Abstract
Background
Heart failure is the leading cause of morbidity and mortality for adults with congenital heart disease (ACHD). Many patients are ineligible for transplantation, and those who are eligible often face long wait times with high wait-list morbidity. Durable mechanical circulatory support (MCS) may be an option for many patients. This systematic review evaluates the published literature on the use of durable MCS in teenagers and adults with congenital heart disease.
Methods
A comprehensive search of MEDLINE (PubMed), EMBASE, and the Cochrane Library was performed electronically in July 2015 and updated in March 2016, guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.
Results
Individual case reports and several case series identified 66 patients with ACHD treated with durable MCS. More than half were INTERMACS 1 or 2 at the time of implantation. Patients with Fontan repairs were more frequently classified as INTERMACS 1 or 2 (89% compared to 59% or less among other groups). Cases published after 2010 showed a trend toward less severe INTERMACS status, and patients were less likely to have received transplants by the time of reporting (31% compared to 61% prior). Durable MCS was implanted as bridge-to-transplant in 77%. Patients with Fontan repair accounted for 14% of cases.
Conclusion
Reports of durable MCS utilization in patients with ACHD are becoming more frequent and devices are being implanted in more stable patients. Reports are mostly case reports or small case series so reporting bias is likely and prospective protocoled reporting is needed.
Keywords: mechanical circulatory support, adult congenital, heart failure
INTRODUCTION
Despite improved survival into adulthood for patients born with congenital heart disease, heart failure remains the leading cause of death for patients with complex disease, and 20% of hospital admissions for patients with adult congenital heart disease (ACHD) are for heart failure (1, 2). The mechanisms and manifestations of heart failure in ACHD are different than those with acquired heart disease. Systolic ventricular failure is often due to failing systemic right ventricles or involves single ventricle physiology. Pulmonary vascular disease, restrictive cardiomyopathy, and shunts also contribute (2).
Heart transplant is the preferred treatment for ACHD patients with end-stage heart failure. However, many are ineligible for transplantation due to high levels of allo-sensitization, pulmonary vascular disease, or comorbidities such as cirrhosis (3). Patients with ACHD listed for heart transplant are typically listed at a lower UNOS priority and have longer wait times than those with acquired disease (4, 5).
In patients with advanced heart failure due to acquired heart disease, durable mechanical circulatory support (MCS) devices such as ventricular assist devices (VADs) and the total artificial heart (TAH) are effective, either as bridge-to-transplant (BTT, both VAD and TAH) or as destination therapy (DT, VAD only) (6, 7). Additionally, MCS allows for preferential listing in the current transplantation algorithms (8), potentially disadvantaging ACHD patients who are less likely to receive MCS.
Publication of MCS in ACHD is limited to case reports and small case series. To the authors’ knowledge, this is the first systematic review of the use durable MCS in ACHD. We hypothesized that the use of durable MCS is becoming more common in ACHD patients, and that most patients would still be awaiting transplant by the time of reporting, revealing MCS as an important bridging option for ACHD patients.
METHODS
The systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on June 14, 2016 (registration number CRD42016041330).
Literature Search and Study Selection
A comprehensive search of MEDLINE (PubMed), EMBASE, and the Cochrane Library was performed electronically in July 2015 and updated in March 2016 using combinations of the following MeSH terms: “congenital heart,” “adult congenital,” “Fontan,” “single ventricle,” “ventricular assist,” “mechanical circulatory support.” This search was performed with guidance from the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines (9). No publication year, study design, or language restrictions were placed on the search criteria. Publications of patients under 13 years of age or animals were excluded, as were papers reporting on temporary support including ECMO. Age 13 was chosen as the lower age limit because most somatic growth (which influences the type of device implanted) is complete by the teenage years. Reference lists of identified publications were examined to identify additional relevant studies. If more than one publication reported data on the same patient, the more complete publication was included. Two reviewers independently screened titles and abstracts, and then examined the full text of relevant publications.
Data Extraction and Analysis
Data were reviewed and extracted by two investigators. Discrepancies were discussed and resolved by consensus. The following data points were extracted: first author, year of publication, site of study group, subject age, sex, congenital heart disease diagnosis, type of corrective surgery, type of ventricular assist device, morphology of ventricle supported, whether this was a systemic or sub-pulmonic ventricle, whether the intention of MCS use was for destination or bridge therapy, whether the patient received a transplant and when, and vital status at the time of report. When possible, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) score was assigned based on patient description. INTERMACS profiles are as follows (10):
Critical cardiogenic shock
Progressive decline on inotropic support
Stable but inotrope dependent
Resting symptoms home on oral therapy
Exertion intolerant
Exertion limited
Advanced NYHA Class III symptoms
Data was analyzed by time period (1999–2010 and 2011–2016) as well as by type of prior surgical repair (atrial switch, Fontan, other/unreported, and none). These roughly 10- and 5-year analysis periods were chosen based on data availability and the timing of approval of the HeartWare HVAD (2012). Surgical repair was chosen rather than underlying congenital heart disease diagnosis because prior repair is most likely to determine needs and surgical considerations at the time of MCS implantation. Vital status was examined overall as well as based on whether the patient received a heart transplant. Survival was defined as alive at the time of reporting. It is important to note that in most cases no long term data are available. Data analysis was performed using Microsoft Excel (2010).
RESULTS
Fifty potential publications were identified in the search. Ten off-topic articles and 3 duplicate cases were excluded. Thirty-seven reports describing 66 patients (3, 11–46), published from 1999 - March 2016 were evaluated. These included contributions by groups in the United States, Japan, Australia, Argentina, and Europe. Most publications were individual case reports (3, 11, 14, 16–21, 23, 24, 26–32, 34, 37, 39, 41–46). Ten were case series (12, 13, 15, 22, 25, 33, 35, 36, 38, 40), the largest of which included seven patients (38). No previous systematic reviews or meta-analyses were identified.
The mean age of reported patients was 36±14 years. Eighty percent were male. The most common diagnoses were dextro- and levo- transposition of the great arteries (D-TGA and L-TGA, 79%). Patients with single ventricle physiology made up only 15% of cases. VADs most commonly described were the HeartWare HVAD (31%), the HeartMate II (28.4%), and the Heart Mate XVE (10%). TAH was described in 2 cases, with the remainder (27%) utilizing a variety of other VADs such as the DeBakey and Jarvik devices. Seventy-nine percent of VADs were inserted into systemic right ventricles. Most patients met criteria for INTERMACS 1 or 2 status at the time of implantation. Seventy-seven percent of patients were BTT, 9% were DT, and 14% were bridge-to-decision (BTD). Fewer than 40% of patients had received heart transplants at the time of report. Overall mortality for both MCS-supported and transplanted patients at the time of report was 24%. The average length of support for patients who received transplants was 275 days. The average length of support for patients who did not receive transplants and who were reported to be alive at the time of publication was 369 days, and for those deceased was 122 days.
Comparison Before and After 2010
Patient data was analyzed as two groups: cases published before 2011 (early group) and those published in 2011 and later (late group). Prior to 2011, 18 patients were reported (1.6 patients per year) compared with 48 patients reported after 2010 (8 patients per year). Patients reported after 2010 were older (mean age 39±13.3 compared to 28±12.4 years). Single ventricle patients or those with Fontan repairs comprised 17% of the early group and 12% of the late group.
Device type changed over time, consistent with the development of newer VADs. The HeartWare HVAD was the most commonly reported VAD after 2010 (44%). Both TAH patients were reported in 2014. Figure 1 shows this trend. Morphologic right ventricles were supported in 63% of the earlier group and 88% of the late group. There was a trend toward VAD implantation in less critically ill patients: more patients in the late group were classified as INTERMACS Class 2 or higher (71% vs 50% prior), shown in Figure 2. In the late group fewer devices were implanted as BTT (71% late vs 94% early). BTD implantations were only reported in the late group, accounting for 19% of cases. Finally, patients described after 2010 were less likely to have received transplants by the time of reporting (31% vs 61%). However, duration between device implantation and reporting was variable and often not reported, so this finding should be interpreted with caution. Survival at the time of reporting was similar over time with 71% of patients reported prior to 2011 alive at the time of reporting and 78% of patients alive in the reports since 2011 (Figure 3).
Figure 1.
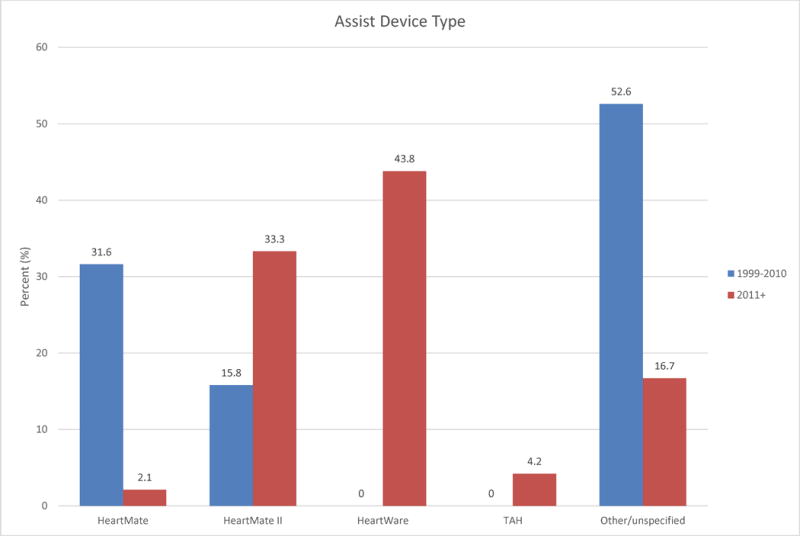
Comparison of assist device type between time periods 1999–2010 and 2011+. TAH = total artificial heart.
Figure 2.
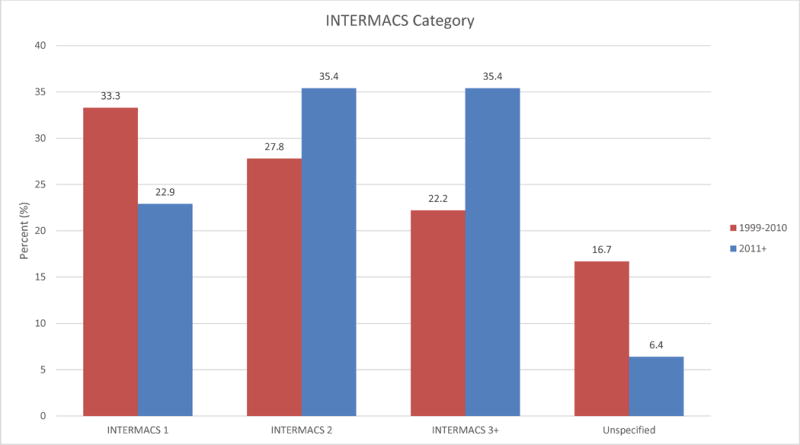
Comparison of INTERMACS scores between time periods 1999–2010 and 2011+. INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support.
Figure 3.
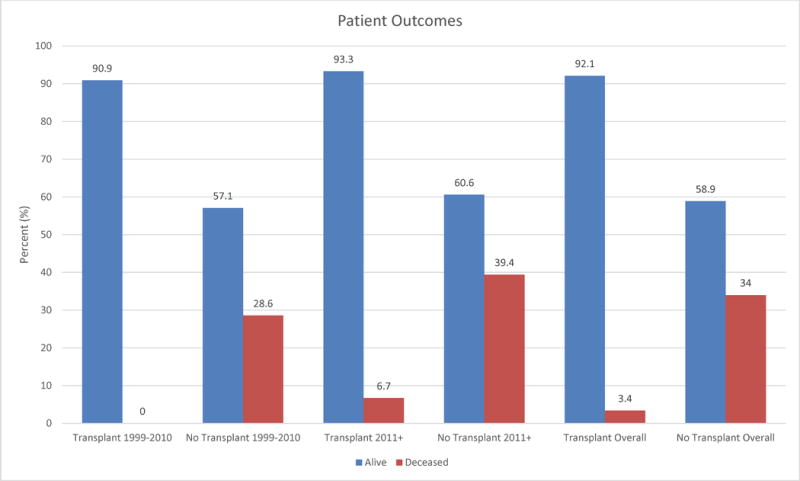
Comparison of patient outcomes regarding transplant and survival between time periods 1999–2010 and 2011+.
Comparison by Prior Surgical Repair
Data was also analyzed according to the type of surgical repair using the following groups: atrial switch procedure (n=32), Fontan operation (n=9), other/unknown (n=14), or none (n=11). Fontan patients were the youngest group, ten years younger than the atrial switch group and 30 years younger than patients with no prior surgery (mean age 20.2±6.4, 32.7±9.3, and 50.9±13 years respectively). Fontan patients were sicker than other groups: 89% were INTERMACS 1–2 compared to less than 60% in the other groups. Across all groups, devices were implanted as BTT 77% of the time. Patients implanted as BTT and who received transplants by the time of reporting were most commonly in the Fontan group (78%), and post-transplant survival for this group was 86%. Post-transplant survival was 100% for the atrial switch and other/unknown groups. Figure 4 displays many of these relationships. Overall survival regardless of transplantation status ranged from 64–78% among these groups.
Figure 4.

Clinical course and vital status (at the time of report) displayed by type of prior surgical repair.
*Vital status is unknown for 1 patient each from the atrial switch and Fontan groups.
Surgical Considerations
A number of publications reported details of surgical implantation. Most cases that offered surgical details reported the use of either epicardial (27) or transesophageal echocardiography to assist with cannula placement (13, 28, 29, 32), given the variability in optimal location compared to the left ventricular apex. The right ventricular free wall and diaphragmatic surface are both alternate options in order to best orient the inflow cannula toward the tricuspid valve (13, 16, 24, 29, 32, 35, 37). Regardless of the epicardial entrance point, implantation of devices into a systemic right ventricle may require resection of trabeculae to provide for unobstructed inflow. To avoid issues with damage to the liver or bowel, or chamber compression upon closing the chest, the device may be placed back-to-front (42), closer to midline (40), or through the right chest (36). Non-sternotomy approaches and non-aortic outflow graft positions have also been employed(45). Pretre et al described the creation of a VAD-driven sub-pulmonic chamber following takedown of a Fontan circuit (39). Valeske and Rossano both describe TAH implantation in Fontan patients, requiring creation of neo right atria that can be problematic due to lack of normal compliance (41, 43).
DISCUSSION
This systematic review reveals that in patients with ACHD, durable MCS utilization may be increasing, and short-term survival rates in published series are approximately 70%. This is in comparison to outcomes for acquired heart disease, where 1-year survival rates are approximately 80% (47).
Despite improvements in devices, surgical techniques, and management, durable MCS is employed far less often in ACHD patients compared to acquired disease patients (48). Reasons for this may include anatomic considerations related to prior complex repairs, heart position in the chest, devices designed for a morphologic left ventricle, and comorbidities of coagulopathy, malnutrition, and end-organ dysfunction. In addition, timing of referral may differ from those of acquired heart disease. ACHD patients are accustomed to living with their disease, and providers may not refer for MCS as aggressively. Because ACHD patients are referred for MCS less frequently and at a later disease state, ACHD patients who are ultimately listed for transplantation may have worse outcomes as a result of lower transplant listing status (4). However, it has been reported that, despite a higher risk profile, patients with ACHD who are bridged to transplant with MCS do not experience worse post-transplant outcomes compared to non-MCS ACHD patients (48). In carefully selected ACHD patients, successful MCS support is possible, further supported by the 66 patients analyzed in this study. Due to limited reported variables and publication bias, this data set is likely incomplete. However, it may guide a better understanding of the use of durable MCS in the ACHD population.
The use of MCS in ACHD patients appears to be increasing over time. This likely represents increased experience in the acquired disease population translating into familiarity with, and willingness to extrapolate use of these devices in the ACHD population. Patients reported since 2011 are approximately 10 years older than those reported prior to 2011, reflecting the aging ACHD population. Likewise, the types of devices reported vary according to availability of new and improved models that may enable increasing use in ACHD (13). INTERMACS scores have trended toward lower acuity, suggesting implantation earlier in the course of advanced heart failure, as has been recommended for patients with acquired disease. Rates of transplantation were lower in more recent years despite the majority of patients implanted with devices as BTT. This may reflect the increased number of patients listed for transplant without concurrent increases in organ availability. It may also attest to the improved durability of newer devices or better outcomes resulting from earlier implantation. Because follow-up on reported patients is inconsistent and incomplete, these findings are speculative.
There are only nine adult patients reported who received durable MCS after prior Fontan operation. The population of adults with Fontan circulation is increasing and will eclipse atrial switch patients as the dominant ACHD heart failure group in the next two decades. The mechanisms of Fontan heart failure are very different compared to heart failure in a biventricular circulation. Fontan patients are more likely to present in a decompensated state, as evidenced by higher INTERMACs scores. They typically have had numerous sternotomies, are chronically anticoagulated, and have hepatic and renal dysfunction related to the obligate elevated central venous pressure associated with long-standing single ventricle physiology (49, 50). Irreversible comorbid conditions and malnutrition are major barriers to the widespread utilization of advanced heart failure therapies in this group. This may explain the smaller numbers of Fontan patients reported, but the fact that twice as many were reported in the second time period compared to the first may represent expansion of durable MCS utilization to more complex populations.
Finally, the rising use of durable MCS in ACHD may help treat transplant-limiting pulmonary hypertension (28), support patients who experience long transplant wait times, and serve as destination therapy when contraindications to transplant are present (12). Shared information regarding provider experience is critical to improving outcomes.
Limitations
This is a retrospective review of the literature and most reports are in the form of individual case reports or small series. Publication bias is a likely limitation, as unsuccessful cases of durable MCS utilization may be underreported. Follow-up is short and in some cases incomplete. Small numbers limit conclusions drawn in this review to descriptive analyses. Patients with less complex ACHD such as coarctation or congenital aortic stenosis may be underreported as these patients more closely mirror those with acquired heart failure. The predominance of male patients examined in this review may be influenced by the prevalence of reported intervention on D-TGA, which is more common in males. Finally, survival described in these case reports is short-term (on average less than 1 year), which does not reflect long-term outcomes.
CONCLUSION
Durable MCS use in the ACHD population is increasing but publications are limited to case reports or small series. Prospective reporting is needed and could be achieved by the creation of an ACHD specific MCS database or by the inclusion of ACHD-specific data in the INTERMACS databases. Increasing device implantation also speaks to the need for multidisciplinary care of patients with ACHD, including advance care planning and treatment at specialized ACHD centers. Complex ACHD is not a contraindication to the use of durable MCS. Therefore, durable MCS should be considered as a treatment option early in the care of these patients. Additional research is needed regarding selection criteria, surgical technique, post-operative management, and long-term outcomes.
Acknowledgments
Funding: None
Footnotes
Conflicts of Interest:
Dr. Mahr: Investigator: St. Jude, HeartWare, SynCardia; Consultant: St. Jude, HeartWare, Abiomed.
Dr. Mokadam: Investigator for Medtronic (HeartWare), St. Jude, SynCardia, Consultant for Medtronic (HeartWare), St. Jude.
Authors not listed above report no relationships that could be construed as a conflict of interest.
References
- 1.Rossano JW, Woods RK, Berger S, Gaynor JW, Ghanayem N, Morales DL, et al. Mechanical support as failure intervention in patients with cavopulmonary shunts (MFICS): rationale and aims of a new registry of mechanical circulatory support in single ventricle patients. Congenit Heart Dis. 2013;8(3):182–6. doi: 10.1111/chd.12053. [DOI] [PubMed] [Google Scholar]
- 2.Krieger EV, Valente AM. Heart failure treatment in adults with congenital heart disease: where do we stand in 2014? Heart. 2014;100(17):1329–34. doi: 10.1136/heartjnl-2014-305667. [DOI] [PubMed] [Google Scholar]
- 3.Agusala K, Bogaev R, Frazier OH, Franklin WJ. Ventricular assist device placement in an adult with D-transposition of the great arteries with prior Mustard operation. Congenit Heart Dis. 2010;5(6):635–7. doi: 10.1111/j.1747-0803.2010.00408.x. [DOI] [PubMed] [Google Scholar]
- 4.Gelow JM, Song HK, Weiss JB, Mudd JO, Broberg CS. Organ allocation in adults with congenital heart disease listed for heart transplant: impact of ventricular assist devices. J Heart Lung Transplant. 2013;32(11):1059–64. doi: 10.1016/j.healun.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alshawabkeh LI, Hu N, Carter KD, Opotowsky AR, Light-McGroary K, Cavanaugh JE, et al. Wait-List Outcomes for Adults With Congenital Heart Disease Listed for Heart Transplantation in the U.S. J Am Coll Cardiol. 2016;68(9):908–17. doi: 10.1016/j.jacc.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 6.Mehta SM, Aufiero TX, Pae WE, Miller CA, Pierce WS. Combined Registry for the Clinical Use of Mechanical Ventricular Assist Pumps and the Total Artificial Heart in conjunction with heart transplantation: sixth official report–1994. J Heart Lung Transplant. 1995;14(3):585–93. [PubMed] [Google Scholar]
- 7.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Organ Procurement and Transplantation Network Policies. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_06: OPTN; 2016 [cited 2016 October 20]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27(10):1065–72. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Frazier OH, Gregoric ID, Messner GN. Total circulatory support with an LVAD in an adolescent with a previous Fontan procedure. Tex Heart Inst J. 2005;32(3):402–4. [PMC free article] [PubMed] [Google Scholar]
- 12.Harper AR, Crossland DS, Perri G, O’Sullivan JJ, Chaudhari MP, Schueler S, et al. Is alternative cardiac surgery an option in adults with congenital heart disease referred for thoracic organ transplantation? Eur J Cardiothorac Surg. 2013;43(2):344–51. doi: 10.1093/ejcts/ezs240. [DOI] [PubMed] [Google Scholar]
- 13.Joyce DL, Crow SS, John R, St Louis JD, Braunlin EA, Pyles LA, et al. Mechanical circulatory support in patients with heart failure secondary to transposition of the great arteries. J Heart Lung Transplant. 2010;29(11):1302–5. doi: 10.1016/j.healun.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Komagamine M, Nishinaka T, Ichihara Y, Nagashima M, Shimizu M, Shinohara T, et al. Ventricular assist device implantation late after double switch operation for L-transposition of the great arteries. Ann Thorac Surg. 2014;98(5):e109–11. doi: 10.1016/j.athoracsur.2014.07.082. [DOI] [PubMed] [Google Scholar]
- 15.Melissa Lyle RD, Grupper Avishay, Schettle Sarah, Stulak John, Joyce Lyle, Park Soon, Kushwaha Sudhir. Left Ventricualr Assist Device Therapy in Patients with Adult Congenital Heart Disease. Journal of the American College of Cardiology. 2014;63(12):A564. [Google Scholar]
- 16.Mohite PN, Popov AF, Garcia D, Hards R, Zych B, Khaghani A, et al. Ventricular assist device outflow graft in congenitally corrected transposition of great arteries - a surgical challenge. J Cardiothorac Surg. 2012;7:93. doi: 10.1186/1749-8090-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales DL, Adachi I, Heinle JS, Fraser CD. A new era: use of an intracorporeal systemic ventricular assist device to support a patient with a failing Fontan circulation. J Thorac Cardiovasc Surg. 2011;142(3):e138–40. doi: 10.1016/j.jtcvs.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Morris CD, Gregoric ID, Cooley DA, Cohn WE, Loyalka P, Frazier OH. Placement of a continuous-flow ventricular assist device in the failing ventricle of an adult patient with complex cyanotic congenital heart disease. Heart Surg Forum. 2008;11(3):E143–4. doi: 10.1532/HSF98.20071180. [DOI] [PubMed] [Google Scholar]
- 19.Newcomb AE, Negri JC, Brizard CP, d’Udekem Y. Successful left ventricular assist device bridge to transplantation after failure of a fontan revision. J Heart Lung Transplant. 2006;25(3):365–7. doi: 10.1016/j.healun.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Petrofski JA, Hoopes CW, Bashore TM, Russell SD, Milano CA. Mechanical ventricular support lowers pulmonary vascular resistance in a patient with congential heart disease. Ann Thorac Surg. 2003;75(3):1005–7. doi: 10.1016/s0003-4975(02)04372-2. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan N, Booth DC, Diaz-Guzman E, Hoopes CW. Successful ventricular assist device placement in transposition of the great arteries with pulmonary hypertension. Ann Thorac Surg. 2013;95(2):e47. doi: 10.1016/j.athoracsur.2012.08.062. [DOI] [PubMed] [Google Scholar]
- 22.Salzberg S, Lachat M, Zünd G, Oechslin E, Schmid ER, DeBakey M, et al. Left ventricular assist device as bridge to heart transplantation–lessons learned with the MicroMed DeBakey axial blood flow pump. Eur J Cardiothorac Surg. 2003;24(1):113–8. doi: 10.1016/s1010-7940(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 23.Schweiger M, Falk V, Biry M, Hübler M, Wilhelm MJ. Biventricular failure in dextro-transposition of the great arteries corrected with the Mustard procedure: VAD support of the systemic ventricle is enough. Int J Artif Organs. 2015;38(4):233–5. doi: 10.5301/ijao.5000400. [DOI] [PubMed] [Google Scholar]
- 24.Sehgal S, Ramachandran S, Leff JD. HeartWare Ventricular Assist Device Placement in a Patient With Corrected Dextro-Transposition of Great Arteries: A Case Report and Its Clinical Challenges. Semin Cardiothorac Vasc Anesth. 2015;19(3):243–7. doi: 10.1177/1089253214566886. [DOI] [PubMed] [Google Scholar]
- 25.Shah NR, Lam WW, Rodriguez FH, Ermis PR, Simpson L, Frazier OH, et al. Clinical outcomes after ventricular assist device implantation in adults with complex congenital heart disease. J Heart Lung Transplant. 2013;32(6):615–20. doi: 10.1016/j.healun.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Stokes MB, Saxena P, McGiffin DC, Marasco S, Leet AS, Bergin P. Successful Bridge to Orthotopic Cardiac Transplantation with Implantation of a HeartWare HVAD in Management of Systemic Right Ventricular Failure in a Patient with Transposition of the Great Arteries and Previous Atrial Switch Procedure. Heart Lung Circ. 2016;25(5):e69–71. doi: 10.1016/j.hlc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Tanoue Y, Jinzai Y, Tominaga R. Jarvik 2000 axial-flow ventricular assist device placement to a systemic morphologic right ventricle in congenitally corrected transposition of the great arteries. J Artif Organs. 2016;19(1):97–9. doi: 10.1007/s10047-015-0866-5. [DOI] [PubMed] [Google Scholar]
- 28.Arendt K, Doll S, Mohr FW. Failing Mustard circulation with secondary pulmonary hypertension: mechanical assist device to achieve reverse pulmonary vascular remodelling for subsequent heart transplantation. Heart. 2010;96(14):1164. doi: 10.1136/hrt.2009.184580. [DOI] [PubMed] [Google Scholar]
- 29.Dakkak AR, Sindermann JR, Dell’Aquila AM, Welp HA, Martens S, Scherer M. Implanting a Nonpulsatile Axial Flow Left Ventricular Assist Device as a Bridge to Transplant for Systemic Ventricular Failure After A Mustard Procedure. Exp Clin Transplant. 2015;13(5):485–7. doi: 10.6002/ect.2014.0043. [DOI] [PubMed] [Google Scholar]
- 30.George RS, Birks EJ, Radley-Smith RC, Khaghani A, Yacoub M. Bridge to transplantation with a left ventricular assist device for systemic ventricular failure after Mustard procedure. Ann Thorac Surg. 2007;83(1):306–8. doi: 10.1016/j.athoracsur.2006.03.119. [DOI] [PubMed] [Google Scholar]
- 31.Gregoric ID, Kosir R, Smart FW, Messner GN, Patel VS, La Francesca S, et al. Left ventricular assist device implantation in a patient with congenitally corrected transposition of the great arteries. Tex Heart Inst J. 2005;32(4):567–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J, Slaughter MS. HeartWare ventricular assist device placement in a patient with congenitally corrected transposition of the great arteries. J Thorac Cardiovasc Surg. 2013;145(2):e23–5. doi: 10.1016/j.jtcvs.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Huebler M, Stepanenko A, Krabatsch T, Potapov EV, Hetzer R. Mechanical circulatory support of systemic ventricle in adults with transposition of great arteries. ASAIO J. 2012;58(1):12–4. doi: 10.1097/MAT.0b013e31823a82df. [DOI] [PubMed] [Google Scholar]
- 34.Jouan J, Grinda JM, Bricourt MO, Iserin L, Fabiani JN. Non-pulsatile axial flow ventricular assist device for right systemic ventricle failure late after Senning procedure. Int J Artif Organs. 2009;32(4):243–5. doi: 10.1177/039139880903200409. [DOI] [PubMed] [Google Scholar]
- 35.Maly J, Netuka I, Besik J, Dorazilova Z, Pirk J, Szarszoi O. Bridge to transplantation with long-term mechanical assist device in adults after the Mustard procedure. J Heart Lung Transplant. 2015;34(9):1177–81. doi: 10.1016/j.healun.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Menachem JN, Swaminathan AC, Bashore TM, Ward CC, Rogers JG, Milano CA, et al. Initial Experience of Left Ventricular Assist Device Support for Adult Patients with Transposition of the Great Vessels. Congenit Heart Dis. 2015;10(5):382–6. doi: 10.1111/chd.12264. [DOI] [PubMed] [Google Scholar]
- 37.Neely RC, Davis RP, Stephens EH, Takayama H, Khalpey Z, Ginns J, et al. Ventricular assist device for failing systemic ventricle in an adult with prior mustard procedure. Ann Thorac Surg. 2013;96(2):691–3. doi: 10.1016/j.athoracsur.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 38.Peng E, O’Sullivan JJ, Griselli M, Roysam C, Crossland D, Chaudhari M, et al. Durable ventricular assist device support for failing systemic morphologic right ventricle: early results. Ann Thorac Surg. 2014;98(6):2122–9. doi: 10.1016/j.athoracsur.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Prêtre R, Häussler A, Bettex D, Genoni M. Right-sided univentricular cardiac assistance in a failing Fontan circulation. Ann Thorac Surg. 2008;86(3):1018–20. doi: 10.1016/j.athoracsur.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Stewart AS, Gorman RC, Pocchetino A, Rosengard BR, Acker MA. Left ventricular assist device for right side assistance in patients with transposition. Ann Thorac Surg. 2002;74(3):912–4. doi: 10.1016/s0003-4975(02)03671-8. [DOI] [PubMed] [Google Scholar]
- 41.Valeske K, Yerebakan C, Mueller M, Akintuerk H. Urgent implantation of the Berlin Heart Excor biventricular assist device as a total artificial heart in a patient with single ventricle circulation. J Thorac Cardiovasc Surg. 2014;147(5):1712–4. doi: 10.1016/j.jtcvs.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Wiklund L, Svensson S, Berggren H. Implantation of a left ventricular assist device, back-to-front, in an adolescent with a failing mustard procedure. J Thorac Cardiovasc Surg. 1999;118(4):755–6. doi: 10.1016/S0022-5223(99)70027-9. [DOI] [PubMed] [Google Scholar]
- 43.Rossano JW, Goldberg DJ, Fuller S, Ravishankar C, Montenegro LM, Gaynor JW. Successful use of the total artificial heart in the failing Fontan circulation. Ann Thorac Surg. 2014;97(4):1438–40. doi: 10.1016/j.athoracsur.2013.06.120. [DOI] [PubMed] [Google Scholar]
- 44.Levin Ricardo Luis, D MA, Salvaggio Flavio, Blanco Norberto, Botbol Alejandro, Balaguer Jorge. Systemic Ventricular Assistance Using a HeartMate 2 Device as Bridge to Transplant in a Congenitally Corrected Transposition of the Great Vessels. Argentine Journal of Cardiology. 2012;80(5) [Google Scholar]
- 45.Hermsen JL, Smith JW, Pal JD, Rubio A, Masri SC, Mokadam NA, et al. Temporary and Durable Mechanical Circulatory Support for Single Ventricular Failure in an Adult. The VAD Journal. 2016 [Google Scholar]
- 46.Halaweish I, Ohye RG, Si MS. Berlin heart ventricular assist device as a long-term bridge to transplantation in a Fontan patient with failing single ventricle. Pediatr Transplant. 2015;19(8):E193–5. doi: 10.1111/petr.12607. Epub 2015/09/26. [DOI] [PubMed] [Google Scholar]
- 47.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–504. doi: 10.1016/j.healun.2015.10.003. Epub 2015/10/08. [DOI] [PubMed] [Google Scholar]
- 48.Maxwell BG, Wong JK, Sheikh AY, Lee PH, Lobato RL. Heart transplantation with or without prior mechanical circulatory support in adults with congenital heart disease. Eur J Cardiothorac Surg. 2014;45(5):842–6. doi: 10.1093/ejcts/ezt498. [DOI] [PubMed] [Google Scholar]
- 49.Pike NA, Evangelista LS, Doering LV, Koniak-Griffin D, Lewis AB, Child JS. Clinical profile of the adolescent/adult Fontan survivor. Congenit Heart Dis. 2011;6(1):9–17. doi: 10.1111/j.1747-0803.2010.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen SB, Ginde S, Bartz PJ, Earing MG. Extracardiac complications in adults with congenital heart disease. Congenit Heart Dis. 2013;8(5):370–80. doi: 10.1111/chd.12080. [DOI] [PubMed] [Google Scholar]


