichinella spiralis in vitro. International journal for parasitology. 2016;46(1):51–8.
Bond A, Vernon A, Reade S, Mayor A, Minetti C, Wastling J, et al. Investigation of Volatile Organic Compounds Emitted from Faeces for the Diagnosis of Giardiasis. J Gastrointestin Liver Dis. 2015;24(3):281–6.
Acknowledgments
We would like to thank the English-speaking experts from BioMed Proofreading LLC for manuscript revision.
Funding Statement
LP received founding by Italian Ministry of Health for Ricerca Corrente 2016 titled “Interpretation of disease phenotypes in term of host-microbiota-exposome interactions: the new role of systems medicine in paediatrics.” The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. Epub 2012/06/16. doi: 10.1038/nature11053 ; PubMed Central PMCID: PMC3376388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375(24):2369–79. Epub 2016/12/16. doi: 10.1056/NEJMra1600266 . [DOI] [PubMed] [Google Scholar]
- 3.Sommer F, Backhed F. The gut microbiota—masters of host development and physiology. Nature reviews Microbiology. 2013;11(4):227–38. Epub 2013/02/26. doi: 10.1038/nrmicro2974 . [DOI] [PubMed] [Google Scholar]
- 4.Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med (Encinitas). 2014;13(6):17–22. ; PubMed Central PMCID: PMCPMC4566439. [PMC free article] [PubMed] [Google Scholar]
- 5.Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22(1):361–8. doi: 10.3748/wjg.v22.i1.361 ; PubMed Central PMCID: PMCPMC4698498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogilvie LA, Jones BV. The human gut virome: a multifaceted majority. Frontiers in microbiology. 2015;6:918 doi: 10.3389/fmicb.2015.00918 ; PubMed Central PMCID: PMCPMC4566309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozena DK, Iwona D, Ilona K. The mycobiome—a friendly cross-talk between fungal colonizers and their host. Ann Parasitol. 2016;62(3):175–84. doi: 10.17420/ap6203.51 . [DOI] [PubMed] [Google Scholar]
- 8.Rook GA. Hygiene hypothesis and autoimmune diseases. Clinical reviews in allergy & immunology. 2012;42(1):5–15. Epub 2011/11/18. doi: 10.1007/s12016-011-8285-8 . [DOI] [PubMed] [Google Scholar]
- 9.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. Epub 2005/04/16. doi: 10.1126/science.1110591 ; PubMed Central PMCID: PMC1395357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. The ISME journal. 2008;2(12):1183–93. Epub 2008/08/02. doi: 10.1038/ismej.2008.76 . [DOI] [PubMed] [Google Scholar]
- 11.Petersen AM, Stensvold CR, Mirsepasi H, Engberg J, Friis-Moller A, Porsbo LJ, et al. Active ulcerative colitis associated with low prevalence of Blastocystis and Dientamoeba fragilis infection. Scandinavian journal of gastroenterology. 2013;48(5):638–9. Epub 2013/03/27. doi: 10.3109/00365521.2013.780094 . [DOI] [PubMed] [Google Scholar]
- 12.Stensvold CR, Clark CG. Current status of Blastocystis: A personal view. Parasitology international. 2016;65(6 Pt B):763–71. doi: 10.1016/j.parint.2016.05.015 . [DOI] [PubMed] [Google Scholar]
- 13.Elliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Annals of the New York Academy of Sciences. 2012;1247:83–96. Epub 2012/01/14. doi: 10.1111/j.1749-6632.2011.06292.x ; PubMed Central PMCID: PMC3744090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berrilli F, Di Cave D, Cavallero S, D'Amelio S. Interactions between parasites and microbial communities in the human gut. Frontiers in cellular and infection microbiology. 2012;2:141 Epub 2012/11/20. doi: 10.3389/fcimb.2012.00141 ; PubMed Central PMCID: PMC3499702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazdanbakhsh M, van den Biggelaar A, Maizels RM. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001;22(7):372–7. . [DOI] [PubMed] [Google Scholar]
- 16.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nature reviews Gastroenterology & hepatology. 2012;9(10):599–608. Epub 2012/08/22. doi: 10.1038/nrgastro.2012.152 . [DOI] [PubMed] [Google Scholar]
- 17.Zaiss MM, Harris NL. Interactions between the intestinal microbiome and helminth parasites. Parasite immunology. 2016;38(1):5–11. doi: 10.1111/pim.12274 ; PubMed Central PMCID: PMCPMC5019230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess SL, Gilchrist CA, Lynn TC, Petri WA Jr. Parasitic protozoa and interactions with the host intestinal microbiota. Infection and immunity. 2017. doi: 10.1128/IAI.00101-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dos Santos BS, da Silva LC, da Silva TD, Rodrigues JF, Grisotto MA, Correia MT, et al. Application of Omics Technologies for Evaluation of Antibacterial Mechanisms of Action of Plant-Derived Products. Frontiers in microbiology. 2016;7:1466 doi: 10.3389/fmicb.2016.01466 ; PubMed Central PMCID: PMCPMC5037136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szymanska E, Saccenti E, Smilde AK, Westerhuis JA. Double-check: validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics. 2012;8(Suppl 1):3–16. Epub 2012/05/18. doi: 10.1007/s11306-011-0330-3 ; PubMed Central PMCID: PMCPMC3337399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguiar-Pulido V, Huang W, Suarez-Ulloa V, Cickovski T, Mathee K, Narasimhan G. Metagenomics, Metatranscriptomics, and Metabolomics Approaches for Microbiome Analysis. Evol Bioinform Online. 2016;12(Suppl 1):5–16. doi: 10.4137/EBO.S36436 ; PubMed Central PMCID: PMCPMC4869604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong W, Abraham PE, Li Z, Pan C, Hettich RL. Microbial metaproteomics for characterizing the range of metabolic functions and activities of human gut microbiota. Proteomics. 2015;15(20):3424–38. doi: 10.1002/pmic.201400571 ; PubMed Central PMCID: PMCPMC4607593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preidis GA, Hotez PJ. The newest "omics"—metagenomics and metabolomics—enter the battle against the neglected tropical diseases. PLoS Negl Trop Dis. 2015;9(2):e0003382 Epub 2015/02/13. doi: 10.1371/journal.pntd.0003382 ; PubMed Central PMCID: PMC4326130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson MW, Menon R, Donnelly SM, Dalton JP, Ranganathan S. An integrated transcriptomics and proteomics analysis of the secretome of the helminth pathogen Fasciola hepatica: proteins associated with invasion and infection of the mammalian host. Molecular & cellular proteomics: MCP. 2009;8(8):1891–907. doi: 10.1074/mcp.M900045-MCP200 ; PubMed Central PMCID: PMCPMC2722771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, Cui SJ, Hu W, Feng Z, Wang ZQ, Han ZG. Excretory/secretory proteome of the adult developmental stage of human blood fluke, Schistosoma japonicum. Molecular & cellular proteomics: MCP. 2009;8(6):1236–51. Epub 2009/03/21. doi: 10.1074/mcp.M800538-MCP200 ; PubMed Central PMCID: PMC2690496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tebeje BM, Harvie M, You H, Loukas A, McManus DP. Schistosomiasis vaccines: where do we stand? Parasites & vectors. 2016;9(1):528 doi: 10.1186/s13071-016-1799-4 ; PubMed Central PMCID: PMCPMC5045607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulvenna J, Sripa B, Brindley PJ, Gorman J, Jones MK, Colgrave ML, et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10(5):1063–78. doi: 10.1002/pmic.200900393 ; PubMed Central PMCID: PMCPMC3038172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaiyadet S, Smout M, Laha T, Sripa B, Loukas A, Sotillo J. Proteomic characterization of the internalization of Opisthorchis viverrini excretory/secretory products in human cells. Parasitology international. 2016. doi: 10.1016/j.parint.2016.02.001 ; PubMed Central PMCID: PMCPMC5149449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rucksaken R, Haonon O, Pinlaor P, Pairojkul C, Roytrakul S, Yongvanit P, et al. Plasma IgG autoantibody against actin-related protein 3 in liver fluke Opisthorchis viverrini infection. Parasite immunology. 2015;37(7):340–8. doi: 10.1111/pim.12188 . [DOI] [PubMed] [Google Scholar]
- 30.Haonon O, Rucksaken R, Pinlaor P, Pairojkul C, Chamgramol Y, Intuyod K, et al. Upregulation of 14-3-3 eta in chronic liver fluke infection is a potential diagnostic marker of cholangiocarcinoma. Proteomics Clin Appl. 2016;10(3):248–56. doi: 10.1002/prca.201500019 . [DOI] [PubMed] [Google Scholar]
- 31.Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17(6):333–51. doi: 10.1038/nrg.2016.49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Chierico F, Ancora M, Marcacci M, Camma C, Putignani L, Conti S. Choice of next-generation sequencing pipelines. Methods Mol Biol. 2015;1231:31–47. doi: 10.1007/978-1-4939-1720-4_3 . [DOI] [PubMed] [Google Scholar]
- 33.Andersen LO, Bonde I, Nielsen HB, Stensvold CR. A retrospective metagenomics approach to studying Blastocystis. FEMS microbiology ecology. 2015;91(7). Epub 2015/07/02. doi: 10.1093/femsec/fiv072 . [DOI] [PubMed] [Google Scholar]
- 34.Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflammatory bowel diseases. 2007;13(6):675–83. Epub 2007/01/31. doi: 10.1002/ibd.20101 . [DOI] [PubMed] [Google Scholar]
- 35.Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, et al. Gut microbiota profiling of pediatric NAFLD and obese patients unveiled by an integrated meta-omics based approach. Hepatology. 2016. doi: 10.1002/hep.28572 . [DOI] [PubMed] [Google Scholar]
- 36.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. doi: 10.1038/nature12506 . [DOI] [PubMed] [Google Scholar]
- 37.Audebert C, Even G, Cian A, The Blastocystis Investigation Group, Loywick A, Merlin S, et al. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci Rep. 2016;6:25255 doi: 10.1038/srep25255 ; PubMed Central PMCID: PMCPMC4857090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Croese J, Gaze ST, Loukas A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. International journal for parasitology. 2013;43(3–4):275–82. Epub 2013/01/08. doi: 10.1016/j.ijpara.2012.12.005 . [DOI] [PubMed] [Google Scholar]
- 39.Loukas A, Hotez PJ, Diemert D, Yazdanbakhsh M, McCarthy JS, Correa-Oliveira R, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:16088 doi: 10.1038/nrdp.2016.88 . [DOI] [PubMed] [Google Scholar]
- 40.Giacomin P, Zakrzewski M, Croese J, Su X, Sotillo J, McCann L, et al. Experimental hookworm infection and escalating gluten challenges are associated with increased microbial richness in celiac subjects. Sci Rep. 2015;5:13797 doi: 10.1038/srep13797 ; PubMed Central PMCID: PMCPMC4585380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cantacessi C, Giacomin P, Croese J, Zakrzewski M, Sotillo J, McCann L, et al. Impact of experimental hookworm infection on the human gut microbiota. The Journal of infectious diseases. 2014;210(9):1431–4. Epub 2014/05/06. doi: 10.1093/infdis/jiu256 ; PubMed Central PMCID: PMC4195438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflammatory bowel diseases. 2010;16(11):1841–9. Epub 2010/09/18. doi: 10.1002/ibd.21299 ; PubMed Central PMCID: PMC2959136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li RW, Wu S, Li W, Navarro K, Couch RD, Hill D, et al. Alterations in the porcine colon microbiota induced by the gastrointestinal nematode Trichuris suis. Infection and immunity. 2012;80(6):2150–7. Epub 2012/04/12. doi: 10.1128/IAI.00141-12 ; PubMed Central PMCID: PMC3370577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Putignani L, Menichella D. Global distribution, public health and clinical impact of the protozoan pathogen cryptosporidium. Interdiscip Perspect Infect Dis. 2010;2010 Epub 2010/08/14. doi: 10.1155/2010/753512 ; PubMed Central PMCID: PMCPMC2913630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SC, Tang MS, Lim YA, Choy SH, Kurtz ZD, Cox LM, et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl Trop Dis. 2014;8(5):e2880 Epub 2014/05/24. doi: 10.1371/journal.pntd.0002880 ; PubMed Central PMCID: PMC4031128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, et al. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog. 2012;8(11):e1003000 Epub 2012/11/21. doi: 10.1371/journal.ppat.1003000 ; PubMed Central PMCID: PMC3499566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harb OS, Roos DS. The Eukaryotic Pathogen Databases: a functional genomic resource integrating data from human and veterinary parasites. Methods Mol Biol. 2015;1201:1–18. doi: 10.1007/978-1-4939-1438-8_1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz EM, Hu Y, Antoshechkin I, Miller MM, Sternberg PW, Aroian RV. The genome and transcriptome of the zoonotic hookworm Ancylostoma ceylanicum identify infection-specific gene families. Nat Genet. 2015;47(4):416–22. doi: 10.1038/ng.3237 ; PubMed Central PMCID: PMCPMC4617383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang YT, Gao X, Rosa BA, Abubucker S, Hallsworth-Pepin K, Martin J, et al. Genome of the human hookworm Necator americanus. Nat Genet. 2014;46(3):261–9. doi: 10.1038/ng.2875 ; PubMed Central PMCID: PMCPMC3978129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hino A, Maruyama H, Kikuchi T. A novel method to assess the biodiversity of parasites using 18S rDNA Illumina sequencing; parasitome analysis method. Parasitology international. 2016;65(5 Pt B):572–5. Epub 2016/01/20. doi: 10.1016/j.parint.2016.01.009 . [DOI] [PubMed] [Google Scholar]
- 51.Tanaka R, Hino A, Tsai IJ, Palomares-Rius JE, Yoshida A, Ogura Y, et al. Assessment of helminth biodiversity in wild rats using 18S rDNA based metagenomics. PLoS ONE. 2014;9(10):e110769 doi: 10.1371/journal.pone.0110769 ; PubMed Central PMCID: PMCPMC4207705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao F, Wang R, Liu M. Trichinella spiralis, potential model nematode for epigenetics and its implication in metazoan parasitism. Front Physiol. 2014;4:410 doi: 10.3389/fphys.2013.00410 ; PubMed Central PMCID: PMCPMC3887316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chauhan A, Quenum FZ, Abbas A, Bradley DS, Nechaev S, Singh BB, et al. Epigenetic Modulation of Microglial Inflammatory Gene Loci in Helminth-Induced Immune Suppression: Implications for Immune Regulation in Neurocysticercosis. ASN Neuro. 2015;7(4). doi: 10.1177/1759091415592126 ; PubMed Central PMCID: PMCPMC4552224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484 ; PubMed Central PMCID: PMCPMC2949280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422(6928):198–207. doi: 10.1038/nature01511 . [DOI] [PubMed] [Google Scholar]
- 56.Wastling JM, Xia D, Sohal A, Chaussepied M, Pain A, Langsley G. Proteomes and transcriptomes of the Apicomplexa—where's the message? International journal for parasitology. 2009;39(2):135–43. Epub 2008/11/11. doi: 10.1016/j.ijpara.2008.10.003 . [DOI] [PubMed] [Google Scholar]
- 57.Aurrecoechea C, Heiges M, Wang H, Wang Z, Fischer S, Rhodes P, et al. ApiDB: integrated resources for the apicomplexan bioinformatics resource center. Nucleic acids research. 2007;35(Database issue):D427–30. Epub 2006/11/14. doi: 10.1093/nar/gkl880 ; PubMed Central PMCID: PMC1669770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Putignani L, Sanderson SJ, Russo C, Kissinger J, Menichella D, Wastiling JM. Proteomic and genomic approaches to understanding the 'power plant' of Cryptosporidium 2009. In: Giardia and Cryptosporidium: From Molecules to Diseases [Internet]. CABI Publishing; [344–59]. [Google Scholar]
- 59.Sanderson SJ, Xia D, Prieto H, Yates J, Heiges M, Kissinger JC, et al. Determining the protein repertoire of Cryptosporidium parvum sporozoites. Proteomics. 2008;8(7):1398–414. Epub 2008/02/29. doi: 10.1002/pmic.200700804 ; PubMed Central PMCID: PMC2770187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snelling WJ, Lin Q, Moore JE, Millar BC, Tosini F, Pozio E, et al. Proteomics analysis and protein expression during sporozoite excystation of Cryptosporidium parvum (Coccidia, Apicomplexa). Molecular & cellular proteomics: MCP. 2007;6(2):346–55. Epub 2006/11/25. doi: 10.1074/mcp.M600372-MCP200 . [DOI] [PubMed] [Google Scholar]
- 61.Wampfler PB, Tosevski V, Nanni P, Spycher C, Hehl AB. Proteomics of secretory and endocytic organelles in Giardia lamblia. PLoS ONE. 2014;9(4):e94089 Epub 2014/04/16. doi: 10.1371/journal.pone.0094089 ; PubMed Central PMCID: PMC3986054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brosson D, Kuhn L, Delbac F, Garin J, C PV, Texier C. Proteomic analysis of the eukaryotic parasite Encephalitozoon cuniculi (microsporidia): a reference map for proteins expressed in late sporogonial stages. Proteomics. 2006;6(12):3625–35. Epub 2006/05/13. doi: 10.1002/pmic.200500796 . [DOI] [PubMed] [Google Scholar]
- 63.Santivañez SJ, Hernandez-Gonzalez A, Chile N, Oleaga A, Arana Y, Palma S, et al. Proteomic study of activated Taenia solium oncospheres. Molecular and biochemical parasitology. 2010;171(1):32–9. doi: 10.1016/j.molbiopara.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcilla A, Sotillo J, Perez-Garcia A, Igual-Adell R, Valero ML, Sanchez-Pino MM, et al. Proteomic analysis of Strongyloides stercoralis L3 larvae. Parasitology. 2010;137(10):1577–83. Epub 2010/04/15. doi: 10.1017/S0031182010000314 . [DOI] [PubMed] [Google Scholar]
- 65.Marcilla A, Garg G, Bernal D, Ranganathan S, Forment J, Ortiz J, et al. The transcriptome analysis of Strongyloides stercoralis L3i larvae reveals targets for intervention in a neglected disease. PLoS Negl Trop Dis. 2012;6(2):e1513 Epub 2012/03/06. doi: 10.1371/journal.pntd.0001513 ; PubMed Central PMCID: PMC3289599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sturbaum GD, Jost BH, Sterling CR. Nucleotide changes within three Cryptosporidium parvum surface protein encoding genes differentiate genotype I from genotype II isolates. Molecular and biochemical parasitology. 2003;128(1):87–90. Epub 2003/04/23. . [DOI] [PubMed] [Google Scholar]
- 67.Biller L, Matthiesen J, Kuhne V, Lotter H, Handal G, Nozaki T, et al. The cell surface proteome of Entamoeba histolytica. Molecular & cellular proteomics: MCP. 2014;13(1):132–44. Epub 2013/10/19. doi: 10.1074/mcp.M113.031393 ; PubMed Central PMCID: PMC3879609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de la Cruz OH, Muniz-Lino M, Guillen N, Weber C, Marchat LA, Lopez-Rosas I, et al. Proteomic profiling reveals that EhPC4 transcription factor induces cell migration through up-regulation of the 16-kDa actin-binding protein EhABP16 in Entamoeba histolytica. Journal of proteomics. 2014;111:46–58. Epub 2014/04/12. doi: 10.1016/j.jprot.2014.03.041 . [DOI] [PubMed] [Google Scholar]
- 69.Leitsch D, Wilson IB, Paschinger K, Duchene M. Comparison of the proteome profiles of Entamoeba histolytica and its close but non-pathogenic relative Entamoeba dispar. Wiener klinische Wochenschrift. 2006;118(19–20 Suppl 3):37–41. Epub 2006/11/30. doi: 10.1007/s00508-006-0675-1 . [DOI] [PubMed] [Google Scholar]
- 70.Davis PH, Zhang X, Guo J, Townsend RR, Stanley SL Jr. Comparative proteomic analysis of two Entamoeba histolytica strains with different virulence phenotypes identifies peroxiredoxin as an important component of amoebic virulence. Molecular microbiology. 2006;61(6):1523–32. Epub 2006/09/14. doi: 10.1111/j.1365-2958.2006.05344.x . [DOI] [PubMed] [Google Scholar]
- 71.Que X, Reed SL. Cysteine proteinases and the pathogenesis of amebiasis. Clinical microbiology reviews. 2000;13(2):196–206. Epub 2001/02/07. ; PubMed Central PMCID: PMC100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wawrzyniak I, Texier C, Poirier P, Viscogliosi E, Tan KS, Delbac F, et al. Characterization of two cysteine proteases secreted by Blastocystis ST7, a human intestinal parasite. Parasitology international. 2012;61(3):437–42. Epub 2012/03/10. doi: 10.1016/j.parint.2012.02.007 . [DOI] [PubMed] [Google Scholar]
- 73.Victor B, Kanobana K, Gabriel S, Polman K, Deckers N, Dorny P, et al. Proteomic analysis of Taenia solium metacestode excretion-secretion proteins. Proteomics. 2012;12(11):1860–9. Epub 2012/05/25. doi: 10.1002/pmic.201100496 . [DOI] [PubMed] [Google Scholar]
- 74.Mulvenna J, Hamilton B, Nagaraj SH, Smyth D, Loukas A, Gorman JJ. Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum. Molecular & cellular proteomics: MCP. 2009;8(1):109–21. doi: 10.1074/mcp.M800206-MCP200 . [DOI] [PubMed] [Google Scholar]
- 75.Navarro S, Pickering DA, Ferreira IB, Jones L, Ryan S, Troy S, et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med. 2016;8(362):362ra143 doi: 10.1126/scitranslmed.aaf8807 . [DOI] [PubMed] [Google Scholar]
- 76.Jiang HY, Zhao N, Zhang QL, Gao JM, Liu LL, Wu TF, et al. Intestinal microbes influence the survival, reproduction and protein profile of Trichinella spiralis in vitro. International journal for parasitology. 2016;46(1):51–8. doi: 10.1016/j.ijpara.2015.08.007 . [DOI] [PubMed] [Google Scholar]
- 77.Santos LN, Gallo MB, Silva ES, Figueiredo CA, Cooper PJ, Barreto ML, et al. A proteomic approach to identify proteins from Trichuris trichiura extract with immunomodulatory effects. Parasite immunology. 2013;35(5–6):188–93. Epub 2013/02/13. doi: 10.1111/pim.12025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santos LN, Silva ES, Santos AS, De Sa PH, Ramos RT, Silva A, et al. De novo assembly and characterization of the Trichuris trichiura adult worm transcriptome using Ion Torrent sequencing. Acta Trop. 2016;159:132–41. doi: 10.1016/j.actatropica.2016.03.036 . [DOI] [PubMed] [Google Scholar]
- 79.Foth BJ, Tsai IJ, Reid AJ, Bancroft AJ, Nichol S, Tracey A, et al. Whipworm genome and dual-species transcriptome analyses provide molecular insights into an intimate host-parasite interaction. Nat Genet. 2014;46(7):693–700. doi: 10.1038/ng.3010 ; PubMed Central PMCID: PMCPMC5012510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jean Beltran PM, Federspiel JD, Sheng X, Cristea IM. Proteomics and integrative omic approaches for understanding host-pathogen interactions and infectious diseases. Mol Syst Biol. 2017;13(3):922 doi: 10.15252/msb.20167062 ; PubMed Central PMCID: PMCPMC5371729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez-Diaz E, Jorda M, Peinado MA, Rivero A. Epigenetics of host-pathogen interactions: the road ahead and the road behind. PLoS Pathog. 2012;8(11):e1003007 doi: 10.1371/journal.ppat.1003007 ; PubMed Central PMCID: PMCPMC3510240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vernocchi P, Del Chierico F, Putignani L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Frontiers in microbiology. 2016;7:1144 doi: 10.3389/fmicb.2016.01144 ; PubMed Central PMCID: PMCPMC4960240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nature chemical biology. 2014;10(6):416–24. Epub 2014/05/20. doi: 10.1038/nchembio.1535 . [DOI] [PubMed] [Google Scholar]
- 84.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nature immunology. 2013;14(7):676–84. Epub 2013/06/20. doi: 10.1038/ni.2640 ; PubMed Central PMCID: PMC4013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535(7610):94–103. doi: 10.1038/nature18850 . [DOI] [PubMed] [Google Scholar]
- 86.Ng JS, Ryan U, Trengove RD, Maker GL. Development of an untargeted metabolomics method for the analysis of human faecal samples using Cryptosporidium-infected samples. Molecular and biochemical parasitology. 2012;185(2):145–50. Epub 2012/09/05. doi: 10.1016/j.molbiopara.2012.08.006 . [DOI] [PubMed] [Google Scholar]
- 87.Ng Hublin JS, Ryan U, Trengove R, Maker G. Metabolomic profiling of faecal extracts from Cryptosporidium parvum infection in experimental mouse models. PLoS ONE. 2013;8(10):e77803 Epub 2013/11/10. doi: 10.1371/journal.pone.0077803 ; PubMed Central PMCID: PMC3800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bond A, Vernon A, Reade S, Mayor A, Minetti C, Wastling J, et al. Investigation of Volatile Organic Compounds Emitted from Faeces for the Diagnosis of Giardiasis. J Gastrointestin Liver Dis. 2015;24(3):281–6. doi: 10.15403/jgld.2014.1121.243.abo . [DOI] [PubMed] [Google Scholar]
- 89.Jeelani G, Sato D, Husain A, Escueta-de Cadiz A, Sugimoto M, Soga T, et al. Metabolic profiling of the protozoan parasite Entamoeba invadens revealed activation of unpredicted pathway during encystation. PLoS ONE. 2012;7(5):e37740 Epub 2012/06/05. doi: 10.1371/journal.pone.0037740 ; PubMed Central PMCID: PMC3360610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Xiao SH, Xue J, Singer BH, Utzinger J, Holmes E. Systems metabolic effects of a Necator americanus infection in Syrian hamster. Journal of proteome research. 2009;8(12):5442–50. Epub 2009/10/09. doi: 10.1021/pr900711j . [DOI] [PubMed] [Google Scholar]
- 91.Wu JF, Holmes E, Xue J, Xiao SH, Singer BH, Tang HR, et al. Metabolic alterations in the hamster co-infected with Schistosoma japonicum and Necator americanus. International journal for parasitology. 2010;40(6):695–703. Epub 2009/12/03. doi: 10.1016/j.ijpara.2009.11.003 . [DOI] [PubMed] [Google Scholar]