Abstract
The main aim of the research was to design a functional impedimetric biosensor able to glycoprofile prostate specific antigen (PSA), a biomarker for prostate cancer (PCa), with high specificity using lectins as glycan recognising proteins. Traditionally, full-length antibody is immobilised on the biosensor interface for specific capture of PSA with subsequent glycoprofiling of PSA by addition of lectins. Since full-length antibodies contain glycans in the Fc domain, particular attention has to be paid to suppress direct binding of lectins to immobilised full-length antibodies, which would compromise accurate glycoprofiling. This issue is addressed here using a recombinant single-chain antibody fragments (scAb), which do not contain any carbohydrate moiety. Surface plasmon resonance was applied to prove negligible interaction of lectins with immobilised scAb fragments, while substantial binding of lectins to full length antibodies was observed. Eight different biosensor designs were tested for their ability to detect PSA. The biosensor device based on scAb fragments covalently immobilised on the gold electrode surface, patterned by a mixed SAM using standard amine coupling chemistry, proved to be the most sensitive. The scAb fragment-based biosensor exhibited sensitivity of 15.9 ± 0.8% decade-1 (R2 = 0.991 with an average RSD of 4.9%), while the full antibody-based biosensor offered sensitivity towards PSA of 4.2 ± 0.1% decade-1 (R2 = 0.999 with an average RSD of 4.8%). Moreover, the selectivity of the scAb-based biosensor was tested using a kallikrein 2 protein, a protein structurally similar to PSA, and the results indicated high selectivity for PSA detection.
Keywords: impedimetric biosensors, full-length antibodies, single-chain antibody fragments (scAb), lectins, glycan analysis, prostate cancer
1. Introduction
Glycans, which consist of chains of carbohydrates, are present on many biological molecules, such as proteins or lipids, but are also found on the surface of cells [1]. More than 160 different biological roles for glycans have been identified, e.g. cell signalling, immune responses and cell adhesion [2]. Glycans also play a major role in development and progress of many diseases including autoimmune disorders and cancer. Aberrant glycosylation is a fundamental characteristic of malignant transformation and tumour development [3]. For this reason, glycomics is receiving great attention in relation to the diagnosis of various diseases including prostate cancer (PCa). Current diagnostic tests for PCa involve digital rectal examination and determination of the concentration of PSA in blood. PSA is a glycoprotein with a protease activity responsible for liquefying seminal fluid. The majority of healthy men have a PSA concentration of about 0.2– 5 mg/mL in seminal fluid while it is below 4 ng/mL in blood [4]. Elevation of PSA levels in PCa is a consequence of disruption of prostate tissue and release of PSA into the blood stream [5].
The most frequent clinical method for detection of PSA is ELISA (Enzyme-linked immunosorbent assays) based on optical reading in a microplate format [6], but there are other bioanalytical methods available for detection of PSA using a diverse range of transducers [7–9] and nanoparticles [1,10]. However, PSA testing has significant drawbacks. More than 20% of men with a PSA below 4 ng/ml were diagnosed with prostate cancer after a biopsy [11]. Moreover, elevated levels of PSA are not PCa-specific, but may be due to benign prostate hyperplasia (enlargement), prostatitis, urinary tract infection or even prostate massage or exercise [12,13]. To circumvent this issue, more specific biomarkers are required. These include prostate stem cell antigen, prostate specific membrane antigen, haptoglobin [14], circulating tumour cells [15], hormones (insulin-like growth factor) [16], gene alterations and gene fusions [17]. In addition, various statistic methods combining results of known analytical approaches (Prostate Health Index, free PSA to total PSA ratio) can provide more accurate diagnosis [6,18]. The study of glycosylation changes has significant potential for PCa diagnostics. The most abundant glycosylation alterations observed were increased branching and fucosylation, especially α-1,2-fucose linked to galactose [5], a significant decrease in α-2,6 sialylation and an increase in terminal α-2,3 sialylated glycans [19]. Precise and specific recognition approaches are required to detect these minute alterations. One of the most reliable methods is mass spectroscopy analysis [20], however, for personalized medical strategies and for point-of-care devices, biosensors and various biochip-based assays are emerging, where antibody-based recognition plays a crucial role.
It is feasible to glycoprofile PSA using biochips/biosensors with immobilised antibodies for a selective capture of PSA from a complex matrix such as blood serum, followed by a final incubation with a lectin in a sandwich configuration. Unfortunately, immunosensors, combined with lectins as a glycoprofiling tool, may provide false positive responses, as a result of interaction of lectins directly with glycans present on the Fc domain of an immobilised full-length antibody [21,22]. For example, Chen et al. addressed this issue by a chemical derivatisation of glycans, which were firstly oxidised by NaIO4 and then blocked by dipeptide [23]. This method, however, required three additional steps in array preparation, was time and labour-intensive and may affect antibody binding.
In this work we investigated an entirely different approach from the protocol developed by Chen et al. [23] i.e. by replacing full-length antibodies with recombinant antibody fragments such as scAb, which do not carry a carbohydrate moiety. Recombinant antibody fragments (scFv - single-chain fragment variable, Fab - fragment antigen binding, scAb - single-chain antibody fragment etc.) may be generated with high reproducibility/reliability, and can be tailored for sensitivity, specificity and stability [24]. In this work SPR was used to demonstrate low background binding of lectins to immobilised scAb fragments, optimisation of the biosensor construction based on scAb fragments and proof-of- concept for glycoprofiling of PSA by the lectin Sambucus nigra agglutinin (SNA).
2. Experimental section
2.1. Material and Methods
Purified free prostate specific antigen (PSA) isolated from human seminal fluid was purchased from Fitzgerald Industries International (USA), anti-PSA mouse monoclonal antibody Ab10185 (specific for total PSA, no cross reactivity for human kallikrein 2) was purchased from Abcam (UK), human kallikrein 2 (KLK2) was purchased from R&D Systems, Inc. (USA), Sambucus nigra agglutinin (SNA, recognising Neu5Acα6 Gal/GalNAc), Phaseolus vulgaris erythroagglutinin (PHA-E, recognising Galβ4GlcNAcβ2Manα6), wheat germ agglutinin (WGA, recognising GlcNAc) and carbo-free blocking solution (CFB) were purchased from Vector laboratories (USA). In designating the glycan preference of the lectins the following abbreviations were applied: Neu5Ac - N-acetylneuraminic acid (sialic acid), Gal - galactose, GalNAc - N-acetylgalactosamine, GlcNAc - N-acetylglucosamine and Man - mannose. A 2-(2-pyridinyldithio) ethaneamine hydrochloride (PDEA) thiol coupling reagent was purchased from GE Healthcare Life Sciences (USA), 2-mercaptoethanol (ME), 6- mercaptohexanol (MH), 11-mercaptoundecanoic acid (MUA), cysteamine, ethanolamine hydrochloride, gelatine from porcine skin (type A), hydrogen peroxide (30% w/w), N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N´-ethylcarbodiimide hydrochloride (EDC), phosphate buffered saline tablet (PBS, one tablet dissolved in 200 mL of deionized water yields 0.01 M phosphate buffer, with 0.0027 M potassium chloride and 0.137 M sodium chloride, pH 7.4, at 25 °C.), potassium ferricyanide (III), potassium ferrocyanide(II) trihydrate, sodium hydroxide, sulphuric acid, Tween 20, and all general chemicals were purchased from Sigma-Aldrich (USA). All solutions were prepared in deionized water (DW) and were filtered prior to use through 0.2 μm sterile filters. Protein solutions were prepared in PBS (10 mM, pH 7.4), unless otherwise stated.
2.2. SPR measurements
Interaction between lectins and full length antibodies or scAb fragments was monitored by a surface plasmon resonance (SPR) dual channel Reichert SR7000DC SPR system, thermostated at 23 °C, operated using SPR Autolink System software (AMETEK, Reichert Technologies, USA). The flow cell was equipped with a three-dimensional carboxymethyldextran hydrogel SPR Sen-sorchip CMD50 m (Xantec bioanalytics, Germany), which was pre-treated by sodium acetate buffer (AcB), pH 5.0, prior to amine coupling, to enhance the immobilisation efficiency. The optimal pH for immobilisation was determined prior to the immobilisation itself (data not shown). The CMD50 m sensorchip was functionalised using the amine coupling protocol recommended by the manufacturer i.e. by covalent attachment of proteins via -NH2 towards activated -COOH groups. Initially, both the working and the reference channel were activated by a 1:1 mixture of 0.2 M EDC and 0.05 M NHS at a flow rate of 20 mL/min for 420 s. After the activation, 20 ug/mL of Ab10185/scAb in 10 mM sodium AcB pH 5.0 was injected into the working channel for 10 min at a flow rate of 20 mL/min. Ethanolamine hydrochloride (1 M, pH 8.5) was then injected into both channels for 10 min to deactivate active carboxylic groups and, finally, 20 mM HCl was injected into the system. SNA, PHA-E and WGA lectins, diluted in a running buffer (PBST) to a concentration of 25 mg/mL, were sequentially injected at a flow rate of 50 mL/min. The injection time was 210 s and the dissociation time was 420 s. Regeneration was achieved with 20 mM NaOH injected for 60 s. The response of the system is represented by arbitrary refractive index units (mRIU, 1 mRIU = 1 pg /mm2 according to the manufacturer) and the monitored interaction is assessed by subtracting the reference response from the signal obtained in the analytical channel. Data obtained were processed by the Scrubber software (BioLogic Software Pty, Australia).
2.3. Electrode pre-treatment
For the electrochemical measurements, polycrystalline gold disc electrodes (1.6 mm diameter, BASi, USA) were cleaned according to the protocol developed by Tkac et al. [25]. Briefly, electrodes were cleaned mechanically, chemically and electro-chemically. Initially, any remaining thiols were removed from the gold surface by electrochemical reductive desorption, under a N2 atmosphere, using cyclic voltammetry (CV) in 100 mM NaOH (100 scans, potential range from -500 mV to -1,500 mV, a scan rate of 100 mV/s) under stirring to dissolve thiol molecules and to prevent subsequent re-adsorption of thiols on the electrode surface. The next step was a mechanical polishing of the electrodes on the polishing pad for 5 min using 1.0 mm alumina slurry, for 5 min using 0.3 mm alumina slurry and for 2 min on a clean pad (all purchased from Buehler, USA). The electrodes were afterwards thoroughly rinsed with DW and ultrasonically cleaned in DW to remove any remaining alumina particles. The cleaned electrodes were then immersed into a freshly prepared piranha solution (mixture of H2O2 and H2SO4 at a volume ratio 1:3, handle with a special care since it is a strongly oxidising agent) for 10 min to remove any organic residual molecules from the electrode surface, rinsed with DW and sonicated for 5 min. This was followed by an electrochemical polishing in 100 mM H2SO4 (50 scans, potential range from -200 mV to +1,500 mV, a scan rate of 100 mV/s). The procedure will electrochemically polish the electrode by lowering the electrode surface roughness, but at the same time delivers a gold oxide layer. In the next step, gold oxide was stripped from the electrode surface by the reduction process in the same solution of H2SO4 (20 scans, potential range from +750 mV to +200 mV, a scan rate of 100 mV/s). Finally, the electrodes were rinsed with DW and ultra-pure ethanol, dried in a N2 atmosphere and were immediately used for the preparation of the biosensor with formation of a SAM layer as the first step.
2.4. Biosensor preparation
In general, two types of biosensor surfaces were prepared - a biosensor with an antibody fragment directly adsorbed on the gold surface and a sensor with an antibody fragment immobilised on alkanethiol-containing self-assembled monolayers (SAMs). A novel single-chain antibody fragment (scAb) specific to free PSA acquired from Dublin City University was produced by addition of the chicken constant domain (Cl) to a single-chain fragment variable (scFv) clone B8 as described by Zapatero-Rodríguez et al. [26]. For direct adsorption of an antibody fragment on gold via chemisorption (biosensors type A and B, see Fig. 4), a C-terminal hinge cysteine (Cys) with free sulphydryl group was added to the antibody constant domain and such an antibody fragment is referred to as HS-scAb. The cleaned gold electrode was incubated with 20 ng/mL solution of HS-scAb in PBS for 20 min and was backfilled for 20 min with 10 mM solution of MH or 10 mM solution of ME, respectively. Alkanethiol solutions for backfilling were prepared in PBS instead of ethanol and were vortexed for 3 min prior to use. Alternatively, the biosensor surface prepared by direct adsorption of 20 ng/mL HS-scAb for 20 min was subsequently blocked by 1 x CFB. The biosensor prepared by co-immobilisation of HS-scAb and MH was prepared by an incubation of a mixture of HS-scAb and MH with a final concentration of 20 ng/mL scAb and 10 mM MH with a gold surface for 20 min. All biosensor configurations stated above were performed by application of 40 μL solutions.
Fig. 4.
Schematic of various biosensor surface types tested. (A) HS-scAb fragments chemisorbed on a gold surface followed by blocking with a carbo-free buffer (CFB), (B) HS-scAb fragments chemisorbed on a gold surface followed by blocking with a thiol (backfilling) or HS-scAb fragments chemisorption in presence of a thiol (co-immobilisation), (C) immobilisation of HS-scAb fragments on a SAM via a disulphide exchange reaction and (D) covalent immobilisation of scAb fragments on a SAM via standard amine coupling with final blocking of the surface with ethanolamine.
In the third approach (using SAMs, biosensor type D, see Fig. 4), the cleaned electrode was immersed into an ethanolic solution of 1 mM MUA and 1 mM MH mixed in a volume ratio of 1:3 or 1:1, respectively. Incubation was conducted overnight at room temperature, in the dark. After incubation, the electrodes were rinsed with ethanol and DW and carboxylic groups of MUA were activated for 15 min with a 1:1 mixture of aqueous solutions of 0.2 M EDC and 0.05 M NHS. The scAb fragment (20 ng/mL in 40 μL) was immobilised on activated SAM during 30 min, rinsed with PBS and then the surface was blocked for 20 min with 40 μL of 0.1% (w/ v) gelatine or 1 M ethanolamine.
The HS-ScAb was also tested for immobilisation in an oriented fashion using a thiol coupling protocol (biosensor type C, Fig. 4) rather than amine coupling. After SAM activation with EDC/NHS, this surface was incubated with 40 mL of 40 mM PDEA in 0.1 M borate, pH 8.5, for 15 min. After rinsing with PBS, HS-scAb (40 μL of a 20 ng/mL protein solution) was immobilised on the interface for 30 min and unreacted PDEA residues were subsequently blocked by cysteamine (40 μL, 50 mM in 1 M NaCl + 0.1 M sodium acetate pH 4.0, for 20 min). For PSA glycan profiling, the biosensor was incubated with 40 μL of 100 ng/mL of the PSA protein (if not specified otherwise) for 20 min and with subsequent addition of the SNA lectin (40 μL of 0.1 mg/mL SNA lectin, for 20 min).
2.5. Electrochemical measurements
Faradaic electrochemical impedance spectroscopy (EIS) was used to monitor an interaction between the biosensor surface and its analyte PSA, as well as between PSA and lectins. EIS measurements were performed on a laboratory potentiostat/galvanostat PGSTAT 128N, controlled by NOVA software 1.11 (both Metrohm Autolab, The Netherlands) at 50 different frequencies ranging from 0.1 Hz to 100 kHz, applying a 200 mV AC voltage. A standard three-electrode configuration was used, with an Ag/AgCl reference electrode (Gamry instruments, USA), platinum auxiliary electrode (BASi, USA) and gold working electrode applied for EIS assays. All measurements were performed in PBS (10 mM, pH 7.4) containing 5 mM ferri/ferrocyanide ([Fe(CN)6]3-/4-). Positive interaction was determined as an increase in a charge transfer resistance (Rct), represented by a semicircle diameter of a Nyquist plot. To extract impedance values from the complex plane plot, Randles equivalent circuit R(Q[RW]) was used for data fitting since the more complete circuit as provided by Davis et al. [27] did not provide better results. The biosensor response is shown as a relative change of the EIS signal as ΔRct in %. All measurements were performed at least in triplicate, on an independent electrode surface.
2.6. Atomic Force Microscopy (AFM) Measurements
AFM was used to visualise dimensions of the immobilised recombinant HS-scAb fragments. A bioscope Catalyst instrument operated by NanoScope 8.15 software (Bruker, USA) in conjunction with an Olympus IX71 microscope were used in a peak force tapping mode (ScanAsyst in air). A square shaped gold chip with dimensions of 10 x 10 mm was used for HS-scAb direct chemisorption and a silicon nitride ScanAsyst-air probe, with a tip radius of 2 nm (Bruker, USA) was employed for the determination of HS-scAb dimensions.
3. Results and discussion
3.1. SPR experiments
SPR experiments were applied to investigate binding of three different types of lectins to commercially available anti-PSA antibody (Ab) or a scAb fragment. Lectin PHA-E with a specificity towards Gal-b4GlcNAc exhibited quite a large response towards Ab with a response of 150 μRIU, while the response towards scAb fragment was 9 μRIU at the end of the association phase (Fig. 1). The SPR assays (run at least in triplicate) of interaction of three lectins (PHA-E, SNA and RCA) with either a covalently immobilised Ab or a scAb fragment revealed that lectins bound only to Ab with a response of 43.7 ± 1.4 μRIU for SNA lectin, a response of 115.6 ± 1.0 μRIU for PHA-E lectin and a response of 102.5 ±1.6 μRIU for WGA lectin with responses measured 120 s after induction of a dissociation phase by flowing a plain buffer through the SPR system. The SPR response after injection of three lectins to the immobilised scAb fragment was 11.5 ± 2.4 μRIU for SNA lectin, 5.7 ± 0.7 μRIU for PHA-E lectin and 13.0 ± 2.9 μRIU for WGA lectin, but such small response could be attributed to a non-specific protein binding to the matrix of the SPR chip (as shown in our previous paper [28]) rather that to the immobilised scAb fragment (Fig. 2).
Fig. 1.
SPR assay showing interaction of PHA-E lectin with either covalently immobilised Ab or scAb fragment. The SPR experiment was run on a carboxymethyldextran hydrogel SPR sensorchip at a flow rate of 50 mL/min while injecting the lectin at a concentration of 25 mg/mL.
Fig. 2.
SPR assays showing binding of three different lectins – SNA, PHA-E and WGA to either covalently immobilised Ab or scAb fragment. The measurements were run in triplicate with a signal response read 120 s after induction of a dissociation phase by flowing a plain buffer through the SPR system. For other conditions see Fig. 2. Measurements were performed in triplicate showing standard deviation of measurements.
3.2. AFM experiments
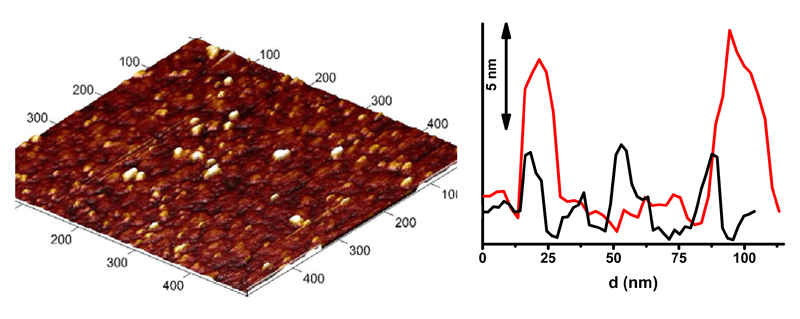
AFM experiments revealed that the HS-scAb fragment after immobilisation on the gold surface is present in two different orientations with features having either approx. 4 nm or approx. 8 nm in height (Fig. 3), in an excellent agreement with the size of scAb fragment of approx. 8 x 4 x 3.5 nm previously described [29]. The experiments suggest that preferentially the scAb fragments are “laying” on the gold surface in a more stable position compared to “standing” of the fragments on the gold surface (Fig. 3). Such observations are consistent with data obtained by others with antibodies immobilised/adsorbed on thiolated SAM [30], silica wafer [31] with flat-on orientation of the antibody, while some identified the height of antibodies of only 2.0-2.5 nm [32], what was explained by compression of the protein molecules. Further, neutron reflection study under aqueous solution provided thickness of an antibody layer of 4.0 nm [33]. Thus, we can conclude that a peak force tapping mode of AFM applied in this study does not compress immobilised antibody fragments.
Fig. 3.
AFM image of HS-scAb fragments adsorbed on a gold surface (left) and a topographical profile of HS-scAb fragments chemisorbed on a gold surface, showing that features with the height of the immobilised proteins is approx. 8 nm (red line), but preferentially the height of protein features on the surface is approx. 4 nm (black line) (right).
3.3. Electrochemical experiments
Several different biosensor configurations were prepared (Fig. 4). Direct chemisorption of the thiolated form of the scAb fragment (HS-scAb) on a gold surface with subsequent stabilisation in a CFB (containing proteins) was tested for biosensor type A. Subsequently, direct chemisorption of a HS-scAb on gold surface with the protein layer stabilised by present self-assembled monolayer (biosensor type B) was tested. Two different protocols were employed for construction of biosensor type B using either a thiol backfilling of the initial HS-scAb layer or simultaneous co-immobilisation of HS-scAb together with a thiol from a solution containing both these components.
The results indicated that the biosensor Au/HS-scAb/CFB (#1, Fig. 5) exhibited only a negligible response (ΔRct) with 100 ng/mL of PSA of 2.5 ± 0.8%. If the chemisorbed HS-scAb layer was backfilled with 6-mercaptohexanol (MH) the biosensor (#2, Fig. 5) exhibited a very similar low response with ΔRct = 1.2 ± 0.7% with 100 ng/mL of PSA. When HS-scAb was chemisorbed together with MH (co-immobilisation, #3, Fig. 5) practically the same low response as in the previous two cases of 2.1 ± 0.6% was observed. There are two possible reasons for such a negligible response of the above-mentioned biosensors: (1) denaturation of HS-scAb upon chemisorption on the gold surface and (2) limited availability of HS-scAb fragment to bind its analyte since the HS-scAb is buried within a layer of a blocking agent (CFB for #1 and MH for #2 and #3, Fig. 5). Co-immobilisation of the HS-scAb fragment together with MH should prevent denaturation during an immobilisation process, since HS-scAb fragment does not have opportunity (time) for lateral spreading of the protein structure on the gold surface, which is a prerequisite for irreversible adsorption and denaturation of the protein [34]. Previously we have shown that it is of importance to control the length of thiols applied to modify gold surfaces as this may significantly affect bioreceptor performance of the constructed biosensor [35]. Thus, in order to prove that limited availability of HS-scAb fragments to bind to its analyte is responsible for a small response, we applied a much shorter thiol 2-mercaptoethanol (ME) to backfill the protein layer (#4, Fig. 5). The results showed that the biosensor could detect 100 ng/mL of PSA with a response ΔRct = 35.8 ± 3.7%, clearly indicating that the antibody fragments are stable on the gold surface during and after chemisorption and that previously demonstrated limited biosensor responses #1, #2 and #3 (Fig. 5) were due to the fact that the biorecognition sites were buried and unavailable.
Fig. 5.
Response of various types of biosensors using various immobilisation strategies (A, B, C and D) as described in Fig. 5 with 100 ng/mL of PSA. The biosensors tested had the following composition: #1: Au/HS-scAb/CFB, #2: Au/HS-scAb/ backfill MH, #3: Au/HS-scAb_co-immob MH, #4: Au/HS-scAb/backfill ME, #5: Au/ SAM1:3/PDEA/HS-scAb/cysteamine, #6: Au/SAM1:1/PDEA/HS-scAb/cysteamine, #7: Au/SAM1:3/EDC-NHS/scAb/gelatine, #8: Au/SAM1:3/EDC-NHS/scAb/ethanol- amine. Abbreviations used: Au – gold electrode, CFB – carbo free buffer, MH – mercaptohexanol, ME – mercaptoethanol, SAM – self-assembled monolayer composed of 11-mercaptioundecanoic acid and 6-mercaptiohexanol, PDEA – 2- (2-pyridinyldithio) ethaneamine hydrochloride, EDC – N-(3-dimethylaminopro- pyl)-N´-ethylcarbodiimide hydrochloride, NHS – N-hydroxysuccinimide. The measurements were performed at least in triplicate with error bars representing standard deviations of such assays.
In the next experiments we tested the hypothesis that oriented immobilisation of the HS-scAb bound on a mixed SAM, via a disulphide exchange reaction can improve the biosensor performance (biosensor type C in Fig. 4). The results showed that when the biosensor was constructed with immobilisation of HS-scAb fragments on the mixed SAM composed of MUA:MH at ratio of 1:3 (#5, Fig. 5), the biosensor exhibited a ΔRct = 24.0 ± 1.8% with 100 ng/mL of PSA. When the biosensor interface was prepared from less diluted functional thiol (MUA) in MH i.e. a mixed SAM at ratio of 1:1 (#6, Fig. 5), the biosensor exhibited a slightly larger response of 32.1 ± 4.6% with 100 ng/mL of PSA compared to biosensor #5. This indicates that a mixed SAM composed of MUA and MH with higher proportion (ratio 1:1) is more beneficial for overall antibody fragment binding ability compared to the binding surface formed on SAM composed of MUA:MH at ratio of 1:3. The last biosensor type was based on covalent immobilisation of scAb fragments via an amine coupling (EDC/NHS coupling chemistry) on a mixed SAM composed of MUA and MH (biosensor type D), since such a mixed SAM with subsequent covalent construction of various types of devices [34–39]. Previously we have shown that for immobilisation of an antibody, SAM composed of MUA and MH at ratio of 1:3 was optimal [37,39] and this is why such a mixed SAM was applied in this work, as well. When the biosensor was prepared by covalent immobilisation of scAb fragments subsequently blocked by a protein layer (#7, Fig. 5), the biosensor response towards 100 ng/mL of PSA was 12.5 ± 1.5%, indicating that the protein blocking layer was too thick with a biorecognition site only moderately accessible for analyte binding, as already discussed above for biosensor types A and B. When the biosensor was blocked by much shorter ethanolamine, the biosensor (#8, Fig. 5) exhibited a ΔRct = 74.7 ± 3.0% with 100 ng/ mL of PSA. Thus, the most sensitive biosensor device for detection of PSA was that based on covalent immobilisation of scAb fragments on a mixed SAM via a standard amine coupling chemistry (Fig. 5).
Finally, we constructed a calibration curve for detection of PSA by the scAb-based biosensor prepared by covalent immobilisation of scAb fragments on a mixed SAM. In Fig. 6 Nyquist plots for analysis of PSA are shown with the final step being an incubation of PSA attached with SNA lectin. After covalent immobilisation of scAb fragments with subsequent blocking by ethanolamine, a typical charge transfer resistance (Rct) was 15.1 ± 0.7 kΩ and this further increased with a higher PSA concentration to a value of 27.8 ± 1.4 kΩ (at 100 ng/mL of PSA) and also after incubation with SNA lectin to a value of 31.2 ± 1.8 kΩ (Fig. 6).
Fig. 6.
Nyquist plots applied for construction of calibration curves using electrochemical impedance spectroscopy as an electrochemical detection platform. Various concentrations of PSA (0.1–100 ng/mL) were applied with a final incubation of the biosensor surface with SNA lectin. The biosensor response (black symbols) is the response of device based on Au/SAM1:3/EDC-NHS/scAb/ethanolamine (biosensor type 8 in Fig. 5) towards plain buffer without PSA.
The results indicate that the biosensor responded very well to increased addition of PSA and that the SNA lectin finally confirmed the presence of Neu5Acα6Gal/GalNAc terminated glycan on PSA, in agreement with previous results [37–39]. Finally, the performance of the scAb-based biosensor towards PSA was directly compared with the performance of a full antibody-based biosensor (Fig. 7), indicating that the scAb-based biosensor exhibited sensitivity towards PSA of 15.9 ± 0.8% decade-1 (R2 = 0.991 with an average RSD of 4.9%), while the full antibody-based biosensor offered sensitivity towards PSA of 4.2 ± 0.1% decade-1 (R2 = 0.999 with an average RSD of 4.8%). Thus, the scAb-based exhibited almost 4-fold higher sensitivity compared to the full antibody-based biosensor towards PSA, while having similar average RSDs of assays. Interestingly, the response of the scAb-based biosensor towards 100 ng/mL during examination of the biosensor calibration was 70.4 ± 4.0% (Fig. 7), what is a value similar to the response obtained in the preliminary optimisation experiments (#8, Fig. 5) exhibited ΔRct = 74.7 ± 3.0% with 100 ng/mL of PSA for the same biosensor design. This indicates a low day-to-day variability of the scAb-based biosensor construction. The extrapolated limit of detection for PSA by the scAb-based biosensor is 0.01 ng/mL. Moreover, the selectivity of the scAb-based biosensor was tested using KLK2 protein, a protein structurally similar to PSA. The results indicate that the biosensor exhibited negligible response towards this protein (Fig. 7), indicating high selectivity for PSA detection by this biosensor device.
Fig. 7.
Calibration plot for detection of PSA biosensor devices constructed with scAb fragments (black line) or full antibodies (Ab, green line). Moreover, selectivity of the scAb-based biosensor was tested with kallikrein 2 (KLK2) protein (red line). The biosensor devices were tested with concentration of PSA, which can be present in the men's blood.
4. Conclusions
The scAb fragment is an ideal biorecognition element for glycoprofiling of PSA with KD for this analyte of 5 nM [26], a value which is much lower than KD of an interaction between full-length antibody and PSA of 17.5 nM [28], both values determined by surface plasmon resonance. The other beneficial features of the scAb fragments are that such fragments do not contain carbohydrate residues and are much smaller (approx. 8 x 4 x 3.5 nm) compared to a full length antibody with the size 15 x 7 x 3.5 nm [29]. These results indicate that the use of scAb fragments in combination with appropriate immobilisation strategies can provide a sensor system that is sensitive and specific and is ideal for use in glycoprofiling of specific cancer-related biomarkers such as PSA, when used with specific lectins.
Acknowledgement
This publication was made possible by funding from the Slovak research and development agency APVV-14-0753 and VEGA2/ 0162/14 is acknowledged. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Program (FP/2007- 2013)/ERC Grant Agreement no 311532. This work was funded by the European Commission FP7 Programme through the Marie Curie Initial Training Network PROSENSE (grant no. 317420, 2012–2016). This publication is the result of the project implementation: Centre for materials, layers and systems for applications and chemical processes under extreme conditions _ Stage I, ITMS No.: 26240120007 supported by the Research & Development Operational Program funded by the ERDF.
References
- [1].Dosekova E, Filip J, Bertok T, Both P, Kasak P, Tkac J. Nanotechnology in Glycomics: Applications in Diagnostics, Therapy, Imaging, and Separation Processes. Medicinal Research Reviews. 2017;37:514–626. doi: 10.1002/med.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nature Reviews in Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- [4].Sävblom C, Malm J, Giwercman A, Nilsson J-Å, Berglund G, Lilja H. Blood levels of free-PSA but not complex-PSA significantly correlates to prostate release of PSA in semen in young men while blood levels of complex-PSA, but not free-PSA increase with age. The Prostate. 2005;65:66–72. doi: 10.1002/pros.20254. [DOI] [PubMed] [Google Scholar]
- [5].Gilgunn S, Conroy PJ, Saldova R, Rudd PM, O'Kennedy RJ. Aberrant PSA glycosylation[mdash]a sweet predictor of prostate cancer. Nature Reviews in Urolog. 2013;10:99–107. doi: 10.1038/nrurol.2012.258. [DOI] [PubMed] [Google Scholar]
- [6].Sharma S, Zapatero-Rodríguez J, O'Kennedy R. Prostate cancer diagnostics: Clinical challenges and the ongoing need for disruptive and effective diagnostic tools. Biotechnology Advances. 2017;35:135–149. doi: 10.1016/j.biotechadv.2016.11.009. [DOI] [PubMed] [Google Scholar]
- [7].Paleček E, Tkáč J, Bartošík M, Bertók TS, Ostatná V, Paleček J. Electrochemistry of Nonconjugated Proteins and Glycoproteins. Toward Sensors for Biomedicine and Glycomics. Chemical Reviews. 2015;115:2045–2108. doi: 10.1021/cr500279h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Santos A, Davis JJ, Bueno PR. Fundamentals and applications of impedimetric and redox capacitive biosensors. Journal of Analytical & Bioanalytical Techniques. 2015:1. [Google Scholar]
- [9].Luo X, Davis JJ. Electrical biosensors and the label free detection of protein disease biomarkers. Chemical Society Reviews. 2013;42:5944–5962. doi: 10.1039/c3cs60077g. [DOI] [PubMed] [Google Scholar]
- [10].Damborska D, Bertok T, Dosekova E, Holazova A, Lorencova L, Kasak P, Tkac J. Nanotechnology in development of biosensors for detection of prostate specific antigen. Microchimica Acta. 2017;184:3049–3067. doi: 10.1007/s00604-017-2410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhu H, Roehl KA, Antenor JA, Catalona WJ. Biopsy of men with PSA level of 2.6 to 4.0 ng/mL associated with favorable pathologic features and PSA progression rate: a preliminary analysis. Urology. 2005;66:547–551. doi: 10.1016/j.urology.2005.03.093. [DOI] [PubMed] [Google Scholar]
- [12].Janbaziroudsari H, Mirzaei A, Maleki N. Association of serum prostate- specific antigen levels with the results of the prostate needle biopsy. Bulletin du Cancer. 2016;103:730–734. doi: 10.1016/j.bulcan.2016.05.006. [DOI] [PubMed] [Google Scholar]
- [13].Shinohara K, Nguyen H, Masic S. Management of an Increasing Prostate- Specific Antigen Level After Negative Prostate Biopsy. Urologic Clinics of North America. 2014;41:327–338. doi: 10.1016/j.ucl.2014.01.010. [DOI] [PubMed] [Google Scholar]
- [14].Belicky S, Tkac J. Can glycoprofiling be helpful in detecting prostate cancer? Chemical Papers. 2015;69:90–111. doi: 10.1515/chempap-2015-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Galletti G, Portella L, Tagawa ST, Kirby BJ, Giannakakou P, Nanus DM. Circulating tumor cells in prostate cancer diagnosis and monitoring: an appraisal of clinical potential. Molecular Diagnosis & Therapy. 2014;18:389–402. doi: 10.1007/s40291-014-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, Languino LR. c-Src, Insulin-Like Growth Factor I Receptor, G-Protein-Coupled Receptor Kinases and Focal Adhesion Kinase are Enriched Into Prostate Cancer Cell Exosomes. Journal of Cellular Biochemistry. 2017;118:66–73. doi: 10.1002/jcb.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mohammed AA. Biomarkers in prostate cancer: new era and prospective. Medical Oncology. 2014;31:140. doi: 10.1007/s12032-014-0140-3. [DOI] [PubMed] [Google Scholar]
- [18].Foley RW, Gorman L, Sharifi N, Murphy K, Moore H, Tuzova AV, Perry AS, Murphy TB, Lundon DJ, Watson RWG. Improving multivariable prostate cancer risk assessment using the Prostate Health Index. BJU International. 2016;117:409–417. doi: 10.1111/bju.13143. [DOI] [PubMed] [Google Scholar]
- [19].Pihíková D, Kasák P, Tkac J. Glycoprofiling of cancer biomarkers: Label-free electrochemical lectin-based biosensors. Open Chemistry. 2015;13:636–655. doi: 10.1515/chem-2015-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haab BB. Using lectins in biomarker research: Addressing the limitations of sensitivity and availability. PROTEOMICS–Clinical Applications. 2012;6:346–350. doi: 10.1002/prca.201200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klukova L, Bertok T, Petrikova M, Sediva A, Mislovicova D, Katrlik J, Vikartovska A, Filip J, Kasak P, Andicsová-Eckstein A, Mosnáček J, et al. Glycoprofiling as a novel tool in serological assays of systemic sclerosis: A comparative study with three bioanalytical methods. Analytica Chimica Acta. 2015;853:555–562. doi: 10.1016/j.aca.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bertok T, Klukova L, Sediva A, Kasák P, Semak V, Micusik M, Omastova M, Chovanova’ L, Vlček M, Imrich R. Ultrasensitive impedimetric lectin biosensors with efficient antifouling properties applied in glycoprofiling of human serum samples. Analytical Chemistry. 2013;85:7324–7332. doi: 10.1021/ac401281t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen S, LaRoche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, Brand RE, Haab BB. Multiplexed analysis of glycan variation on native proteins captured by antibody microarrays. Nature Methods. 2007;4:437–444. doi: 10.1038/nmeth1035. [DOI] [PubMed] [Google Scholar]
- [24].Spain E, Gilgunn S, Sharma S, Adamson K, Carthy E, O’Kennedy R, Forster RJ. Detection of prostate specific antigen based on electrocatalytic platinum nanoparticles conjugated to a recombinant scFv antibody. Biosensors and Bioelectronics. 2016;77:759–766. doi: 10.1016/j.bios.2015.10.058. [DOI] [PubMed] [Google Scholar]
- [25].Tkac J, Davis JJ. An optimised electrode pre-treatment for SAM formation on polycrystalline gold. Journal of Electroanalytical Chemistry. 2008;621:117–120. [Google Scholar]
- [26].Zapatero-Rodríguez J, Liébana S, Sharma S, Gilgunn S, Drago GA, O’Kennedy R. Detection of Free Prostate-Specific Antigen Using a Novel Single- Chain Antibody (scAb)-Based Magneto-Immunosensor. BioNanoScience. 2016:1–10. [Google Scholar]
- [27].Johnson A, Song Q, Ko Ferrigno P, Bueno PR, Davis JJ. Sensitive Affimer and Antibody Based Impedimetric Label-Free Assays for C-Reactive Protein. Analytical Chemistry. 2012;84:6553–6560. doi: 10.1021/ac300835b. [DOI] [PubMed] [Google Scholar]
- [28].Damborský P, Madaboosi N, Chu V, Conde JP, Katrlík J. Surface plasmon resonance application in prostate cancer biomarker research. Chemical Papers. 2015;69:143–149. [Google Scholar]
- [29].Viguier C, Crean C, O'Kennedy R. Trends and perspectives in immunosensors. Antibodies Applications and New Development. 2012:184–208. [Google Scholar]
- [30].Lee K-B, Park S-J, Mirkin CA, Smith JC, Mrksich M. Protein nanoarrays generated by dip-pen nanolithography. Science. 2002;295:1702–1705. doi: 10.1126/science.1067172. [DOI] [PubMed] [Google Scholar]
- [31].Wang X, Wang Y, Xu H, Shan H, Lu JR. Dynamic adsorption of monoclonal antibody layers on hydrophilic silica surface: a combined study by spectroscopic ellipsometry and AFM. Journal of Colloid and Interface Science. 2008;323:18–25. doi: 10.1016/j.jcis.2008.04.024. [DOI] [PubMed] [Google Scholar]
- [32].Raab A, Han W, Badt D, Smith-Gill SJ, Lindsay SM, Schindler H, Hinterdorfer P. Antibody recognition imaging by force microscopy. Nature Biotechnology. 1999;17:901–905. doi: 10.1038/12898. [DOI] [PubMed] [Google Scholar]
- [33].Xu H, Zhao X, Grant C, Lu JR, Williams DE, Penfold J. Orientation of a Monoclonal Antibody Adsorbed at the Solid/Solution Interface: A Combined Study Using Atomic Force Microscopy and Neutron Reflectivity. Langmuir. 2006;22:6313–6320. doi: 10.1021/la0532454. [DOI] [PubMed] [Google Scholar]
- [34].Davis JJ, Tkac J, Laurenson S, Ferrigno PK. Peptide aptamers in label-free protein detection: 1. Characterization of the immobilized scaffold. Analytical Chemistry. 2007;79:1089–1096. doi: 10.1021/ac061863z. [DOI] [PubMed] [Google Scholar]
- [35].Davis JJ, Tkac J, Humphreys R, Buxton AT, Lee TA, Ko Ferrigno P. Peptide aptamers in label-free protein detection: 2. Chemical optimization and detection of distinct protein isoforms. Analytical Chemistry. 2009;81:3314–3320. doi: 10.1021/ac802513n. [DOI] [PubMed] [Google Scholar]
- [36].Kveton F, Blšáková A, Hushegyi A, Damborsky P, Blixt O, Jansson B, Tkac J. Optimization of the Small Glycan Presentation for Binding a Tumor-Associated Antibody: Application to the Construction of an Ultrasensitive Glycan Biosensor. Langmuir. 2017;33:2709–2716. doi: 10.1021/acs.langmuir.6b04021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pihikova D, Belicky S, Kasak P, Bertok T, Tkac J. Sensitive detection and glycoprofiling of a prostate specific antigen using impedimetric assays. Analyst. 2016;141:1044–1051. doi: 10.1039/c5an02322j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pihikova D, Kasak P, Kubanikova P, Sokol R, Tkac J. Aberrant sialylation of a prostate-specific antigen: Electrochemical label-free glycoprofiling in prostate cancer serum samples. Analytica Chimica Acta. 2016;934:72–79. doi: 10.1016/j.aca.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pihikova D, Pakanova Z, Nemcovic M, Barath P, Belicky S, Bertok T, Kasak P, Mucha J, Tkac J. Sweet characterisation of prostate specific antigen using electrochemical lectin-based immunosensor assay and MALDI TOF/TOF analysis: Focus on sialic acid. Proteomics. 2016;16:3085–3095. doi: 10.1002/pmic.201500463. [DOI] [PMC free article] [PubMed] [Google Scholar]