Abstract
BACKGROUND
Maraviroc (MVC) is a candidate drug for HIV PrEP.
OBJECTIVE
To assess the safety/tolerability of MVC-containing PrEP in U.S. women at-risk for HIV over 48 weeks.
DESIGN
Phase 2 randomized, controlled, double-blinded study of four PrEP regimens (#NCT01505114).
SETTING
Twelve clinical research sites of the HIV Prevention Trials Network and AIDS Clinical Trials Group.
PARTICIPANTS
HIV-uninfected women reporting condomless vaginal or anal intercourse with ≥1 HIV-infected or unknown-serostatus man within 90 days.
INTERVENTIONS
MVC alone, MVC+emtricitabine (FTC), MVC+tenofovir disoproxil fumarate (TDF), and TDF+FTC (control).
MEASUREMENTS
At each visit, clinical and laboratory (including HIV) assessments were conducted. Primary outcomes were grade 3–4 adverse events and time to permanent regimen discontinuation. Analyses were conducted on all randomized participants, according to original regimen assignment.
RESULTS
Among 188 participants, 85% completed follow-up, 11% withdrew early, and 4% were lost-to-follow-up; 19% discontinued their regimen prematurely. Number discontinuing and time-to-discontinuation did not differ among regimens. Grade 3/4 adverse events occurred in 5 (MVC), 13 (MVC+FTC), 9 (MVC+TDF) and 8 (TDF+FTC) participants; rates did not differ among regimens. One death occurred (suicide; MVC+FTC), judged not regimen-related. Of available samples at week 48 (n=126), 60% demonstrated detectable drug concentrations. No new HIV infections occurred.
LIMITATIONS
Participants were not necessarily high-risk for HIV. Regimen was 3 pills daily. Study was not powered for efficacy.
CONCLUSIONS
MVC-containing PrEP regimens were safe and well-tolerated compared to the control regimen of TDF+FTC in U.S. women. No new HIV infections occurred, although whether this was due to low risk of the population or to protection from the study regimens is not certain. MVC-containing PrEP for women may warrant further study.
FUNDING SOURCE
U.S. National Institutes of Health
INTRODUCTION
Over 2 million new HIV infections occur annually worldwide with about half in women (1). Effective strategies to prevent HIV acquisition among women are urgently needed, yet studies of HIV pre-exposure prophylaxis (PrEP) in women demonstrate conflicting results: Partners PrEP, conducted in African serodiscordant heterosexual couples, showed tenofovir disoproxil fumarate (TDF)-containing PrEP was associated with a 67%–75% reduction in HIV infections overall and a 66%–71% reduction in women (2). TDF2, conducted in sexually active heterosexual adults in Botswana, showed TDF/FTC was associated with a 62% reduction in HIV overall and a 49% reduction in women (3). However, FEM-PrEP and VOICE, both conducted exclusively in women, failed to demonstrate efficacy of oral TDF-containing PrEP over placebo in reducing HIV, mainly due to suboptimal participant adherence to study drugs (4, 5). In contrast, studies of PrEP with TDF/FTC among men who have sex with men (MSM), demonstrated a 44% reduction in HIV acquisition in iPrEx (6) and an 86% reduction in Ipergay (7) and PROUD (8).
Current guidelines recommend daily oral HIV PrEP for individuals at “substantial” risk of HIV, including women (9–11.) Currently, the only FDA-approved PrEP regimen is daily oral TDF/FTC, but tenofovir diphosphate drug concentrations are >100-fold lower in the female genital tract compared to colorectal mucosa (12, 13). TDF/FTC PrEP also was associated with significantly more gastrointestinal side effects than placebo in women in FEM-PrEP (4) and decreased renal function was significantly more common in the TDF-containing arms compared to placebo in women in Partners PrEP (2, 14) and VOICE (5). Also, TDF/FTC was associated with a small, but statistically significant, loss of bone mineral density in men and women in some PrEP studies (3, 15, 16). For these reasons, alternative PrEP regimens are needed.
Maraviroc (MVC) is a CCR5-antagonist HIV entry inhibitor approved for treatment of HIV infection based on randomized clinical trials in HIV-infected, treatment-experienced participants, however only 11% of participants were women (17). Maraviroc is active against R5 (CCR5-tropic) HIV which is associated with the majority of acute HIV transmissions (18), and has additional favorable properties as an HIV PrEP drug for women: Compared to plasma, maraviroc is concentrated 2–3-fold higher in cervicovaginal fluid, 2-fold higher in vaginal tissue (19), and 8–26 fold higher in rectal tissue (20). Maraviroc has no clinically relevant interactions with the oral contraceptives ethinyloestradiol and levonorgestrel (21). Maraviroc is prescribed infrequently for HIV treatment (22) and does not select for drug resistance to recommended first-line antiretroviral drugs. Maraviroc was generally safe in HIV-infected individuals for at least 5 years (23) and was associated with less bone loss than TDF in a comparative study of combination antiretroviral regimens in HIV-infected individuals (24). Hypothesizing that maraviroc could prevent chemokine-induced CCR5 activation (and resultant tissue/joint destruction), maraviroc was investigated in a 12-week pilot study of 126 HIV-uninfected participants with rheumatoid arthritis (81% women) and was generally well tolerated (25). In a parallel study of at-risk HIV-uninfected U.S. MSM and transgender women, maraviroc-containing PrEP regimens were generally safe/tolerable compared to standard TDF+FTC (26). Here, we compared the safety/tolerability of maraviroc-containing HIV PrEP regimens to TDF+FTC in at-risk U.S. women.
METHODS
Design Overview
This was a prospective, randomized, multicenter study of four antiretroviral regimens for HIV PrEP in HIV-uninfected women at-risk for HIV infection. The primary objective was to assess the safety/tolerability of the study regimens over 48 weeks. Secondary objectives included assessing adherence and incident HIV infections. The study was approved by the institutional review board (IRB) at each participating site and all participants provided written informed consent prior to any data collection. A parallel study was conducted earlier in MSM using the same protocol [26].
Setting and Participants
The study was conducted in the U.S. and Puerto Rico with participants recruited from the communities of 12 clinical research sites and was sponsored by the Division of AIDS of the U.S. National Institutes of Health through the HIV Prevention Trials Network (HPTN) and co-sponsored by the AIDS Clinical Trials Group. Participants enrolled from March 2013 to December 2014; study follow-up concluded in November 2015.
Eligible participants were born female, at least 18 years old, and self-reported condomless vaginal or anal intercourse with ≥1 man known to be HIV-infected or of unknown HIV-serostatus within 90 days prior to study entry. Eligible participants agreed to use one form of contraception (condoms, diaphragm/cervical cap, IUD or hormonal) during and for 30 days after the study and had adequate baseline safety laboratory results within 45 days of study entry including a calculated creatinine clearance ≥70 mL/min (Cockcroft-Gault), a negative serum or urine pregnancy test within 48 hours, and a non-reactive HIV antibody test (tested at the site with the local standard-of-care assay and subsequently confirmed at the HPTN Laboratory Center [Baltimore, MD] with the ARCHITECT HIV Ag/Ab Combo Assay [4th generation, Abbott Laboratories, Abbott Park, IL]) and a plasma HIV RNA level below detection within 14 days. Participants were excluded if they used any antiretroviral drug within 90 days (e.g., for HIV PrEP or post-exposure prophylaxis), reported active injection drug use, or had a positive hepatitis B surface antigen because of the virologic activity of TDF and FTC against hepatitis B virus.
Randomization and Interventions
Eligible participants were enrolled and randomly assigned with equal probability to receive one of four regimens: (1) maraviroc (MVC, Selzentry, ViiV Healthcare, Brentford, UK); (2) MVC plus emtricitabine (FTC, Emtriva, Gilead Sciences, Foster City, California); (3) MVC plus tenofovir disoproxil fumarate (TDF, Viread, Gilead Sciences); or (4) TDF plus FTC (control arm). Doses of the study drugs were: MVC 300 mg, FTC 200 mg, and TDF 300 mg. The study regimen was a total of 3 pills that included matching placebos, taken together orally once-daily.
The computerized randomization method was developed, implemented, and monitored by the Statistical Center for HIV/AIDS Research and Prevention (SCHARP; Fred Hutchinson Cancer Research Center, Seattle), and was stratified by site using block randomization with a block size of 12 such that approximately equal numbers of participants were assigned to each arm within each site. Site staff requested randomization and the site pharmacist received the randomized treatment assignment. All other site staff and study participants were blinded to randomization assignment until completion of follow-up.
Outcomes and Follow-up
The primary safety outcome was grade 3 (severe) and 4 (life-threatening) adverse events, as assessed by the site investigators (27). The primary tolerability outcome was the time to permanent study drug discontinuation through 48 weeks of follow-up. Secondary outcomes included adherence, assessed by detectable plasma drug concentrations, and incident HIV infections, assessed by laboratory testing. Following enrollment and randomization, participants were seen at weeks 2, 4, 8 and then every 8 weeks through week 48. Study drugs were discontinued at week 48 and a final visit was conducted at week 49. At each visit, interval history, targeted physical examination, safety laboratory tests including a serum or urine pregnancy test, blood plasma stored for drug concentration measurements, a self-reported adherence questionnaire, and site-standard risk-reduction counseling were conducted and study drugs and condoms were dispensed. HIV pre/post counseling and testing was conducted at each visit and whenever HIV infection was suspected. Adherence support was provided at each visit through semi-structured, counselor-guided discussions encouraging participant-centered problem-solving strategies.
Testing for sexually transmitted infections (STI; chlamydia and gonorrhea nucleic acid testing of cervical and rectal swabs, urine, and syphilis serology) was done at entry, week 16 or 24, and week 40 or 48, and whenever a participant reported suggestive symptoms; participants with positive STI test results continued study drugs and were referred for treatment. Participants were evaluated at each visit for HIV exposure; those who requested post-exposure prophylaxis (PEP) for HIV exposure were referred locally. If the participant started PEP, they were instructed to hold study medications and undergo repeat HIV testing at least 14 days after completing PEP; if HIV-negative, they could resume their study regimen. Each site was required to have centrally approved standard operating procedures for data management and quality assurance in place prior to study initiation.
Specialized testing and quality assurance was performed at the HPTN Laboratory Center, including HIV testing and confirmation of HIV seroconversion. Antiretroviral drug concentration testing was performed on available plasma samples from study participants collected at weeks 24 and 48; testing laboratory personnel were unblinded to regimen assignment and only assayed for assigned study drugs. MVC, FTC, and tenofovir (TFV) were quantified via validated liquid-chromatographic-tandem mass spectrometric (LC-MS/MS) methods, with assay limits of quantification of 0.5 ng/mL (MVC) and 0.3 ng/mL (FTC, TFV)(28,29). Quantitative drug concentrations were reported and then designated as detectable (or not) based on drug concentrations above assay-specific lower limits of quantification. In addition, drug concentrations were assessed in a subset of participants randomized to TDF- or FTC-containing study regimens who participated in a substudy (n=32) and compared to established adherence benchmarks based on directly observed dosing and 90% sensitivity threshold for each adherence stratum (30). Assays were validated in accordance with U.S. FDA Guidance for Industry, Bioanalytical Method Validation recommendations, and reviewed by the NIH-supported Clinical Pharmacology Quality Assurance (CPQA) Program; biannual external proficiency testing was also performed.
Statistical Analysis
The planned enrollment was 200 participants (50 per arm) with an assumed 5% loss-to-follow-up rate. The length of the study was estimated at 2 years, with 9 months for accrual and 12-month follow-up of each participant. This sample size was selected in order to provide estimates for the true value of the safety endpoint (occurrence of ≥grade 3 adverse events through 48 weeks) and a reasonable power to detect differences between arms for the tolerability endpoint (time-to-permanent-discontinuation of study drugs.) With 50 participants per arm, if an observed safety adverse event rate was 20%, the estimate would have 95% confidence intervals (CIs) of 9% to 31%. The power to detect differences for the tolerability endpoint was 80% for TDF/FTC vs. each of the 3 individual MVC-containing arms when the true hazard ratio is 2.8 and the incidence rate is 30%.
Primary data analysis was performed for all enrolled participants, according to original randomized study regimen assignment. The rates of grade 3/4 adverse events per person-year of observation (number of events per year) was summarized and compared between any 2 arms. Kaplan-Meier estimates were calculated to summarize the distribution of permanent treatment discontinuation.
Summary statistics were calculated in proportions, means/medians, or event rates in person-years, depending on the variable being binary, continuous, or count, respectively. Except for the medians, associated 95% confidence intervals (CI) were calculated based on the underlying distribution to be binomial, normal, or Poisson, respectively. For the medians, inter-quartile ranges (IQR) were calculated. HIV incidence was calculated using the exact method for Poisson counts. The study was reviewed biannually by an independent study monitoring committee of the HPTN.
The study was registered at clinicaltrials.gov: NCT01505114.
Role of the Funding Source
The Division of AIDS of the National Institute of Allergy and Infectious Diseases of the U.S. NIH funded the study; Gilead Sciences and ViiV Healthcare supplied study drugs. Representatives from the pharmaceutical companies served on the protocol team but did not contribute to final study interpretation. The investigators designed the study; collected, analyzed, and interpreted the data; wrote the manuscript; and made the decision to submit for publication with input from the protocol team. The corresponding author had full access to the data and made the final decision to submit the manuscript.
RESULTS
Study Population
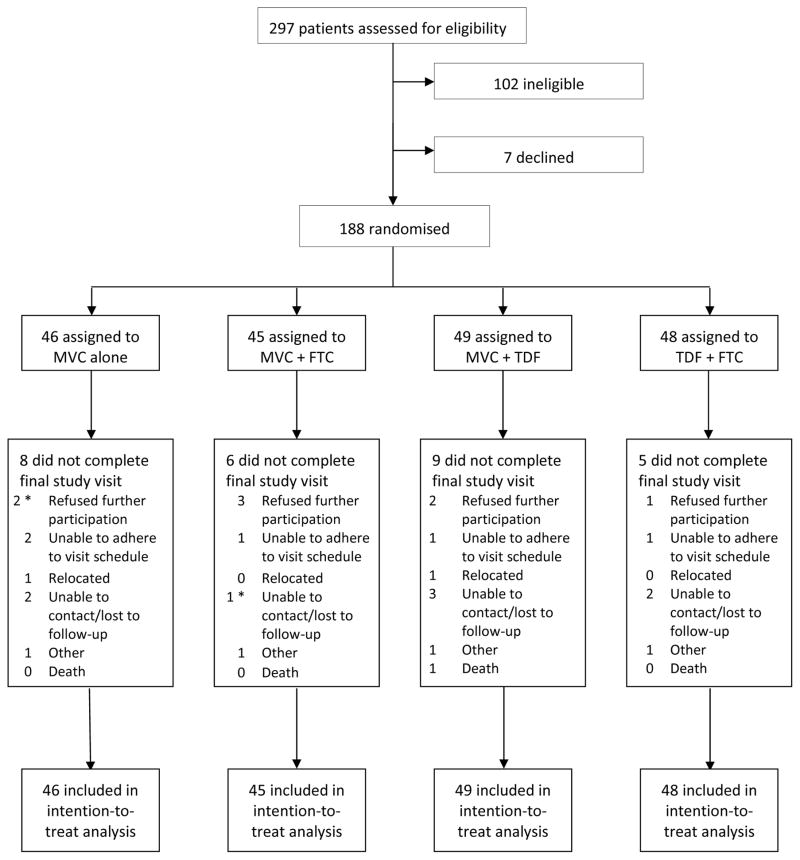
A total of 188 participants were enrolled in the study with similar enrollment across the 12 sites, and randomized to one of four study regimens (Figure 1); all but two started study drugs. The study population was 100% female at birth, with a median age of 35 years (Table 1). Study participants were 65% Black, 17% Latina (of any race), 27% White and 8% other race/ethnicity. Demographic characteristics were balanced among the study arms. During screening, prior to enrollment, 7 (4%) study participants were diagnosed with sexually transmitted infections: 3 (2%) with chlamydia and 4 (2%) with syphilis.
FIGURE 1. STUDY FLOW DIAGRAM.
Legend: MVC, maraviroc; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate. * Two participants were randomized, but never started their study regimen (one randomized to MVC alone who refused further participation and one to MVC+FTC who was unable to be contacted).
TABLE 1.
BASELINE CHARACTERISTICS OF THE STUDY PARTICIPANTS
| MVC | MVC+FTC | MVC+TDF | TDF+FTC | TOTAL | |
|---|---|---|---|---|---|
| Enrolled (N) | 46 | 45 | 49 | 48 | 188 |
| Sex (% born female) | 100% | 100% | 100% | 100% | 100% |
| Age, median (years) | 40 | 36 | 37 | 37 | 35 |
| Age, min, max (years) | 18, 61 | 19, 57 | 22, 60 | 18, 60 | 18, 61 |
| Race (%)* | |||||
| American Indian/Alaskan Native | 0% | 4% | 2% | 4% | 3% |
| Asian | 2% | 0% | 2% | 0% | 1% |
| Black | 65% | 69% | 65% | 62% | 65% |
| Pacific Islander | 0% | 0% | 0% | 2% | 1% |
| White | 26% | 29% | 29% | 25% | 27% |
| Other | 9% | 9% | 4% | 10% | 8% |
| Ethnicity – Latina (%) | 20% | 13% | 16% | 19% | 17% |
| Marital status (%) | |||||
| Married/partnership | 6% | 11% | 8% | 21% | 12% |
| Living with primary partner | 26% | 20% | 18% | 10% | 19% |
| Not living with primary partner | 13% | 4% | 10% | 15% | 11% |
| Single/divorced/widowed | 54% | 62% | 61% | 54% | 58% |
| Other | 0% | 2% | 2% | 0% | 1% |
| Employment status (%) | |||||
| Full-time | 20% | 33% | 22% | 19% | 23% |
| Part-time | 37% | 22% | 16% | 15% | 22% |
| Unemployed | 43% | 44% | 61% | 67% | 54% |
| Education (%) | |||||
| Less than high school | 15% | 11% | 8% | 15% | 12% |
| High school/trade school | 35% | 40% | 37% | 37% | 37% |
| Some college | 33% | 29% | 31% | 35% | 32% |
| Finished college | 13% | 13% | 22% | 10% | 15% |
| Advanced degree | 4% | 7% | 2% | 2% | 4% |
MVC, maraviroc, FTC, emtricitabine, TDF, tenofovir disoproxil fumarate.
Participants could self-identify as more than one race.
Study Disposition
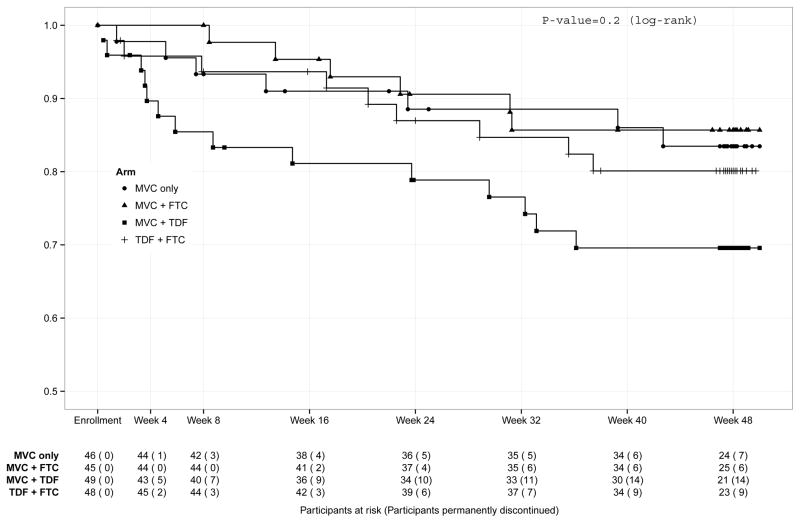
Of 188 participants randomized, 160 (85%) completed study follow-up, 20 (11%) withdrew from the study early, and 8 (4%) were lost-to-follow-up (Figure 1). The most common reasons for withdrawing from the study early were participant refusal (n=8) and inability to adhere to the visit schedule (n=5). There was one death from suicide on the MVC+FTC arm, judged not related to study drugs. Thirty-six (19%) participants permanently discontinued the study regimen prior to week 48; of these, 3 also terminated study participation while 33 completed study follow-up off study medications. The most common reasons for early discontinuation of the study regimen were participant request (n=16) and pregnancy (n=9). In total, 34% of participants were off study drugs by week 48. There was no difference among the study regimens in the proportion of participants who permanently discontinued study drugs (Table 2) or in the time to permanent study drug discontinuation (Table 2 and Figure 2).
TABLE 2.
ADVERSE EVENTS AND PERMANENT STUDY DRUG DISCONTINUATIONS
| MVC | MVC+FTC | MVC+TDF | TDF+FTC | TOTAL | |
|---|---|---|---|---|---|
| Enrolled (N) | 46 | 45 | 49 | 48 | 188 |
| Deaths | 0 | 0 | 1 | 0 | 1 |
| Permanent study drug discontinuations (participants, percent of total) | 7 (15%) | 6 (13%) | 14 (29%) | 9 (19%) | 36 (19%) |
| Time to permanent study drug discontinuation (median, [25th, 75th percentile], days) | 89 [36, 275] | 142 [94, 218] | 51 [25, 207] | 143 [55, 202] | 112 [34, 213] |
| Permanently discontinued study drugs due to adverse events | 0 | 1 | 4 | 2 | 7 |
| Serious adverse events (SAEs) | 1 | 4 (one participant with 2 events) | 8 (one participant with 2 occurrences of suicidal ideation) | 3 | 16 |
| Grade 3–4 adverse events (participants [%]; events) | 5 [11%]; 6 | 13[29%]; 16 | 9 [18%]; 15 | 8 [17%]; 11 | 35 [19%]; 48 |
| Grade 3 events – related to study drug*† | 1 | 5 | 1 | 4 | 11 |
| Grade 3 events – not related to study drug* | 4 | 10 | 11 | 6 | 31 |
| Grade 4 events – related to study drug* | 0 | 0 | 0 | 0 | 0 |
| Grade 4 events – not related to study drug* | 1 | 1 | 3 | 1 | 6 |
| Grade 3–4 Adverse event rate (per person-year; 95% confidence interval) | 0.15 (0.06, 0.36) | 0.41 (0.25, 0.69) | 0.37 (0.13, 0.54) | 0.26 (0.12, 0.58) | |
| Selected adverse events (grade 2–4, participants, percent‡) | |||||
| diarrhea | 0% | 0% | 5, 10% | 1, 2% | 6, 3% |
| nausea | 1, 2% | 3, 7% | 3, 6% | 0% | 7, 4% |
| vomiting | 0% | 1, 2% | 2, 4% | 3, 6% | 6, 3% |
| unintentional weight loss | 0% | 2, 4% | 0% | 1, 2% | 3, 2% |
| hypophosphatemia | 5, 11% | 8, 18% | 5, 10% | 6, 12% | 24, 13% |
| increased creatinine | 1, 2% | 1, 2% | 0% | 0% | 2, 1% |
MVC, maraviroc, FTC, emtricitabine, TDF, tenofovir disoproxil fumarate.
The study investigators assessed the relationship of adverse event to study drug.
Grade 3 related adverse events were: abnormal weight loss (MVC+FTC), back pain (MVC+FTC), congenital anomaly in offspring (n=2, MVC+FTC, TDF+FTC), depression (MVC+FTC), headache (TDF+FTC), hypophosphatemia (TDF+FTC), increased low-density lipoprotein (LDL) (TDF+FTC), spontaneous abortion (n=2, MVC+FTC, MVC+TDF), vitamin D deficiency (MVC only), upper limb fracture (MVC+TDF)
All events listed were predetermined to be of interest given prior reports of toxicities associated with the study medications; all are grade 2 with the exception of two grade 3 hypophosphatemia and one grade 3 weight loss.
FIGURE 2. TIME TO PERMANENT STUDY DISCONTINUATION.
Legend: Kaplan-Meier plot. MVC, maraviroc; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
Among participants with available plasma specimens across the 4 study arms, all drug(s) in the study regimen were detected in 65% (91/141) at week 24 and 60% (75/126) at week 48, without significant differences among the study arms. Among a subset of women tested who had detectable study drugs (n=32), more than 90% had drug concentrations consistent with daily adherence (30) without differences between study regimens or time (week 24 or 48); the maraviroc-only arm was not assessed because MVC-concentration benchmarks for adherence have not been established. In an intent-to-treat (missing value = undetectable) analysis, 48% (91/188) of participants at week 24 and 40% (75/188) at week 48 had study drugs detected. Participants self-reported they took a median of 94% and 92% of their study medications as recommended at weeks 24 and 48, respectively, without differences among the study arms.
Adverse Events and HIV Acquisition
Thirty-five (19%) participants experienced a total of 48 grade 3/4 adverse events; there was no difference among the 4 study regimens in the occurrences or rates of these events and <25% of events were judged related to study drug(s)(Table 2). Rates of pre-selected gastrointestinal and renal grade 2–4 adverse events also were similar among the study regimens (Table 2). Overall creatinine clearance decreased a median 3.3% from baseline to week 48, without differences among the study arms. Among 16 on-study pregnancies, there were 2 congenital abnormalities (Table 3). During study follow-up, 4 (2%) participants were diagnosed with sexually transmitted infections: 1 (1%) gonorrhea and 3 (2%) chlamydia. One participant reported using HIV PEP. No participant acquired HIV infection during the study; overall annualized incidence of HIV was 0% [95% CI: 0%, 2.3%].
TABLE 3.
PREGNANCY OUTCOMES
| Participant | Study Regimen | Study week with positive pregnancy test | Outcome of pregnancy | Disposition of study regimen |
|---|---|---|---|---|
| 1 | TDF+FTC | 2 | premature live birth; congenital anomaly (hydrocephalus) | discontinued due to pregnancy |
| 2 | MVC+TDF | 4 | spontaneous abortion | discontinued due to participant request |
| 3 | TDF+FTC | 8 | premature live birth of twins | discontinued due to pregnancy |
| 4 | TDF+FTC | 8 | ectopic pregnancy | completed |
| 5 | TDF+FTC | 16 | elective abortion | discontinued due to pregnancy |
| 6 | MVC alone | 16 | premature live birth of twins | discontinued due to pregnancy |
| 7 | TDF+FTC | 24 | full-term live birth | discontinued due to pregnancy |
| 8 | MVC+TDF | 32 | full-term live birth | discontinued due to toxicity |
| 9 | MVC alone | 40 | unknown | discontinued due to pregnancy |
| 10 | MVC+TDF | 40 | premature live birth | discontinued due to pregnancy |
| 11 | MVC alone | 48 | unknown | discontinued due to pregnancy |
| 12 | MVC+FTC | 48 | unknown | discontinued due to pregnancy |
| 13 | MVC+FTC | 48 | full-term live birth, congenital anomaly (syndactyly) | completed |
| 14 | MVC+FTC | 48 | stillbirth | completed |
| 15 | TDF+FTC | 49 | full-term live birth | completed |
| 16 | TDF+FTC | 49 | elective abortion | completed |
MVC, maraviroc, FTC, emtricitabine, TDF, tenofovir disoproxil fumarate.
CONCLUSIONS
In this phase 2, prospective, randomized, controlled, double-blinded, multicenter 48-week study of at-risk U.S. women, we showed that maraviroc-containing regimens were generally safe/well-tolerated compared to the control regimen of TDF+FTC when assessed for use as HIV PrEP. The number of participants discontinuing study medications and the time-to-discontinuation did not differ among regimens and the rates of grade 3/4 and specific adverse events of interest, including gastrointestinal side effects, hypophosphatemia, and increased creatinine, were similar among the study arms. Our team reported similar safety/tolerability results for HIV PrEP with maraviroc-containing regimens vs. TDF+FTC in at-risk U.S. MSM (26).
About a third of the women discontinued study drugs by week 48, including 11% who withdrew from the study early, 4% who were lost to follow-up, and 19% who discontinued study drugs prematurely, including 9 women for pregnancy as required by the protocol. The reasons for this relatively high discontinuation rate are likely multifactorial, including pregnancy, drug toxicities, pill burden, and/or a perception of low HIV risk. A total of 19% of women experienced grade 3/4 adverse events on the study regimens, although only one quarter of the grade 3 events and none of the grade 4 events were judged related to study drugs. U.S. women who practice condomless sex and who live in high HIV prevalence neighborhoods have an annual HIV incidence rate of 0.32%, and may benefit from effective HIV PrEP(9, 31). However, HIV PrEP regimens must be safe, well-tolerated, and perceived as necessary for women to use them consistently.
Of 16 on-study pregnancies, there were 2 congenital abnormalities (Table 3). The most recent interim report from the Antiretroviral Pregnancy Registry states the rate of birth defects in women exposed to antiretroviral drugs is 2.8/100 live births, which is not significantly different from the rate in the general population (32). In addition, the report states that sufficient numbers of first-trimester exposures have been monitored to detect at least a 1.5-fold increase in risk of birth defects for a number of antiretroviral drugs including TDF and FTC, and that no such increases have been detected (32). There currently are not enough reports with MVC to discern if there is an increased risk in pregnancy compared to other antiretroviral drugs. In animal reproduction studies, no evidence of adverse developmental outcomes with maraviroc was observed; maraviroc is FDA pregnancy category B (33).
Adherence is critical for HIV PrEP efficacy (34, 35). Adherence to study medications on FEM-PrEP and VOICE, conducted at a time when HIV PrEP was an unproven strategy for women, was less than 30–40% (4, 5). Participants in our study, conducted after studies reported PrEP efficacy, detected study drugs in 60–65% of plasma samples tested; the 34% premature study drug discontinuation rate impacts this estimate. While a detectable TFV or FTC concentration indicates only one or more doses taken in the prior week, in a subset of women tested with detectable drug levels, more than 90% demonstrated TFV or FTC concentrations consistent with daily adherence in the prior week, suggestive of adherence levels consistent with a protective effect against HIV acquisition (30). Tenofovir diphosphate drug concentrations are >100-fold lower in the female genital tract compared to the colorectal mucosa (12, 13). A model predicted that 85% adherence to TDF/FTC PrEP (≥6 daily doses per week) would be necessary for women to achieve target exposure in the female genital tract vs. only 28% adherence (≥2 daily doses per week) needed to achieve target exposure in colorectal tissue (36–38); suboptimal adherence compromises TDF/FTC PrEP efficacy, particularly in women. Further investigation of alternative agents with more favorable pharmacokinetics for HIV PrEP regimens in women is needed.
This phase 2 safety study was not designed to assess the efficacy of the study regimens to prevent HIV; we recruited study participants who were “at-risk” for HIV rather than necessarily “high risk”. While no study participant acquired HIV on our study, it is not clear if the study regimens prevented HIV acquisition or if the study population simply was at low risk for infection (or both). The low number of other new sexually transmitted infections during this 48-week study (n=4) and the fact that only one participant reported PEP use, suggests that our participants were at low risk for acquiring HIV during the study. Of note, in our study of 406 MSM testing the same study regimens, there were 5 seroconversions (4 in men randomized to MVC alone, 1 to MVC+FTC); of these, 2 participants had no detectable drug concentrations at any study visit and 3 others had low or variable drug concentrations (26). We cannot make conclusions about the efficacy of maraviroc-containing PrEP regimens from these studies.
There are strengths and limitations of this study. This was a comparative HIV PrEP study conducted in women at-risk for HIV in the U.S. Our study population was racially and ethnically similar to that of women living with HIV infection in the U.S., with significant representation from women of color. The active-controlled design ensured that every participant received an antiretroviral drug regimen (i.e., there was no all-placebo arm). Frequent follow-up allowed ongoing risk-reduction counseling, adherence support, and testing for HIV and other sexually transmitted infections that may have influenced adherence. Study limitations include the study population that was “at-risk” for HIV rather than necessarily “high-risk,” based on self-reported behavior; self-assessed HIV risk may have influenced adherence to the study drugs. The study regimen consisted of three pills (two more than the standard one-pill TDF/FTC PrEP regimen) and used 300 mg daily dosing of maraviroc, although the drug is approved for HIV treatment with twice-daily dosing. Finally, our study was not powered to detect differences in specific adverse events, not designed to assess efficacy, and had limited 48-week follow-up.
In summary, we found that maraviroc-containing PrEP regimens were generally safe/well-tolerated in U.S. women at-risk for HIV when compared to the standard-of-care TDF/FTC HIV PrEP regimen. Study drugs were taken by more than 60% of women on study as demonstrated by detectable study drug levels in available plasma samples and in a subset analysis, more than 90% had drug concentrations historically associated with high levels of adherence and protection against HIV acquisition (30). While no HIV infections occurred in the study, the study was not designed to assess efficacy. Given concerns about suboptimal tenofovir levels in the female genital tract, additional PrEP drug choices are needed. Maraviroc-containing oral PrEP regimens, with demonstrated comparable safety/tolerability to the standard-of-care regimen of TDF+FTC, may warrant further study in fully powered efficacy studies for HIV PrEP.
Supplementary Material
Acknowledgments
The authors would like to recognize the participants, their partners and families, other members of the study team (Todd T. Brown, Albert Liu, Jonathan Lucas, Kate MacQueen, Joseph Margolick, Ana Martinez, Bijal Patel, Bruce R. Schackman, Usha Sharma, and Fulvia Veronese), FHI 360, the pharmaceutical sponsors who provided study drugs and the study staff at the following participating sites: Case Western Reserve, Cleveland, OH (UM1-AI-069501); Fenway/Harvard Medical School, Boston, MA (UM1-AI-069412); George Washington University, Washington, DC (UM1-AI-069503; AI-117970 [DC CFAR]); Johns Hopkins University, Baltimore, MD (UM1-AI-069465); New Jersey Medical School, Newark, NJ (UM1-AI-069466); University of California, Los Angeles, CA (UM1-AI-069424); University of North Carolina, Chapel Hill, NC (UM1- AI-069423, UL1-TR-001111, P30-AI-50410); University of Pennsylvania, Philadelphia, PA (UM1-AI-069534 and P30-AI-045008); University of Pittsburgh, Pittsburgh, PA (UM1-AI-069494, UL1-RR-024153, UL1-TR-000005); University of Puerto Rico, San Juan, PR (UM1-AI-069415); University of Washington, Seattle, WA (UM1-AI-69481); and Weill Cornell Medicine, New York, NY (UM1-AI-069419, UL1-RR-024996).
GRANT SUPPORT: This work was supported by the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) through the HIV Prevention Trials Network (HPTN) [UM1-AI068619, UM1-AI068613, UM1-AI068617] and the AIDS Clinical Trials Group (ACTG) [UM1-AI-068636]. Gilead Sciences and ViiV Healthcare provided study drugs.
Footnotes
PREVIOUS PRESENTATION: The preliminary study results were presented at the 21st International AIDS Conference, Durban, South Africa, July 18–22, 2016, abstract #TUAC0102.
CONTRIBUTORS: All authors were involved in the design and conduct of the study. RMG was the Principal Investigator. RMG, TJW, MBM, and KHM drafted the protocol, consent forms, and manuscript with input from other authors. LMC was responsible for data management, and YQC and AMY were responsible for statistical analysis and interpretation. SHE, CWH, MAM, and PR were responsible for overseeing laboratory testing, analysis, and interpretation. All authors contributed to writing the paper and approved the final version.
DISCLOSURES: RMG, YQC, AMY, PR, MAM, LMC, CM, KLK, WC, RAS, YCM, MS, PA, KH, JDS, and MBM have no conflicts of interest. TJW has received research grants (to Weill Cornell) from Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline/ViiV and served as an ad-hoc consultant to GlaxoSmithKline/ViiV. RJL has received drug supplies, consulting fees and travel costs from Gilead Sciences. KRA has received an educational grant from Gilead Sciences (to the University of Michigan) and served as an ad-hoc consultant to Gilead Sciences. CWH has received a research grant (through Johns Hopkins) from Gilead Sciences and served as an ad hoc consultant to GlaxoSmithKline/ViiV. SHE has participated in collaborative research studies with Monogram Biosciences. IM has received consulting fees from ABIVAX, Aelix Therapeutics, and Novicol Life Sciences and research grants from Janssen R and D and ViiV Healthcare Inc. AA received an honorarium from BMS for a CME program and research grants from Gilead and GSK for investigator-initiated studies. ARR is an employee and stockholder in ViiV Healthcare. JFR is an employee and stockholder in Gilead Sciences. IF has received honoraria for serving on an advisory board to Gilead Sciences and received a research grant (to the University of Pennsylvania) from GlaxoSmithKline/ViiV. JS has received research grants, advisory board and speaker honoraria from Gilead, Merck, and ViiV. SS received honoraria for serving on Advisory Boards to Gilead Sciences and received research grants (to Rutgers New Jersey Medical School) from Gilead Sciences. SH has received honoraria for serving on advisory boards to Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/Viiv, and Janssen Therapeutics and received a research grant (to Rutgers University and West Virginia University) from Gilead Sciences for a PrEP demonstration project. KHM has received unrestricted research grants (to Fenway Health) from Gilead Sciences and Glaxo/SmithKline/ViiV Healthcare.
Contributor Information
Roy M. Gulick, Department of Medicine, Weill Cornell Medicine, New York, NY 10065 USA.
Timothy J. Wilkin, Department of Medicine, Weill Cornell Medicine, New York, NY 10010 USA.
Ying Q. Chen, Statistical Center for HIV/AIDS Research and Prevention (SCHARP), Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA.
Raphael J. Landovitz, Department of Medicine, University of California, Los Angeles, Los Angeles, CA 90025 USA.
K. Rivet Amico, Department of Health Behavior and Health Education, School of Public Health, University of Michigan, Ann Arbor, Michigan 48109-2029 USA.
Alicia M. Young, Statistical Center for HIV/AIDS Research and Prevention (SCHARP), Fred Hutchinson Cancer Research Center, Seattle, WA 98109 USA.
Paul Richardson, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD 21287 USA.
Mark A. Marzinke, Department of Medicine, Johns Hopkins University School of Medicine, 600 North Wolfe St, Baltimore, MD 21287.
Craig W. Hendrix, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21287 USA.
Susan H. Eshleman, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD 21205 USA.
Ian McGowan, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, 15213 USA.
Leslie M. Cottle, Statistical Center for HIV/AIDS Research and Prevention (SCHARP), Fred Hutchinson Cancer Research Center, Seattle, WA 98109 USA.
Adriana Andrade, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205 USA.
Cheryl Marcus, Department of Medicine, University of North Carolina, Chapel Hill NC 27599 USA.
Karin L. Klingman, HIV Research Branch, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, 20892-9830 USA.
Wairimu Chege, Clinical Prevention Research Branch, Prevention Sciences Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892-9831 USA.
Alex R. Rinehart, ViiV Healthcare, 5 Moore Drive, Durham, NC 27709.
James F. Rooney, Gilead Sciences, Foster City, CA 94404 USA.
Philip Andrew, FHI 360, Durham, NC 27713 USA.
Robert A. Salata, Department of Medicine, Case Western Reserve University, Cleveland, OH 44106 USA.
Marc Siegel, Department of Medicine, The George Washington University, Washington, DC 20037 USA.
Yukari C. Manabe, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205 USA.
Ian Frank, Department of Medicine, Perelman School of Medicine of the University of Pennsylvania, Philadelphia, PA 19104 USA.
Ken Ho, Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15213.
Jorge Santana, Department of Medicine, University of Puerto Rico School of Medicine, San Juan, PR 00935 USA.
Joanne D. Stekler, Department of Medicine, University of Washington, Seattle, WA 98104-2499 USA.
Shobha Swaminathan, Department of Medicine, Rutgers New Jersey Medical School, Newark, NJ 07103 USA.
Marybeth McCauley, FHI 360, 1825 Connecticut Avenue NW, Washington, DC 20009 USA.
Sally Hodder, West Virginia Clinical and Translational Science Institute, West Virginia University, P.O. Box 9102, Morgantown, WV 26506 USA.
Kenneth H. Mayer, Fenway Health, Department of Medicine, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, MA 02215 USA.
References
- 1.UNAIDS. [accessed 6/8/17];AIDS By The Numbers. 2016 Available at: http://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf.
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373:2237–46. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 8.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:54–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Centers for Disease Control and Prevention. [accessed 6/8/17];Recommendations for HIV Prevention for Adults and Adolescents with HIV in the United States. 2014 Available at: http://www.cdc.gov/hiv/guidelines/index.html.
- 10.Günthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2016 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. [accessed 6/8/17];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection – recommendations for a public health approach – Second Edition. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/
- 12.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, Cohen MS, Kashuba AD. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louissaint NA, Cao YJ, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, Anderson JR, Everts S, Bakshi R, Fuchs EJ, Hendrix CW. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–50. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mugwanya KK, Wyatt C, Celum C, Donnell D, Kiarie J, Ronald A, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015 Feb;175:246–54. doi: 10.1001/jamainternmed.2014.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasonde M, Niska RW, Rose C, Henderson FL, Segolodi TM, Turner K, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014 Mar 13;9:e90111. doi: 10.1371/journal.pone.0090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan K, Glidden DV, Anderson PL, Liu A, McMahan V, Gonzales P, et al. Effects of Emtricitabine/Tenofovir on Bone Mineral Density in HIV-Negative Persons in a Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis. 2015;61:572–80. doi: 10.1093/cid/civ324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–89. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009 Aug 15;51(5):546–53. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown KC, Patterson KB, Malone SA, Shaheen NJ, Prince HM, Dumond JB, et al. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis. 2011;203:1484–90. doi: 10.1093/infdis/jir059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel S, Russell D, Whitlock LA, Ridgway CE, Muirhead GJ. Effect of maraviroc on the pharmacokinetics of midazolam, lamivudine/zidovudine, and ethinyloestradiol/levonorgestrel in healthy volunteers. Br J Clin Pharmacol. 2008;65(Suppl):19–26. doi: 10.1111/j.1365-2125.2008.03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed 6/8/17]. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 23.Gulick RM, Fatkenheuer G, Burnside R, Hardy WD, Nelson MR, Goodrich J, et al. Five-year safety evaluation of maraviroc in HIV-1-infected treatment-experienced patients. J Acquir Immune Defic Syndr. 2014;65:78–81. doi: 10.1097/QAI.0b013e3182a7a97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taiwo BO, Chan ES, Fichtenbaum CJ, Ribaudo H, Tsibris A, Klingman KL, et al. Less bone loss with maraviroc- versus tenofovir-containing antiretroviral therapy in the AIDS Clinical Trials Group A5303 Study. Clin Infect Dis. 2015;61:1179–88. doi: 10.1093/cid/civ455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleishaker DL, Garcia Meijide JA, Petrov A, Kohen MD, Wang X, Menon S, et al. Maraviroc, a chemokine receptor-5 antagonist, fails to demonstrate efficacy in the treatment of patients with rheumatoid arthritis in a randomized, double-blind placebo-controlled trial. Arthritis Res Ther. 2012;14:R11. doi: 10.1186/ar3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulick RM, Wilkin TJ, Chen YQ, Landovitz RJ, Amico KR, Young AM, et al. Phase 2 study of the safety and tolerability of maraviroc-containing regimens to prevent HIV infection in men who have sex with men (MSM)(HPTN 069/ACTG A5305) J Infect Dis. 2017;215:238–246. doi: 10.1093/infdis/jiw525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Division of AIDS (DAIDS) Table for Table for Grading the Severity of Adult and Pediatric Adverse Events Version 1.0. Dec, 2004. [clarification dated August 2009.]) [Google Scholar]
- 28.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emory JF, Seserko LA, Marzinke MA. Development and bioanalytical validation of a liquid chromatographic-tandem mass spectrometric (LC-MS/MS) method for the quantification of the CCR5 antagonist maraviroc in human plasma. Clin Chim Acta. 2014 Apr 20;431:198–205. doi: 10.1016/j.cca.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, Anderson PL, Bushman LR, Fuchs EJ, Wiggins I, Radebaugh C, Prince HA, Bakshi RP, Wang R, Richardson P, Shieh E, McKinstry L, Li X, Donnell D, Elharrar V, Mayer KH, Patterson KB. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066) AIDS Res Hum Retroviruses. 2016;32:32–43. doi: 10.1089/aid.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodder SL, Justman J, Hughes JP, Wang J, Haley DF, Adimora AA, et al. HIV acquisition among women from selected areas of the United States: a cohort study. Ann Intern Med. 2013;158:10–18. doi: 10.7326/0003-4819-158-1-201301010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antiretroviral Pregnancy Registry. [accessed 6/8/16];Interim Report, 1 January 1989 through 31 January 2016, issued June 2016. Available at: http://apregistry.com/forms/interim_report.pdf.
- 33. [accessed 6/8/17];Maraviroc full prescribing information. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Selzentry/pdf/SELZENTRY-PI-MG-IFU.PDF 11/20/16.
- 34.Amico KR. Adherence to preexposure chemoprophylaxis: the behavioral bridge from efficacy to effectiveness. Curr Opin HIV AIDS. 2012;7:542–8. doi: 10.1097/COH.0b013e3283582d4a. [DOI] [PubMed] [Google Scholar]
- 35.Amico KR, Stirratt MJ. Adherence to preexposure prophylaxis: current, emerging, and anticipated bases of evidence. Clin Infect Dis. 2014;59:S55–60. doi: 10.1093/cid/ciu266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, McMahan V, Bushman LR, Casapía M, Montoya-Herrera O, Veloso VG, Mayer KH, Chariyalertsak S, Schechter M, Bekker LG, Kallás EG, Grant RM iPrEx Study Team. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell. 2013;155:515–518. doi: 10.1016/j.cell.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 38.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis. 2016;214:55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.