Abstract
Background
We investigated whether adherence to breast screening would yield a clinical benefit even among patients with small breast cancer (≤2 cm) by comparing differences between those who did and did not adhere to breast screening.
Methods
Patients who were diagnosed with invasive T1 breast cancer and treated at Gangnam Severance Hospital from January 2006 to June 2014 were included. Of the 632 study patients, 450 and 182 were classified as screen-adherent and non-adherent. Adherence to the breast screening program was defined as the completion of breast screening examinations within 3 years before cancer diagnosis. Recurrence-free survival (RFS) and metastasis-free survival (MFS) were compared between the groups. Propensity score matching were applied to compare survival outcome.
Results
Adherent patients were more likely to have a lower histologic grade (P < 0.001), high estrogen receptor expression (P = 0.040), and lower HER2-positivity (P = 0.026). The adherent group had more favorable subtypes compared to the non-adherent group, with a greater percentage of Luminal/HER2-negative subtype (66.7% vs. 56.5%) and a lower percentage of HER2 subtype (8.3% vs. 16.7%). The RFS and MFS were significantly better in the adherent group (P = 0.003, 0.010, respectively). In the case-matched cohort, superior survival of the adherent group was maintained.
Conclusions
Adherence to breast screening in patients with small breast tumors was associated with more favorable tumor biology and better prognosis. Our findings suggest that adherence to breast screening might offer clinical benefits in terms of tumor biology as well as early detection.
Introduction
The goal of cancer screening is early tumor detection, followed by appropriate treatment. Regular screening mammography for breast cancer has been widely recommended and is supported by solid evidence: most population-based randomized controlled trials have reported reduced mortality among women who undergo screening mammography [1–5]. As in other developed countries, a national health screening program in the Republic of Korea initiated a population-based mammography screening program in 1999 [6].
Breast tumors detected by mammography screening are known to be smaller than those detected outside of screening [7–11]; accordingly, better prognosis is largely observed in populations wherein cancer is detected through screening programs. Of note, studies have also shown that mammographic cancer detection is associated with a better prognosis than that of similarly sized tumors found outside of screening [7–9,11–17]. These studies suggest that breast screening itself could be considered a favorable prognostic variable independent of the primary tumor size, axillary metastasis, age at cancer diagnosis, and tumor grade. Additionally, even among patients with ductal carcinoma in situ (DCIS), those with screening-detected tumors had better outcomes than did those with symptom-detected tumors [18].
In this study, we investigated whether adherence to a breast screening program would yield clinical benefit even among patients with small invasive breast cancers, as outcomes might be less affected by the detection method. We compared the pathological and biological differences, including the proportions of immunohistochemistry (IHC)-based subtypes, between groups of patients who did and did not adhere to a screening program.
Materials and methods
Study population
The institutional review board of Gangnam Severance Hospital, Yonsei University, Seoul, Korea, approved this study, which was conducted in accordance with good clinical practice guidelines and the Declaration of Helsinki. The need for informed consent was waived because of the retrospective design, under the approval of the institutional review board. Patients with T1 breast cancer who were treated at Gangnam Severance Hospital between January 2006 and June 2014 were identified. We excluded patients with bilateral breast cancer and those who were treated with neoadjuvant chemotherapy. Women younger than age 40 years were also excluded because general population screening was recommended from age 40 years. Raw data of these patients is provided online (S1 Data).
Tumor detection method
Physicians in the Breast Cancer Center of Gangnam Severance Hospital interviewed patients and documented information about the tumor detection method in the medical charts. Adherence to the breast screening program was defined as the completion of breast screening examinations within 3 years prior to the diagnosis of breast cancer because the Korean national health screening program offers biennial mammography screening. The completion of private mammography was also considered adherence to the breast screening program. Patients who had never participated in a breast screening program or completed screening ≥3 years before a diagnosis of breast cancer were classified as non-adherent. The patients for whom information about the breast screening program was not available were considered unclassified.
With a consideration of mammographic density which affects results of screening program, we included mammographic density category evaluated by Breast Imaging Reporting and Data System (BI-RADS).
Immunohistochemistry markers
For our IHC study, we stained formalin-fixed, paraffin-embedded tissue sections obtained from surgical specimens using appropriate antibodies specific for four markers: estrogen receptor (ER; 1:100 dilution, clone 6F11; Novocastra, Newcastle upon Tyne, UK), progesterone receptor (PR; clone 16; Novocastra, UK), human epidermal growth factor receptor 2 (HER2; 4B5 rabbit monoclonal antibody; Ventana Medical Systems, Tucson, AZ, USA), and Ki-67 (MIB-1; Dako, Glostrup, Denmark). Patients were stratified by ER and PR IHC test results into four groups using the modified Allred system: strong, Allred score 7–8; moderate, Allred score 5–6; weak, Allred score 2–4; and negative, Allred score 0–1 [19]. The HER2 status was defined as positive with a score of 3+ and negative with a score of 0 or 1+ [20]. Tumors with scores of 2+ were sent for fluorescent in situ hybridization (FISH) analysis, according to the protocol given by the supplier (PathVysion kit; Vysis, Downers Grove, IL, USA or HER2 inform; Ventana). Ki67 expression was measured by an experienced pathologist and reported as a percentage of positive tumor cells (range: 0–100%).
IHC-based subtype
Tumors were classified into four molecular subtypes based on ER, PR, HER2 expression: luminal-HER2 negative (ER+ and/or PR+, HER2-), luminal-HER2-positive (ER+ and/or PR+, HER2+), HER2 (ER-, PR-, HER2+), and triple-negative breast cancer (TNBC; ER-, PR-, HER2-).
Statistical analysis
Continuous variables such as age and tumor size were compared by Mann–Whitney U test. Discrete variables were compared using the chi-square test. Recurrence-free survival (RFS) was measured from the date of the first curative surgery to the date of the first loco-regional recurrence or distant metastasis. Metastasis-free survival (MFS) was measured from the date of the first curative surgery to the date of the first distant metastasis. The Kaplan–Meier method was used to estimate the RFS and MFS, and the estimated survival curves were compared using the log-rank test. Using Harrell c-statistic, the concordance index (c-index) was calculated to measure the concordance for time-to event data, in which increasing values between 0.5 and 1.0 indicated improved prediction. The Cox’s regression-hazard model was used for univariate and multivariable survival analyses.
To adjust for potential confounding factors, we performed an individual propensity score-matching method using a Greedy algorithm in which randomly selected individuals in the adherent group were paired with comparable individuals in the non-adherent group. The one control per one case was selected based on tumor size, lymph node metastasis, histologic grade, ER, and HER2.
All analyses were performed using SPSS version 18 (SPSS; Chicago, IL, USA) and SAS (version 9.4, SAS Inc., Cary, NC, USA). Statistical significance was defined as a P-value < 0.05 or a 95% confidence interval (CI) that did not include 1.
Results
Comparisons of clinical and pathologic characteristics
A total of 684 patients were included; of these, 450, 182, and 52 were categorized as screen-adherent, non-adherent, and unclassified, respectively. We further compared the clinical and pathological characteristics between the adherent and non-adherent groups (Table 1). Patients who adhered to the breast screening were more likely to have smaller tumors (P < 0.001) than were the non-adherent patients. Although 490 patients (77.5%) had node-negative disease, the non-adherent group was more likely to have a higher nodal stage (P = 0.004). The non-adherent patients had more advanced stage disease (P < 0.001), higher histologic grade (P < 0.001), higher ER-negative rate (P = 0.040), and higher HER2 positive rate (P = 0.026). Regarding adjuvant treatments, the adherent group was more likely to receive breast-conserving surgery (P < 0.001) and endocrine therapy (P = 0.007), and less likely to receive chemotherapy (P < 0.001).
Table 1. Clinical and pathologic characteristics according to the screen-adherence.
| Original Cohort | Case-matched cohort | |||||
|---|---|---|---|---|---|---|
| Variables | Screening-adherence (n = 450) | Screening-non-adherence (n = 182) | P-value | Screening-adherence (n = 137) | Screening-non-adherence (n = 137) | P-value |
| Age, median (range) | 51 (40–87) | 49 (40–82) | 0.055 | 50 (40–84) | 49 (40–82) | 0.462 |
| Tumor size, median (range) | 1.2 (0.1–2.0) | 1.5 (0.3–2.0) | <0.001 | 1.5 (0.3–2.0) | 1.5 (0.3–2.0) | 0.872 |
| Mammographic density a, b | 0.094 | 0.348 | ||||
| I | 11 (2.7) | 2 (1.2) | 4 (3.1) | 1 (0.8) | ||
| II | 51 (12.4) | 32 (18.6) | 16 (12.5) | 22 (17.1) | ||
| III | 286 (69.8) | 120 (69.8) | 91 (71.1) | 93 (72.1) | ||
| IV | 62 (15.1) | 18 (10.5) | 17 (13.3) | 13 (10.1) | ||
| T stage | <0.001 | 0.878 | ||||
| T1a | 47 (10.4) | 6 (3.3) | 6 (4.4) | 6 (4.4) | ||
| T1b | 115 (25.6) | 24 (13.2) | 19 (13.9) | 22 (16.1) | ||
| T1c | 288 (64.0) | 152 (83.5) | 112 (81.8) | 109 (79.6) | ||
| N stage | 0.004 | 0.802 | ||||
| 0 | 356 (79.1) | 133 (73.1) | 103 (75.2) | 106 (77.4) | ||
| N1 | 89 (19.8) | 38 (20.9) | 32 (23.4) | 30 (21.9) | ||
| N2 | 4 (0.9) | 8 (4.4) | 2 (1.5) | 1 (0.7) | ||
| N3 | 1 (0.2) | 3 (1.6) | ||||
| Stage | <0.001 | 0.988 | ||||
| I | 366 (81.3) | 136 (74.7) | 109(79.6) | 108 (78.8) | ||
| II | 80 (17.8) | 35 (19.2) | 26 (19.0) | 27 (19.7) | ||
| III | 4 (0.9) | 11 (6.0) | 2 (1.5) | 2 (1.5) | ||
| Grade a | <0.001 | 0.881 | ||||
| I or II | 373 (86.9) | 126 (72.0) | 110 (80.3) | 108 (78.8) | ||
| III | 56 (13.1) | 49 (28.0) | 27 (19.7) | 29 (21.2) | ||
| Estrogen receptor c | 0.040 | 0.398 | ||||
| Positive | 334 (74.2) | 120 (65.9) | 95 (69.3) | 92 (67.2) | ||
| Negative | 116 (25.8) | 62 (34.1) | 42 (30.7) | 45 (32.8) | ||
| Progesterone receptor c | 0.471 | 0.623 | ||||
| Positive | 280 (62.2) | 107 (58.8) | 84 (61.3) | 79 (57.7) | ||
| Negative | 170 (37.8) | 75 (41.2) | 53 (42.3) | 58 (42.3) | ||
| HER2 | 0.026 | 0.352 | ||||
| Negative | 351 (81.2) | 122 (72.6) | 115 (83.9) | 108 (78.8) | ||
| Positive | 81 (18.8) | 46 (27.4) | 22 (16.1) | 29 (21.2) | ||
| Ki67 a | 0.194 | 1.000 | ||||
| ≥20 | 87 (19.4) | 44 (24.2) | 32 (23.4) | 32 (23.4) | ||
| <20 | 362 (80.6) | 138 (75.8) | 105 (76.6) | 105 (76.6) | ||
| Surgery | <0.001 | 0.182 | ||||
| BCS | 287 (63.8) | 87 (47.8) | 81 (59.1) | 69 (50.4) | ||
| TM | 163 (36.2) | 95 (52.2) | 56 (40.9) | 68 (49.6) | ||
| Chemotherapy | <0.001 | 0.146 | ||||
| Not given | 248 (55.1) | 70 (38.7) | 70 (51.1) | 57 (41.6) | ||
| Given | 202 (44.9) | 111 (61.3) | 67 (48.9) | 80 (58.4) | ||
| Endocrine therapy | 0.007 | 0.256 | ||||
| Not given | 83 (18.4) | 52 (28.6) | 28 (20.4) | 37 (27.0) | ||
| Given | 367 (81.6) | 130 (71.4) | 109 (79.6) | 100 (73.0) | ||
| Radiotherapy | 0.040 | 0.394 | ||||
| Not given | 170 (37.8) | 85 (46.7) | 56 (40.9) | 64 (46.7) | ||
| Given | 280 (62.2) | 97 (53.3) | 81 (59.1) | 73 (53.3) | ||
a Missing value
b Mammographic density was categorized according to Breast Imaging Reporting and Data System
c Positive, Allred score 2–8; Negative, Allred score 0–1.
Abbreviations: HER2, human epidermal growth factor receptor 2; BCS, breast-conserving surgery; TM, total mastectomy
Moreover, we described our two groups in relation to the presence of symptoms (S1 Fig). All of the non-adherent had positive symptoms, while 161 (35.8%) of the adherent had positive symptoms. Details of symptoms in relation to the screening-adherence were presented in S1 Table.
Comparisons for IHC-based subtypes
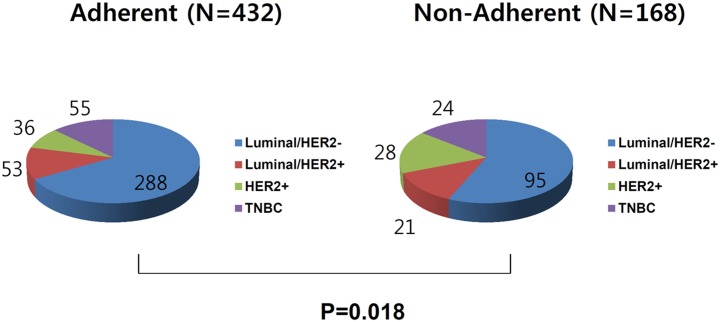
In intergroup comparisons of IHC-based subtype frequencies, the adherent group had more favorable subtypes compared to the non-adherent group, with a greater percentage of Luminal/HER2-negative subtype (66.7% vs. 56.5%) and a lower percentage of HER2 subtype (8.3% vs. 16.7%; Fig 1).
Fig 1. Comparisons of immunohistochemistry (IHC)-based subtypes between groups that did or did not adhere to breast screening.
Screening-adherent patients more frequently presented with a favorable subtype (66.7% vs. 56.5% for Luminal/HER2-negative), and less frequently presented with an aggressive subtype, compared with non-adherent patients (8.3% vs. 16.7% for HER2).
Clinical outcomes
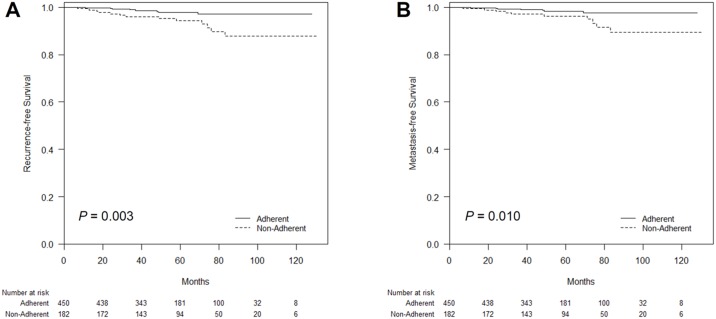
During a median follow-up period of 59 (range: 12–131) months, there were 22 recurrences occurred, including 5 loco-regional and 17 distant metastases. The RFS and MFS differed significantly according to adherence to the breast screening program based on the log-rank test (P = 0.003 and P = 0.010, respectively; Fig 2).
Fig 2. Kaplan–Meier plots of recurrence-free survival (RFS) and metastasis-free survival (MFS) according to adherence to breast screening.
(A) The RFS and (B) MFS differed significantly according to the adherence status (P = 0.003 and P = 0.010, respectively, log-rank test).
To demonstrate the adherence to screening as an independent prognostic factor, we conducted our analyses in two ways. First, multivariate models for RFS and MFS were constructed using the Harrell c-statistic. In a multivariate analysis using a Cox regression hazard model, adherence to breast screening (adjusted hazard ratio: 2.974; 95% CI: 1.280–6.909) was identified as a prognostic factor for RFS, independent of the nodal status, tumor size, nodal status, grade, ER status (Table 2). In a multivariate model for MFS, the adherence showed a marked trend toward a significant prognostic factor (adjusted hazard ratio: 2.464; 95% CI: 0.876–6.932).
Table 2. Multivariate survival analyses of recurrence-free survival and metastasis-free survival.
| Variables | Recurrence-free survival | Metastasis-free survival | ||
|---|---|---|---|---|
| Multivariate (P) | Hazard ratio (95% CI) | Multivariate (P) | Hazard ratio (95% CI) | |
| T stage | 0.762 | 0.796 | ||
| T1a /b | Reference | Reference | ||
| T1c | 1.195 (0.379–3.766) | 0.853 (0.255–2.849) | ||
| N stage | 0.717 | 0.260 | ||
| Negative | Reference | Reference | ||
| Positive | 1.196 (0.452–3.164) | 1.814 (0.644–5.106) | ||
| ER | 0.442 | 0.277 | ||
| Positive | Reference | Reference | ||
| Negative | 1.569 (0.497–4.949) | 2.814 (0.534–8.933) | ||
| Histologic grade | 0.460 | 0.763 | ||
| I and II | Reference | Reference | ||
| III | 1.1.536 (0.492–4.793) | 1.239 (0.308–4.977) | ||
| Adherence | 0.033 | 0.087 | ||
| Adherence | Reference | Reference | ||
| Non-Adherence | 2.974 (1.280–6.909) | 2.464 (0.876–6.932) | ||
Multivariate P values: Cox regression hazard model
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2
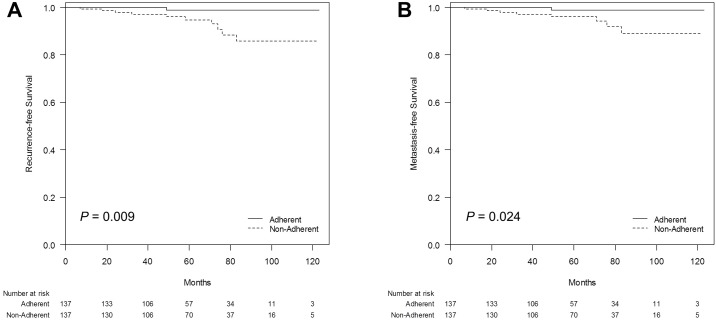
In addition, to adjust for potential confounding factors including adjuvant treatments, we adopted case-matching method, and assigned 137 patients for each group. Of the 274 patients in the propensity-matched cohorts, tumor characteristics and adjuvant treatments were not different according to the adherence to screening (Table 1). In the matched cohort, 12 women showed recurrences, with 2 loco-regional and 10 distant recurrences. Improved RFS and MFS of the adherent group was still observed (P = 0.009 for RFS, P = 0.024 for MFS; Fig 3).
Fig 3. Kaplan–Meier plots of recurrence-free survival (RFS) and metastasis-free survival (MFS) according to adherence to breast screening in the case-matched cohort.
(A) The RFS and (B) MFS differed significantly according to the adherence status (P = 0.009 and P = 0.024, respectively, log-rank test).
Discussions
Even in this modern era characterized by advances in systemic therapy, screening mammography for the detection of early-stage breast cancers remains associated with a reduction in breast cancer-related mortality [5,21]. In our selected group of patients with small tumors, who were selected to circumvent the effects of early detection via screening programs, the positive effect of breast screening was substantiated in terms of tumor biology and clinical outcome. We found that when compared with tumors diagnosed outside of a breast screening program, those detected via screening had more favorable biologic characteristics and, consequently, a better prognosis, despite the small tumor size (≤2 cm).
Previous studies have shown a link between screening-detected tumors and a less aggressive biological profile, including a lower histologic grade and mitotic count, strong ER and PR expression, reduced HER2 expression, and a lower cell proliferation rate, compared with symptom-detected tumors. In support of these findings, another study used a novel tumor genotyping approach to demonstrate that symptom-detected tumors have higher copy number gains, compared with screen-detected tumors [22]. Likewise, our findings regarding the better biologic characteristics of tumors diagnosed via breast screening were concordant with findings from previous studies.
A previous study of DCIS compared the subtype frequencies among screen-detected tumors and symptom-detected tumors [18]. In that study, favorable subtypes were more frequent among screen-detected DCIS cases, compared with symptom-detected cases. In accordance with this study, we found that the adherent group had a higher rate of luminal/HER2-negative and a lower rate of TNBC, compared with the non-adherent group.
A lower rate of chemotherapy given could be addressed as another advantage of the adherence to breast screening (45.4% in the adherence vs.62.3% in the non-adherence). In our study, the patients with adherence to breast screening showed a better outcome than the patients with non-adherence despite of a lower rate of adjuvant chemotherapy. It is reasonable because the non-adherence group had higher rates of HER2 expression and TNBC subtype. The higher chance of sparing toxicity from chemotherapy in the adherence group would be recognized as the benefit of breast screening program.
We recognize that our study had some limitations. One major caveat is the retrospective design, as well as the inclusion of a small number of patients from a single institute. Future studies involving multi-institutional databases are warranted to affirm our results. Also, another limitation is to acknowledge that clinical outcome can be affected by various variables including duration of endocrine treatments and complexity of clinical behavior, which are not fully considered in our analyses. Thus, our findings should be carefully integrated into current knowledge on pro and cons of breast screening program. Despite these limitations, one strength of our study is the population included in the study, which represents patients treated in daily practice because the definition of breast screening included private practice breast screening, as well as the national screening program. Furthermore, our findings obtained among selected patients with small, early-phase tumors further support the use of breast screening, as tumors detected using this modality remained associated with better tumor biologic characteristics and a favorable prognosis.
Conclusions
Breast cancers identified under adherence to breast screening are associated with a more favorable tumor biology and better prognosis, even during the early phase. Our findings suggest that adherence to breast screening, in addition to providing early detection, may yield clinical benefits due to more favorable tumor biology and outcomes.
Supporting information
(XLSX)
(TIF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Basic Science Research Program through the NRF, funded by the Ministry of Science, ICT, & Future Planning (NRF-2015R1C1A1A02037104), and grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1520120). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. Jama. 2014;311: 1327–1335. doi: 10.1001/jama.2014.1398 [DOI] [PubMed] [Google Scholar]
- 2.Alexander FE, Anderson TJ, Brown HK, Forrest AP, Hepburn W, Kirkpatrick AE, et al. 14 years of follow-up from the Edinburgh randomised trial of breast-cancer screening. Lancet. 1999;353: 1903–1908. [DOI] [PubMed] [Google Scholar]
- 3.Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years' follow-up: a randomised controlled trial. Lancet. 2006;368: 2053–2060. doi: 10.1016/S0140-6736(06)69834-6 [DOI] [PubMed] [Google Scholar]
- 4.Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359: 909–919. doi: 10.1016/S0140-6736(02)08020-0 [DOI] [PubMed] [Google Scholar]
- 5.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367: 1998–2005. doi: 10.1056/NEJMoa1206809 [DOI] [PubMed] [Google Scholar]
- 6.Lee EH, P B, Kim N-S, Seo H-J, Ko KL, Min JW, Shin M-H, Lee K, Lee S, Choi N, Hur MH, Kim DI, Kim MJ, Kim SY, Sung S, Dang JY, Kim SY, Kim Y, Lee W-C, Jeong J. The Korean guideline for breast cancer screening. J Korean Med Assoc. 2015;5: 408–419. [Google Scholar]
- 7.Crosier M, Scott D, Wilson RG, Griffiths CD, May FE, Westley BR. Differences in Ki67 and c-erbB2 expression between screen-detected and true interval breast cancers. Clin Cancer Res. 1999;5: 2682–2688. [PubMed] [Google Scholar]
- 8.Klemi PJ, Parvinen I, Pylkkanen L, Kauhava L, Immonen-Raiha P, Rasanen O, et al. Significant improvement in breast cancer survival through population-based mammography screening. Breast. 2003;12: 308–313. [DOI] [PubMed] [Google Scholar]
- 9.Moody-Ayers SY, Wells CK, Feinstein AR. "Benign" tumors and "early detection" in mammography-screened patients of a natural cohort with breast cancer. Arch Intern Med. 2000;160: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 10.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91: 2020–2028. [DOI] [PubMed] [Google Scholar]
- 11.Yassin MM, Peel AL, Thompson WD, Patton J, Ashton V, Leaper DJ. Does screen-detected breast cancer have better survival than symptomatic breast cancer? Asian J Surg. 2003;26: 101–107. doi: 10.1016/S1015-9584(09)60229-3 [DOI] [PubMed] [Google Scholar]
- 12.Burrell HC, Sibbering DM, Wilson AR, Pinder SE, Evans AJ, Yeoman LJ, et al. Screening interval breast cancers: mammographic features and prognosis factors. Radiology. 1996;199: 811–817. doi: 10.1148/radiology.199.3.8638010 [DOI] [PubMed] [Google Scholar]
- 13.Dong W, Berry DA, Bevers TB, Kau SW, Hsu L, Theriault RL, et al. Prognostic role of detection method and its relationship with tumor biomarkers in breast cancer: the university of Texas M.D. Anderson Cancer Center experience. Cancer Epidemiol Biomarkers Prev. 2008;17: 1096–1103. doi: 10.1158/1055-9965.EPI-08-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliland FD, Joste N, Stauber PM, Hunt WC, Rosenberg R, Redlich G, et al. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst. 2000;92: 743–749. [DOI] [PubMed] [Google Scholar]
- 15.Groenendijk RP, Bult P, Tewarie L, Peer PG, van der Sluis RF, Ruers TJ, et al. Screen-detected breast cancers have a lower mitotic activity index. Br J Cancer. 2000;82: 381–384. doi: 10.1054/bjoc.1999.0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joensuu H, Lehtimaki T, Holli K, Elomaa L, Turpeenniemi-Hujanen T, Kataja V, et al. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. Jama. 2004;292: 1064–1073. doi: 10.1001/jama.292.9.1064 [DOI] [PubMed] [Google Scholar]
- 17.Lee AK, Loda M, Mackarem G, Bosari S, DeLellis RA, Heatley GJ, et al. Lymph node negative invasive breast carcinoma 1 centimeter or less in size (T1a,bNOMO): clinicopathologic features and outcome. Cancer. 1997;79: 761–771. [PubMed] [Google Scholar]
- 18.Koh VC, Lim JC, Thike AA, Cheok PY, Thu MM, Tan VK, et al. Characteristics and behaviour of screen-detected ductal carcinoma in situ of the breast: comparison with symptomatic patients. Breast Cancer Res Treat. 2015;152: 293–304. doi: 10.1007/s10549-015-3472-6 [DOI] [PubMed] [Google Scholar]
- 19.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17: 1474–1481. doi: 10.1200/JCO.1999.17.5.1474 [DOI] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31: 3997–4013. doi: 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 21.Saadatmand S, Bretveld R, Siesling S, Tilanus-Linthorst MM. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. Bmj. 2015;351: h4901 doi: 10.1136/bmj.h4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewster AM, Thompson P, Sahin AA, Do K, Edgerton M, Murray JL, et al. Copy number imbalances between screen- and symptom-detected breast cancers and impact on disease-free survival. Cancer Prev Res (Phila). 2011;4: 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.