Abstract
Objectives
Orthotopic left lung transplantation in the mouse, recently developed by our laboratory, represents a physiological model for studies in transplantation biology. However, due to the limited overall respiratory contribution of the murine left lung, left lung transplant recipients remain healthy despite immune-mediated graft necrosis. We sought to develop a lung transplant model where animal survival depends on graft function.
Methods
Orthotopic vascularized right lung transplants utilizing cuff techniques were performed in syngeneic and allogeneic strain combinations. Grafts were assessed histologically or functionally by measuring arterial blood gases from 7 to 28 days after transplantation. In a parallel set of experiments syngeneic and immunosuppressed allogeneic hosts underwent a left pneumonectomy two weeks following right lung transplantation with assessment of graft function one week later.
Results
We performed 40 right lung transplantations with a survival rate of 87.5%. Syngeneic grafts remain free of inflammation as far as 28 days after transplantation. On day 7, arterial oxygen levels in syngeneic recipients (481 ± 90 mmHg) are equivalent to those in naïve mice (503 ± 59 mmHg) following left hilar occlusion. Alternatively, allogeneic grafts develop histological evidence of acute rejection and arterial oxygen levels are significantly lower after left hilar clamping (53.3 ± 10.3 mmHg). Syngeneic as well as immunosuppressed allogeneic right lung recipients tolerate a left pneumonectomy.
Conclusions
Right lung transplantation followed by left pneumonectomy represents the first survival model of vascularized lung transplantation in the mouse and will therefore allow for the design of novel studies in experimental lung transplantation.
Introduction
Physiological animal models have been instrumental for the advancement of clinical lung transplantation. To this end, technical refinements as well as immunosuppressive studies in canine models of pulmonary auto- or allotransplantation have paved the way for the first successful human lung transplants (1-3). Introduction of cuff techniques has allowed for the widespread use of orthotopic lung transplantation in the rat (4). Undoubtedly, important insights into lung transplantation biology have been gained using the rat model. However, the paucity of transgenic and knockout strains in the rat limits the use of this model for the design of mechanistic studies. Extending microsurgical techniques established in the rat model our laboratory was the first to describe a method for orthotopic vascularized left lung transplantation in the mouse (5). Using this model we have reported important differences between requirements for allograft rejection in the lung compared to other organs (6). Notably, there exist marked similarities between observations in mouse and human lung allografts pointing out the potential translational value of the mouse model (5, 7).
Other mouse transplant models have clearly defined indicators of graft rejection such as cessation of heart beat in the case of vascularized cardiac transplantation, animal death in the case of kidney transplantation with removal of the recipient’s native kidneys or sloughing and necrosis of the graft in the case of skin transplantation (8-10). One limitation of the orthotopic left lung transplant model in the mouse is that recipient animals survive and appear healthy even after alloimmune-mediated necrosis of their allograft (11). Therefore, graft assessment relies on histological examination of the transplanted left lung. We sought to develop a mouse lung transplant model where survival depends on the function of the transplanted lung. Here, we took advantage of the fact that the right mouse lung is markedly larger than the left lung. The right mouse lung, which is composed of four lobes, accounts for approximately 70% of the total lung volume (12). Here, for the first time we report the development of a technique for orthotopic vascularized right lung transplantation in the mouse. Syngeneic recipients have fully functional grafts and are able to survive a left pneumonectomy hereby providing the first description of a survival model for experimental lung transplantation in the mouse.
Materials and Methods
Animals
Male inbred C57BL/6 (H-2b) (B6) and Balb/c (H-2d) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals weighing 22-28 grams were used as donors and mice weighing 26-30 grams were used as recipients. Syngeneic transplants were performed in the B6 → B6 and allogeneic transplants were performed in the B6 → Balb/c as well as Balb/c → B6 strain combinations. Some allogeneic transplants received treatment with MR1 (250 μg intraperitoneally, day 0) and CTLA4-Ig (200 μg intraperitoneally, day 2). All animal procedures were approved by The Animal Studies Committee at Washington University School of Medicine. Animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health and the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research.
Donor procedure
Donor mice are anesthetized with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg), intubated with a 20 G angiocatheter through a tracheostomy and then placed on a rodent ventilator. After injection of 100 units of heparin into the penile vein, the chest is exposed through a median laparosternotomy. Right atrium, left atrium and inferior vena cava are incised to vent the heart and subsequently 3 ml of cold low-potassium dextran glucose solution are injected into the main pulmonary artery to flush the lungs. The trachea is ligated after the lungs are inflated to end-tidal volume with room air and the heart-lung block is excised. The right lung is then prepared for the placement of cuffs. A 22 G angiocatheter is used for the pulmonary artery, a 20 G angiocatheter is utilized for pulmonary veins and an 18 G angiocatheter is used for the right mainstem bronchus. Due to anatomical differences between the right and the left lungs in the mouse, we were forced to modify our previously described technique for the donor portion of the left lung transplant (5). The pulmonary vein is located anterior to the pulmonary artery and the right mainstem bronchus courses posterior to the pulmonary artery. The anatomic arrangement of these hilar structures necessitates a careful dissection to avoid damage. Importantly, while the left lung is made up of a single lobe, the right lung is composed of four lobes, namely the apical lobe, the azygous lobe, the cardiac lobe and the diaphragmatic lobe (Figure 1A). The pulmonary veins draining the lobes of the right lung converge in close proximity to the left atrium resulting in a relatively short right pulmonary vein. Therefore, the length of the cuff for the right pulmonary vein (0.3 mm excluding extension handle) needs to be shorter than for left lung transplantation (0.6 mm) (Figure 1B). The length of the cuff for the pulmonary artery is 0.6 mm. After introduction of these structures through the cuffs and everting their ends, the cuffs are secured with a circumferential 10-0 nylon ligature. Of note, as previously described for the left lung transplant procedure, we cuff the bronchus at the time of implantation. The length of the bronchial cuff is 0.6 mm. The donor procedure takes approximately 40 minutes. The donor lung is stored in low-potassium dextran glucose solution at 4°C until implantation.
Figure 1.
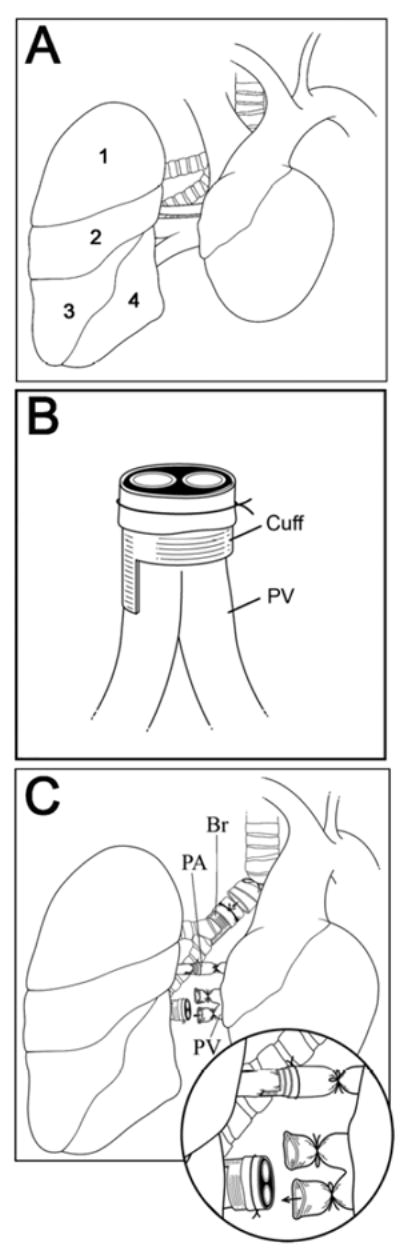
(A) Right lung anatomy. 1: apical lobe. 2: azygous lobe, 3: cardiac lobe, 4: diaphragmatic lobe. (B) Donor pulmonary vein (PV) after placement of the cuff. (C) Implantation of donor right lung is initiated by insertion of the cuffed donor pulmonary artery (PA) into the recipient pulmonary artery. Only the inferior branch is used for later insertion of the cuffed donor pulmonary vein. Br = bronchus.
Recipient procedure
Recipient mice are anesthetized with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg), intubated orotracheally with a 20 G intravenous catheter and then placed in a left lateral decubitus position. A right thoracotomy is performed, a portion of the third rib is excised to enhance exposure and a chest retractor is placed. Dissection is initiated in the superior portion of the pulmonary hilum where the right mainstem bronchus is gently freed from the pulmonary artery. Of note, the right mainstem bronchus is markedly shorter than the left mainstem bronchus. Therefore, during dissection of the right mainstem bronchus special attention needs to be directed toward avoiding injury to the left bronchus. The right bronchus is occluded close to the carina with a slip knot (8-0 silk ligature) leading to a collapse of the right lung, which facilitates the subsequent dissection. To further improve exposure we then excise the diaphragmatic lobe and attach a clamp to the remainder of the right lung for lateral retraction. The pulmonary artery is dissected free from the pulmonary vein and then occluded with a slipknot (10-0 silk ligature). The superior branch of the pulmonary vein is divided between two 10-0 silk ties. We then occlude the inferior branch of the pulmonary vein with a slipknot (8-0 silk ligature) close to its confluence with the superior branch. The recipient pulmonary artery is incised and the cuffed donor pulmonary artery is inserted and secured as previously described for the left lung transplant procedure (Figure 1C) (13). The donor lung is rotated medially to expose the recipient right mainstem bronchus. We then incise the recipient bronchus, insert the cuffed donor bronchus and secure it with a circumferential ligature (10-0 nylon). Only the superior branch of the recipient pulmonary vein is used for insertion of the cuffed donor pulmonary vein. The pulmonary venous anastomosis is also secured with a circumferential ligature (10-0 nylon). The recipient right lung is excised and the slipknots on pulmonary veins, pulmonary artery and bronchus are released to reperfuse the lung. The recipient procedure takes approximately 70-80 minutes. The cold ischemic period lasts approximately 50 minutes and the implantation of the donor lung, during which time period the graft is covered with an ice cold sponge, takes approximately 15 minutes.
Left pneumonectomy
Two weeks after right lung transplantation, mice are placed in a right lateral decubitus position and a left thoracotomy is performed. The chest is entered through the third intercostal space. The left hilar structures are mass ligated with a 6-0 silk tie and the left lung is excised. A piece of gelfoam is placed into the left chest cavity prior to closure in order to stabilize the mediastinum. Postoperatively, mice are maintained on 100% oxygen for 30 minutes.
Histology
At the time of sacrifice, lung grafts were harvested, inflation fixed in formaldehyde, embedded in paraffin, sectioned and stained with hematoxylin and eosin.
Functional graft assessment
After a 4 min-period of occlusion of the left pulmonary hilum arterial blood was drawn from the left ventricle while mice were ventilated with FiO2 of 1.0. Blood gases were measured using a iSTAT Portable Clinical Analyzer (iMale STAT Corp, East Windsor, NJ).
Statistical analysis
Statistical analysis was performed with student’s t test. Oxygen levels are expressed as mean ± standard error of the mean.
Results
Macroscopic and microscopic appearance of syngeneic and allogeneic grafts
A total of 40 right lung transplantations were performed with a survival rate of 87.5%. Five animals died intraoperatively secondary to bleeding (3) or in the immediate postoperative period due to pneumothoraces (2). Syngeneic B6 grafts were ventilated at 7 days (n=10) and appeared grossly normal up to 28 days (n=16) after transplantation (Figure 2). On histological examination these grafts had no evidence of inflammation and were comparable to unoperated lungs. On the other hand, allogeneic Balb/c → B6 right lung grafts were not ventilated at 7 days after transplantation and had histological evidence of acute rejection with perivascular cuffing and peribronchiolar mononuclear infiltrates (n=3) (Figure 3). B6 → Balb/c (n=3) right lung grafts were also acutely rejected 7 days after transplantation (data not shown).
Figure 2.
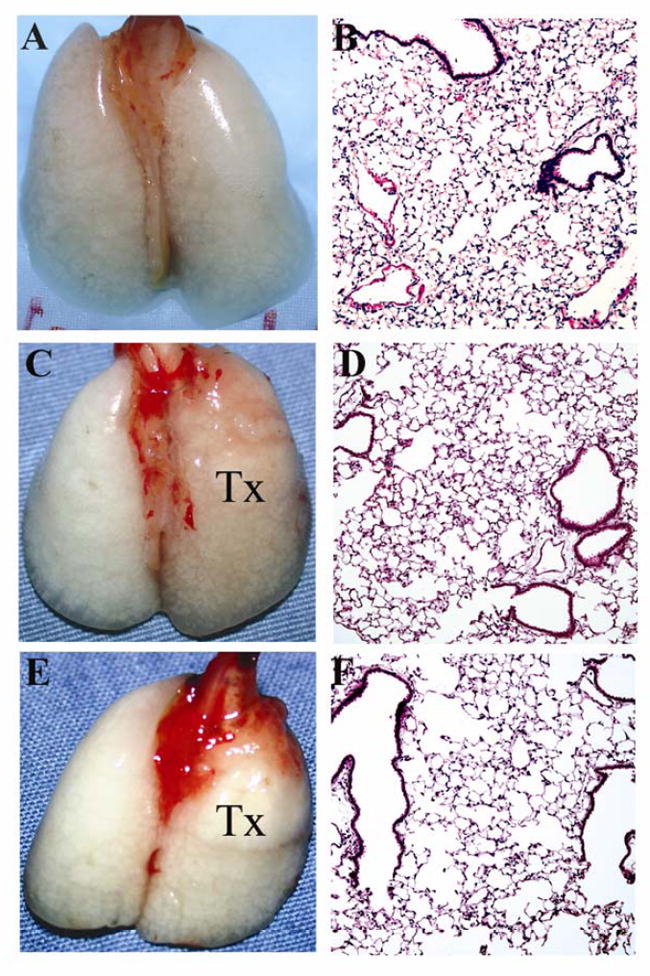
Gross appearance and histology (H&E, 100x) of unoperated right B6 lung (A, B) as well as right B6 lung 7 (C, D) (n=10) and 28 (E, F) days (n=3) after transplantation into B6 recipient. Tx = right lung graft.
Figure 3.
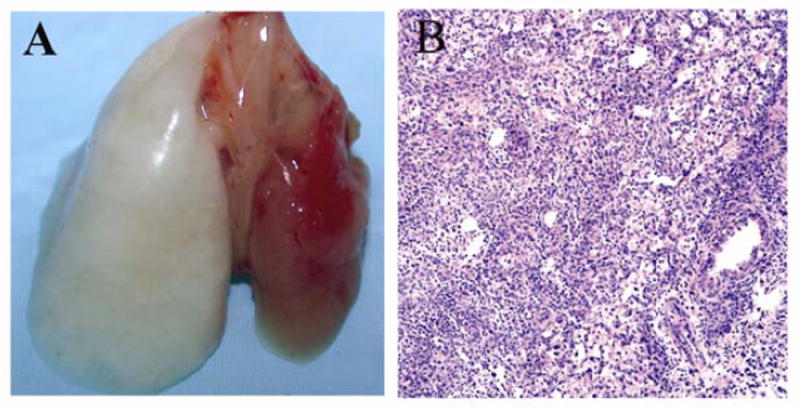
Gross appearance (A) and histology (H&E, 100x) (B) 7 days after transplantation of right Balb/c lung into B6 recipient. Results are representative of three independent experiments. Tx = right lung graft.
Functional assessment of right lung grafts
To assess the grafts functionally we compared arterial oxygen levels in naïve B6 mice and syngeneic B6 → B6 right lung transplant recipients 7 days following engraftment after occlusion of the left pulmonary hilum. We found no significant differences between oxygen levels in these two groups following either 4 minutes (PaO2 503 ± 59 mmHg in naïve B6 mice (n=4); PaO2 481 ± 90 mmHg in B6 → B6 right lung transplant recipients (n=4), p>0.1) or 10 minutes of left hilar occlusion (PaO2 399.2 ± 16.9 mmHg in naïve B6 mice (n=3) PaO2 362.5 ± 6.9 mmHg in B6 → B6 right lung recipients ((n=3), p>0.1) indicating that the syngeneic right lung transplants are functional (Figure 4). However, oxygen levels were significantly lower in allogeneic Balb/c → B6 right lung recipients compared to both naïve B6 mice and syngeneic right lung recipients following 4 minutes of left hilar clamping (53.3 ± 10.3 mmHg, n=3; p<0.01 compared to syngeneic transplants and naïve mice).
Figure 4.
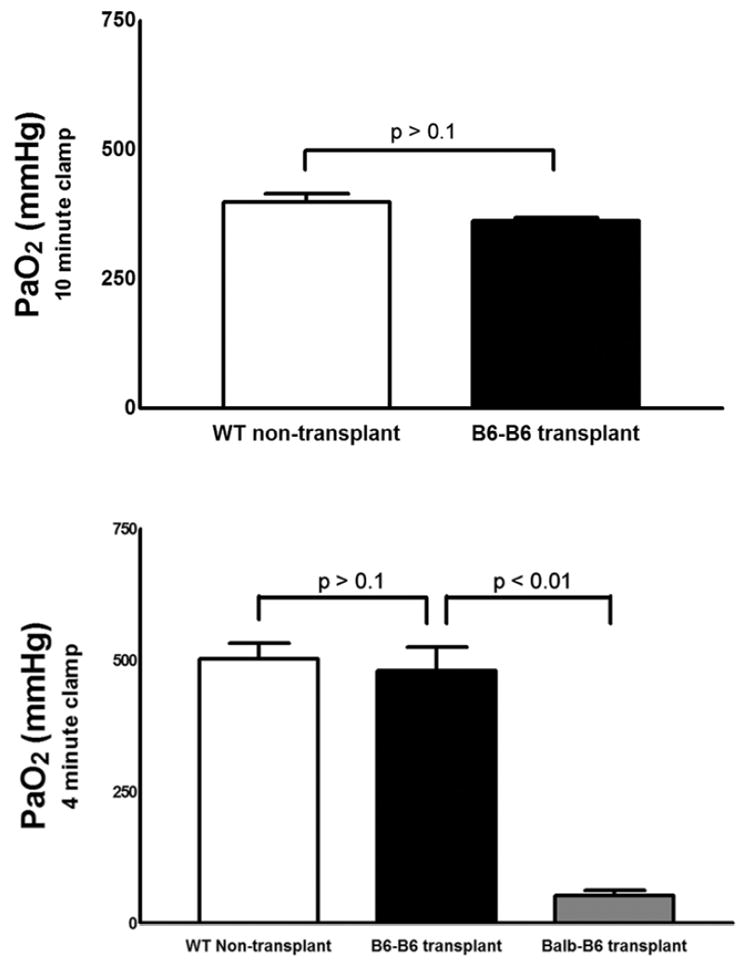
(A) There were no statistically significant differences in arterial PaO2 levels between wild-type B6 mice (n=3) and B6 recipients of syngeneic right B6 lungs 7 days after transplantation (n=3) after 10 minutes of left hilar occlusion. (B) PaO2 levels were significantly lower in allogeneic Balb/c → B6 right lung recipients (n=3) than in either syngeneic B6 → B6 right lung recipients (n=4) or naïve B6 mice (n=4) after a 4-minute period of left hilar occlusion (*p<0.01). Following 4 minutes of left hilar occlusion the pH was 7.37 ± 0.02 for naïve B6 mice, 7.29 ± 0.02 for B6 → B6 transplants and 7.19 ± 0.02 for Balb/c → B6 transplants. The pCO2 was 33.3 ± 2.1 mmHg for naïve B6 mice, 46.9 ± 3.9 for B6 → B6 transplants and 46.7 ± 2.6 for Balb/c → B6 transplants. The base deficit was 3.3 ± 0.7 for naïve B6 mice, 3.6 ± 1.4 for B6 → B6 transplants and 8.7 ± 0.8 for Balb/c → B6 transplants. Tx = right lung transplantation.
Right lung transplantation followed by left pneumonectomy
We measured arterial oxygen levels in syngeneic B6 right lung recipients 7 days following a left pneumonectomy. There were no statistically significant differences between these mice and naïve B6 mice 7 days after a left pneumonectomy (PaO2 534 ± 61 mmHg in naïve B6 mice after left pneumonectomy (n=3), PaO2 461 ± 67 mm Hg in B6 → B6 right lung transplant recipients after left pneumonectomy (n=3), p= 0.23) (Figure 5). We have previously reported that in left lung transplants perioperative blockade of CD40/CD40 Ligand and B7/CD28 costimulatory pathways prevents graft necrosis and grafts stay ventilated for time periods exceeding 100 days (11). Balb/c → B6 right lung recipients that received this regimen tolerated a left pneumonectomy. Their arterial oxygen levels seven days following left pneumonectomy were 373.1 ± 16.3 mmHg (n=3) (p>0.1 vs. syngeneic B6 right lung recipients following left pneumonectomy, p<0.05 vs. naïve B6 mice following left pneumonectomy.)
Figure 5.
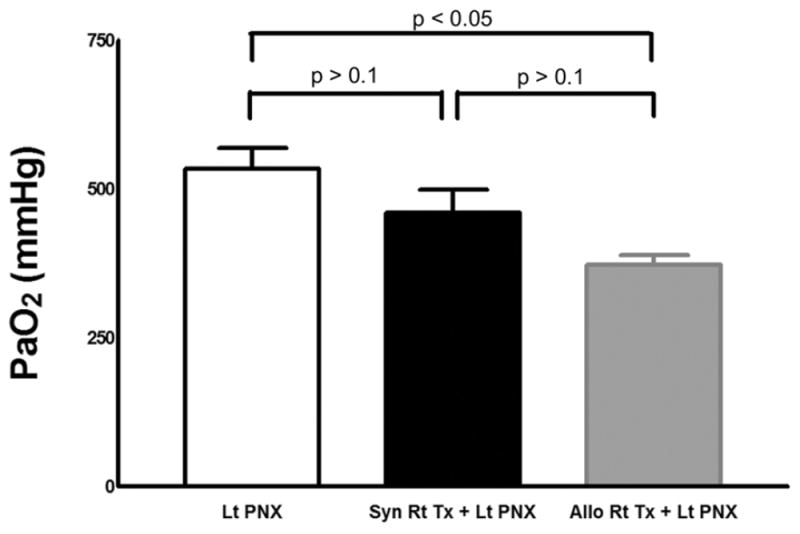
There were no statistically significant differences in PaO2 levels between wild-type B6 mice (n=3) and B6 recipients of syngeneic right B6 lungs 7 days after left pneumonectomy (n=3) (p>0.1). Lt PNX = left pneumonectomy; Syn Rt Tx + Lt PNX = right syngeneic lung transplantation + left pneumonectomy. PaCO2 levels were 35.2 ± 2.7 mmHg and 28 ± 6 mmHg for Lt PNX and Syn Rt Tx + Lt PNX, respectively. pH was 7.38 ± 0.02 and 7.35 ± 0.02 for Lt PNX and Syn Rt Tx + Lt PNX, respectively. Base deficits were 2.9 ± 1.8 and 8.5 ± 2.6 for Lt PNX and Syn Rt Tx + Lt PNX, respectively. PaO2 levels in MR1- & CTLA4-Ig-treated Balb/c → B6 right lung recipients following left pneumonectomy (n=3) (Allo Rt Tx + Lt PNX) were 373.1 ± 16.3 mmHg (p>0.1 vs. Syn Rt Tx + Lt PNX and p<0.05 vs. Lt PNX)., pCO2 levels were 28.3 ± 5.9, pH was 7.36 ± 0.03 and the base deficit was 8.3 ± 1.6.
Discussion
Outcomes after lung transplantation are markedly worse than those after transplantation of all other organs. Development of new experimental models in the mouse will be critical for the design of novel investigations in lung transplant biology. To this end, we and others have recently reported microsurgical techniques for orthotopic left lung transplantation in the mouse (5, 13, 14). Heterotopic mouse tracheal transplantation has been widely used as a model for lung transplantation (15). However, this model is not likely to mirror immunological and non-immunological events that lead to failure of vascularized human lung grafts. Unlike human lung transplants, tracheal transplants are not vascularized and are not exposed to the external environment. Airway epithelial cells in tracheal allografts undergo apoptosis leading to fibroproliferation and occlusion of the lumen. In stark contrast, we have recently reported that airway epithelial cells remain intact, maintain differentiation markers and also upregulate anti-apoptotic proteins in nonimmunosuppressed vascularized allografts that have undergone extensive vascular necrosis (11). These observations suggest that airway epithelial cells may be able to protect themselves and that insults in addition to the alloimmune response may be necessary to initiate changes in airway epithelial cells that eventually lead to fibrotic changes seen in bronchiolitis obliterans.
We have observed several similarities between vascularized mouse lung transplants and human lung transplants. Histological changes of acute rejection in mouse lungs are analogous to those observed in humans (5). Moreover, compared to orthotopic lung transplant models in the rat, important advantages of the mouse model are the wider availability of transgenic and knockout strains as well as reagents for cellular and molecular analysis. The mouse model has already allowed us to gain some new insight into requirements for acute lung rejection. Unlike the case for other organs, CD8+ T cells outnumber CD4+ T cells in both mouse and human lungs that undergo acute rejection (7, 16). Notably, CD4+ T cells are not necessary for the activation of alloreactive CD8+ T cells and for the acute rejection of mouse lung grafts (7). Clinical observations in combined heart-lung transplants in the 1980’s have revealed that lung rejection is initiated at earlier time points than cardiac rejection (17). A recent study by our group has provided a potential mechanism for this observation (6). While secondary lymphoid organs are critically important for the rejection of other organs including heart and skin alloreactive T cells can be primed locally within lung grafts (18). In fact, we have observed clustering of recipient T cells around graft-resident dendritic cells at early time points after engraftment indicating that the lung allograft represents a suitable environment for the activation of alloreactive T cells.
In this study we have modified techniques that we have previously described for the transplantation of the left lung (5, 13). Due to anatomic differences we found right lung transplantation to be technically more challenging, which also resulted in longer operative times for both donor and recipient procedures. Therefore, ischemic times are longer for right compared to left lung transplants when both donor and recipient operations are performed consecutively by the same surgeon. Specifically, the right pulmonary vein is markedly shorter than the left pulmonary vein. We found it technically impossible to insert the cuffed donor pulmonary vein into the main right pulmonary vein of the recipient animal or perform separate inferior and superior pulmonary venous anastomoses as has been previously described in the rat (19). Therefore, we opted to ligate the superior pulmonary venous branch and use the inferior branch for insertion of the donor vessel. We did not experience pulmonary venous outflow obstruction with this technique.
Bilateral lung transplantation in mice would most closely mirror bilateral sequential lung transplantation in humans, which is currently the most commonly employed technique worldwide. There are no published reports of successful bilateral lung transplantation in the rat and, in our hands, attempts at bilateral sequential lung transplantation in the mouse have resulted in intraoperative animal death. While further developments of microsurgical techniques may eventually result in successful bilateral lung transplantation in rodents, we had to rely on the removal of the native left lung in order to limit the recipient’s lung function to the transplanted graft.
Unlike previous descriptions for left pneumonectomies in naïve mice, we found it critical to place a piece of gelfoam into the left chest cavity of right lung recipients at the time of the left pneumonectomy in order to prevent mediastinal shifting, which in our hands led to the death of the animal in the immediate postoperative period (20). Similar to a previous report of right lung transplantation followed by left pneumonectomy in the rat we performed the left pneumonectomy after a two-week recovery period following the transplantation of the right lung (19). Attempts at left pneumonectomies less than two weeks after right lung transplantation resulted in death of the animals, which may be due to transiently impaired graft function secondary to reperfusion injury (21). A better understanding of molecular mechanisms contributing to ischemia reperfusion-related graft dysfunction may allow right lung recipients to tolerate a left pneumonectomy at earlier time points.
To our knowledge, there exist no reports of successful right pneumonectomies in the mouse, which is likely related to an inability of the left lung to compensate for the sudden loss of 70% of overall lung function. To this end, left lung transplant recipients did not survive a right pneumonectomy even after prolonged recovery periods. Allogeneic right lung recipients undergoing acute graft rejection were alive at 7 days after transplantation, but displayed respiratory distress. It is possible that a gradual loss of right graft function in these nonimmunosuppressed allogeneic recipients may allow for the development of some compensatory mechanisms by the native left lung (22).
It is important to point out that compensatory growth of the right lung has been well described after left pneumonectomies in mice. This process involves expansion of multiple cell populations such as endothelial and epithelial cells and results in complete restoration of the original lung volume (23). Compensatory lung growth is associated with the rapid upregulation of multiple genes and transcription factors (24). Therefore, processes regulating compensatory growth of the graft can influence right lung graft function following a left pneumonectomy and should be taken into consideration when designing studies and interpreting results utilizing this model. Similarly, compensatory lung growth may affect graft function after lobar transplantation in the pediatric population (25).
In conclusion, this is the first description of vascularized aerated transplantation of the right lung in the mouse. Histopathological changes observed in syngeneic and allogeneic grafts are virtually identical to those that we have reported after transplantation of the left lung (5). An important advantage of the right lung transplantation is that right lung recipients tolerate a left pneumonectomy where graft failure can result in animal death. Therefore, as we have demonstrated that right allograft recipients that are treated with costimulatory blockade can tolerate a left pneumonectomy, this model may be valuable for long term studies evaluating graft survival in immunosuppressed hosts. Thus, this model represents an important addition to the armamentarium in experimental lung transplantation.
Acknowledgments
We would like to thank Arlene Ligori for medical illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hardy JD, Eraslan S, Dalton ML, Jr, Alican F, Turner MD. Re-implantation and homotransplantation of the lung: laboratory studies and clinical potential. Ann Surg. 1963 May;157:707–18. doi: 10.1097/00000658-196305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan E, Lima O, Goldberg M, Ferdman A, Luk SK, Cooper JD. Successful revascularization of totally ischemic bronchial autografts with omental pedicle flaps in dogs. J Thorac Cardiovasc Surg. 1982 Aug;84(2):204–10. [PubMed] [Google Scholar]
- 3.Unilateral lung transplantation for pulmonary fibrosis. Toronto Lung Transplant Group. N Engl J Med. 1986 May 1;314(18):1140–5. doi: 10.1056/NEJM198605013141802. [DOI] [PubMed] [Google Scholar]
- 4.Mizuta T, Kawaguchi A, Nakahara K, Kawashima Y. Simplified rat lung transplantation using a cuff technique. J Thorac Cardiovasc Surg. 1989 Apr;97(4):578–81. [PubMed] [Google Scholar]
- 5.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007 Jun;7(6):1672–9. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 6.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009 Apr 1;182(7):3969–73. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008 Apr 1;180(7):4754–62. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Han WR, Murray-Segal LJ, Mottram PL. Modified technique for kidney transplantation in mice. Microsurgery. 1999;19(6):272–4. doi: 10.1002/(sici)1098-2752(1999)19:6<272::aid-micr3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Boisgerault F, Liu Y, Anosova N, Dana R, Benichou G. Differential roles of direct and indirect allorecognition pathways in the rejection of skin and corneal transplants. Transplantation. 2009 Jan 15;87(1):16–23. doi: 10.1097/TP.0b013e318191b38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki M, Gelman AE, Tietjens JR, Ibricevic A, Kornfeld CG, Huang HJ, et al. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. Am J Respir Cell Mol Biol. 2007 Dec;37(6):625–30. doi: 10.1165/rcmb.2007-0257RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voswinckel R, Motejl V, Fehrenbach A, Wegmann M, Mehling T, Fehrenbach H, et al. Characterisation of post-pneumonectomy lung growth in adult mice. Eur Respir J. 2004 Oct;24(4):524–32. doi: 10.1183/09031936.04.10004904. [DOI] [PubMed] [Google Scholar]
- 13.Krupnick AS, Lin X, Li W, Okazaki M, Lai J, Sugimoto S, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4(1):86–93. doi: 10.1038/nprot.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jungraithmayr WM, Korom S, Hillinger S, Weder W. A mouse model of orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg. 2009 Feb;137(2):486–91. doi: 10.1016/j.jtcvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol. 1993 Jun;142(6):1945–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Donnenberg VS, Burckart GJ, Zeevi A, Griffith BP, Iacono A, McCurry KR, et al. P-glycoprotein activity is decreased in CD4+ but not CD8+ lung allograft-infiltrating T cells during acute cellular rejection. Transplantation. 2004 Jun 15;77(11):1699–706. doi: 10.1097/01.tp.0000131163.43015.85. [DOI] [PubMed] [Google Scholar]
- 17.McGregor CG, Baldwin JC, Jamieson SW, Billingham ME, Yousem SA, Burke CM, et al. Isolated pulmonary rejection after combined heart-lung transplantation. J Thorac Cardiovasc Surg. 1985 Oct;90(4):623–6. [PubMed] [Google Scholar]
- 18.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000 Jun;6(6):686–8. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi AT, Mizuta T, Shirai M, Ishibashi-Ueda H, Machida H, Kawashima Y. Right lung transplantation followed by left pneumonectomy in the rat. Eur J Cardiothorac Surg. 1996;10(11):1011–4. doi: 10.1016/s1010-7940(96)80406-4. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai MK, Greene AK, Wilson J, Fauza D, Puder M. Pneumonectomy in the mouse: technique and perioperative management. J Invest Surg. 2005 Jul-Aug;18(4):201–5. doi: 10.1080/08941930591004485. [DOI] [PubMed] [Google Scholar]
- 21.Marck KW, Prop J, Wildevuur CR. Lung transplantation in the rat. III. Functional studies in iso- and allografts. J Surg Res. 1983 Aug;35(2):149–58. doi: 10.1016/0022-4804(83)90137-3. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai MK, Lee S, Arsenault DA, Nose V, Wilson JM, Heymach JV, et al. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2007 Mar;292(3):L742–7. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez LG, Le Cras TD, Ruiz M, Glover DK, Kron IL, Laubach VE. Differential vascular growth in postpneumonectomy compensatory lung growth. J Thorac Cardiovasc Surg. 2007 Feb;133(2):309–16. doi: 10.1016/j.jtcvs.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Fernandez LG, Dodd-o J, Langer J, Wang D, Laubach VE. Upregulation of hypoxia-induced mitogenic factor in compensatory lung growth after pneumonectomy. Am J Respir Cell Mol Biol. 2005 Mar;32(3):185–91. doi: 10.1165/rcmb.2004-0325OC. [DOI] [PubMed] [Google Scholar]
- 25.Binns OA, DeLima NF, Buchanan SA, Lopes MB, Cope JT, Marek CA, et al. Mature pulmonary lobar transplants grow in an immature environment. J Thorac Cardiovasc Surg. 1997 Aug;114(2):186–94. doi: 10.1016/S0022-5223(97)70143-0. [DOI] [PubMed] [Google Scholar]


