Abstract
The Coronin family of proteins were first noted for their role in pathogen–host interactions and for modulating actin dynamics. Recently, however, Coronins have been found in a greater variety of cell types, and novel roles for the Coronins within the nervous system have been discovered. In the immune system, Coronin-1a enables Mycobacterium tuberculosis to evade lysosomal destruction. This activity appears to be analogous to protection of the NGF–TrkA signaling endosome during sympathetic nervous system development that is required for survival signaling. Similarly, others have implicated Coronin-1a in GPCR signaling during the formation of excitatory connections in the central nervous system. Its role in multiple signaling pathways suggests that it may influence cross talk between key pathways (TrkA, GPCRs) during neurodevelopment. Here, we review the role of Coronin-1a in neural development and function.
1. INTRODUCTION
Coronin family members have been principally described to play critical roles in regulating actin filament dynamics and cargo internalization (BoseDasgupta & Pieters, 2014a, 2014b; Chan, Creed, & Bear, 2011; Gandhi, Achard, Blanchoin, & Goode, 2009; Yan, Di Ciano-Oliveira, Grinstein, & Trimble, 2007). Beyond regulating cytoskeletal rearrangements, there is an emergent role for Coronin-1a in mediating a range of signaling events. A role for Coronin-1a in signal transduction was first described in the context of pathogen–host interaction by Pieters and colleagues who found Coronin-1a to be a phagosomal coat protein for Mycobacterium tuberculosis engulfed by macrophages (Ferrari, Langen, Naito, & Pieters, 1999; Jayachandran et al., 2007). By recruiting Coronin-1a, these pathogenic phagosomes induce calcium–calcineurin signaling to evade fusion with lysosomes, thereby allowing for propagation of the disease. Coronin family members are also highly expressed in the nervous system, yet their roles in neuronal signaling are only beginning to be described. We and others have found that in neurons Coronin-1a facilitates second messenger pathways such as IP3-calcium and cAMP-PKA. These findings appear to be unraveling new functions of Coronin within the nervous system. This chapter will review: (1) the Coronin family of proteins, (2) Coronin-1a as a cytoskeletal regulator, and (3) Coronin-1a as an effector protein of several different signaling pathways. The emphasis will be on signaling within the nervous system; however, we will draw on findings from nonneuronal cells such as fibroblasts and immune cells to illustrate conserved functions of the Coronin family and to elucidate future research directions.
2. THE CORONIN FAMILY OF PROTEINS
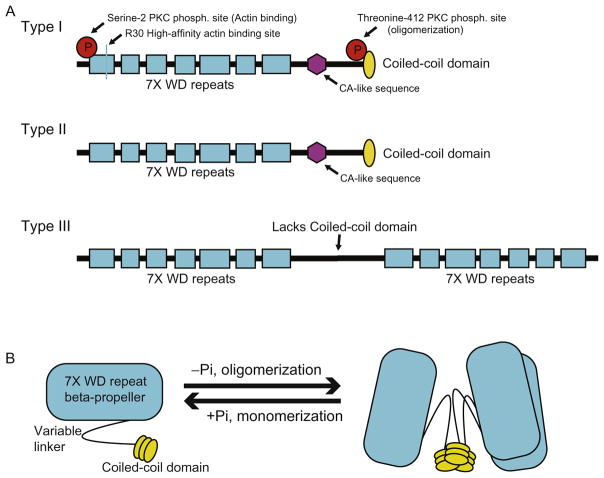
The highly conserved Coronin family of proteins has roles in cell motility, phagocytosis, vesicular trafficking, and cytokinesis (Chan et al., 2011; de Hostos et al., 1993; Yan et al., 2007). Humans express seven paralogs of Coronin, grouped into types I, II, and III. Structurally, all seven Coronin family members contain an N-terminal seven-bladed beta-propeller region made up of seven WD40 repeats followed by a variable linker region. Types I and II Coronins contain a coiled-coil C-terminus necessary for homodimer and trimer formation, while type III has an additional WD40 repeat domain at the C-terminus (Fig. 1A and B) (Appleton, Wu, & Wiesmann, 2006; BoseDasgupta & Pieters, 2014a, 2014b; Yee et al., 2014). de Hostos (1999) named Coronins 1–5, but the discovery of new Coronin family members and the expanding knowledge of their structure and function has led to the replacement of this nomenclature with the naming system established by The Human Genome Organization Committee. Coronin family members are now named based on their phylogenetic relationships. The official names and common alternatives are shown in Table 1. The structural differences, expression profile, subcellular localization, and general function of each Coronin type will be briefly elaborated upon here. However, the majority of this chapter will focus on Coronin-1a as a critical signaling effector protein in nervous system development and function.
Fig. 1.
(A) Structural characteristics of each Coronin family subtype. Highlighted are the conserved phosphorylation sites and high-affinity F-actin binding site (R30) on type I Coronins. (B) Coronin oligomerization occurs via coiled-coil domain interactions. Phosphorylation disrupts coiled-coil interactions and induces monomerization.
Table 1.
Coronin Family Nomenclature
| Species | HGNC Name | NCBI Gene ID | Gene Symbol | Type | Aliases | de Hostos Naming |
|---|---|---|---|---|---|---|
| Homo sapiens | Coronin-1a | 11151 | CORO1A | Type I | p57, IMD8, TACO, CLABP, ClipinA | Coronin1 |
| Coronin-1b | 57175 | CORO1B | Type I | Coronin-2 | Coronin2 | |
| Coronin-1c | 23603 | CORO1C | Type I | HCRNN4 | Coronin3 | |
| Coronin-2a | 7464 | CORO2A | Type II | IR10, WDR2, ClipinB | Coronin4 | |
| Coronin-2b | 10391 | CORO2B | Type II | ClipinC | Coronin5 | |
| Coronin-6 | 84940 | CORO6 | Type I | ClipinE, Coro1D | N/A | |
| Coronin-7 | 79585 | CORO7 | Type III | CRN7, POD1 | N/A | |
| Drosophila | POD-1 | 31620 | pod1 | Type III | Coronin-7, CG4532 | N/A |
| Coronin | 35596 | Coro | Type I-like | Coronin-8, CG9446 | N/A |
2.1 Type I Coronin Family
Type I Coronin family members include Coronin-1a, -1b, -1c, and -6. Type I family members display broad overlapping and nonoverlapping expression profiles. While Coronin-1b and -1c are ubiquitously expressed, Coronin-1a seems to have a more restricted expression profile. Of particular note is the expression of Coronin-1a in T cells, thymocytes, macrophages, neutrophils, neurons, and endothelial cells (Jayachandran & Pieters, 2015; Moshous & de Villartay, 2014; Suo et al., 2014). Interestingly, many of the cells expressing Coronin-1a are polarized and require active cytoskeleton rearrangement and protein transport.
The Coronin family members are structurally similar and contain a WD40-repeat containing beta-propeller surface capable of binding F-actin. This surface has been most highly characterized in type I Coronins. High-affinity F-actin binding in type I Coronins is conferred by conserved residues along the beta-propeller surface (Gandhi, Jangi, & Goode, 2010). Type I family members are typically localized to protrusions of the cell membrane where they have been reported to modulate actin dynamics in nonpolarized cells (Chan et al., 2011; Terzi, Kocaefe, & Ayter, 2014). The localization and activity of type I Coronins are typically regulated by protein kinase C (PKC) phosphorylation. Phosphorylation at an N-terminal serine residue disrupts interactions with actin (Ser2 in Coronin-1a, -1b, and -6), while phosphorylation at a threonine in the coiled-coil trimerization domain (Thr412 in Coronin-1a, variations in Coronin-1c and -6) prevents Coronin oligomerization (BoseDasgupta & Pieters, 2014a, 2014b; Cai, Holowecky, Schaller, & Bear, 2005; Oku et al., 2012; Yee et al., 2014) (Fig. 1B). Importantly, Ser2 phosphorylation has not been observed in Coronin-1c. This suggests that Coronin family members may have overlapping but distinct roles in part due to differences in the regulation of their activity. Other regulatory sites include acetylation at the N-terminus of Coronin-1b and Coronin-6 and at the C-terminus of Coronin-1c, but a specific regulating acetyltransferase has not been identified (Chan et al., 2011). As a monomer, Coronin is incapable of binding F-actin and is redistributed from the subcortical network to the cytosol. This cytosolic localization has been shown to impact its normal function in the immune response as well as to inactivate its ability to regulate actin dynamics (BoseDasgupta, Suzette, Jenoe, & Pieters, 2015; Chan et al., 2011). Implications of this redistribution will be further discussed in Section 4.
2.2 Type II Coronins
Type II Coronin family members include Coronin-2a and -2b. Coronin-2a is expressed ubiquitously, while Coronin-2b is enriched in the nervous system (Chan et al., 2011; Marshall, Aloor, & Bear, 2009). Though lacking the same high-affinity actin binding sites in the propeller region seen in type I Coronins, both Coronin-2a and -2b bind F-actin via uncharacterized beta-propeller surfaces and induce ADF/cofilin activity along ADP-actin-containing filaments while promoting Arp2/3 nucleation activity along ADP+Pi-actin filaments (Cai, Makhov, & Bear, 2007). This process is further elaborated upon in Section 3. Because of this dual role in actin dynamics, Coronin-2a is known to mediate focal adhesion turnover during cell migration and assist in chemokine-mediated T-cell migration (Huang et al., 2011; Shiow et al., 2008). Coronin-2b is not well characterized; however, in brain tissue samples, Coronin-2b coimmunoprecipitates with a focal adhesion protein, vinculin. Additionally, immunocytochemistry shows that Coronin-2b localizes to neurite tips (Nakamura, Takeuchi, Muraoka, Takezoe, & Mori, 1999). These data suggest that Coronin-2b may perform a similar role as Coronin-2a in actin reorganization, but acts within the nervous system to affect neurite growth and growth cone advance.
2.3 Type III Coronin
The Type III classification includes the long and structurally distinct isoform of Coronin, Coronin-7. Unlike type I and type II Coronins, Coronin-7 lacks a coiled-coil domain and instead contains two beta-propeller WD-repeat regions connected by a flexible linker (Rybakin et al., 2004). This Coronin family member is the most highly conserved across species, but interestingly has different functions in mammalian vs nonmammalian organisms. In Drosophila, Coronin-7 localizes to growth cones and cross-links actin filaments and microtubules (Rothenberg, Rogers, Vale, Jan, & Jan, 2003). Mammalian Coronin-7 does not bind actin, but instead has been localized to the cis-Golgi network and is suspected to play a role in secretory vesicle trafficking. This seems likely due to its WD-repeat sequence homologies with other secretory proteins, namely rabconnectin3 and α- and β′-COP (Rybakin et al., 2004). Coronin-7 also seems to be ubiquitously expressed, with the highest expression in the thymus and the brain. Specifically, Coronin-7 is most abundant in the hypothalamus, striatum, and locus coeruleus. Interestingly, its upregulation has been associated with increased food intake and obesity in mouse models (Eriksson et al., 2015).
3. TYPE I CORONINS AS ACTIN REGULATORS
Coronin-1a was first characterized by its role in modulating actin filament organization and dynamics (see Box 1 for general description of actin assembly) (de Hostos, Bradike, Lottspeich, Guggenheim, & Gerisch, 1991; de Hostos et al., 1993). Coronin promotes the formation of protrusions at the leading edge of migrating cells, aids in remodeling actin during the formation of cellular junctions, and is involved in endocytosis and endosomal tubulation (Chan et al., 2011). In order to enable these processes to occur, Coronin functions both to stabilize and to destabilize actin filaments (Gandhi et al., 2009; Liu, Needham, May, & Nolen, 2011; Mikati, Breitsprecher, Jansen, Reisler, & Goode, 2015). Coronin accomplishes these dual roles by influencing the binding of actin filament modifiers to filaments (e.g., cofilin and Arp2/3) in a manner that depends on the adenine nucleotide associated with individual actin subunits of filaments, and by recruiting the regulators of these actin modulators (e.g., Slingshot) (Cai et al., 2007; Gandhi et al., 2009). Coronin binds to ADP+Pi- and ADP-actin subunits via two different surfaces on its beta-propeller (Gandhi et al., 2009). When bound to ADP-actin, Coronin promotes actin destabilization through two mechanisms. First, Coronin enhances filament severing by providing additional and more favorable binding surfaces for cofilin (Gandhi et al., 2009). Second, Coronin recruits the phosphatase activator of cofilin, Slingshot (Gandhi et al., 2009). The opposite effect has been observed in yeast when Coronin is bound to ADP+Pi-actin. The conformation Coronin adopts sterically hinders cofilin binding, and the C-terminal coiled-coil provides an additional handle for Arp2/3 binding (Liu et al., 2011; Mikati et al., 2015). Several other studies in yeast have demonstrated that removing the coiled-coil domain enhances cofilin activity while impairing Arp2/3 binding, confirming that the coiled-coil domain interacts differently with these actin modifiers (Gandhi et al., 2009; Mikati et al., 2015).
BOX 1. Actin Assembly.
Actin assembly is a continuous, highly dynamic process that can be divided into three general phases: nucleation, elongation, and steady state (Cooper, 2000). Actin itself comes in two forms, a monomeric unit, G-actin, and a polymeric filament, F-actin. In both forms, actin subunits can be bound to ATP, ADP+Pi, or ADP, and these different ATP-binding states determine which actin modifiers are bound, and thus whether assembly or disassembly is favored (Rottner & Stradal, 2016). Actin monomers bound to ATP exhibit higher affinity for one another and typically promote assembly (Cooper, 2000). Because actin is an ATPase, it slowly hydrolyzes ATP to ADP, thus favoring its disassembly over time. Nucleation involves the polymerization of three or more ATP-bound G-actin monomers into an F-actin filament. This assembly is spontaneous, but it is facilitated in vivo by factors like the Arp2/3 complex, which, together with nucleation promoting factors (NPFs) such as WASP and Scar/WAVE, promotes filament initiation along the sides of existing actin filaments (Rottner & Stradal, 2016). Filament elongation is promoted largely by the formin family of proteins or Ena/VASP proteins. Some formins are involved in the reallocation of monomers to the plus end to establish an actin monomer concentration that favors actin assembly. New actin branches formed during elongation are stabilized by cortactin, an NPF subtype (Cooper, 2000). The third actin phase is steady state, also referred to as “treadmilling,” and it occurs when monomers are removed from the minus end, at the same rate monomers are assembled at the plus end (Cooper, 2000). This “treadmilling” effect is considered an in vitro concept, however, because actin modulators will continuously alter this steady state. Actin disassembly is mediated by ADF/cofilin proteins, with accessory factors, that sever actin filaments, thereby increasing the number of minus ends and promoting (Mikati et al., 2015).
It should be noted that the majority of our understanding of the role of Coronin in actin modification has been established in yeast. Mammalian systems suggest that it may play a broader and more dynamic role. For one, Coronin stabilization of Arp2/3 binding at ADP+Pi-actin filaments has not been confirmed to occur in mammals; however, it has been shown that Coronin interacts with and replaces Arp2/3 at ADP+Pi filaments (Cai, Makhov, Schafer, & Bear, 2008). This would provide several advantages. For one, Coronin would act as a temporary stabilizer of newly formed branches that is also capable of recruiting Slingshot, thus enabling the dynamic nature of actin by allowing for the gradual disassembly of branches and turnover of actin filaments (Cai et al., 2008). Another key aspect of Coronin replacing Arp2/3 is its flexibility. Normally new actin branches form at a 70-degree angle when bound to Arp2/3, but stabilization of branches by Coronin allows for a greater variety of angles to be established (Cai et al., 2008). This is advantageous in that it allows the cell to rapidly adjust the actin network in order to accommodate newly formed cellular adhesions, trafficking of vesicles, or unique morphological events (such as those that occur during apoptosis) (Cai et al., 2008).
Whether the cellular functions Coronin promotes during actin dynamics bears a more significant and interrelated effect on cell signaling requires first that our understanding of its interactions with different actin modulators is more thoroughly understood. Evidence, thus far, heavily implicates Coronin, cofilin, and other actin modifiers in recycling and transcytosis events in neurons, but how these functions relate to neurotrophin signaling remains an open question (Box 2).
BOX 2. Neurotrophin Signaling in the Development of the PNS.
In vertebrates, formation of a functional peripheral nervous system is achieved by an overproduction of neurons followed by a 50% die off. Which neurons are destined to live or die? Upon final target organ innervation, a competition ensues whereby neurons compete for target-derived trophic factors. The neurons that receive sufficient trophic factor survive and those that do not undergo apoptosis (Levi-Montalcini & Booker, 1960). Nerve growth factor is one such trophic factor that has been broadly studied in the context of sympathetic and sensory neuron development. NGF transduces its signal by binding to the TrkA receptor tyrosine kinase at the distal axon. For survival signaling to occur, the NGF–TrkA complex must internalize into what is commonly referred to as the signaling endosome, and then traffic back to the cell body via dynein to induce survival pathways like the phosphorylation of CREB (Lonze & Ginty, 2002).
Given that axons often arrive to targets nearly simultaneously and have comparable access and responsiveness to trophic factor, what determines which neurons win and which neurons lose? We demonstrated that a series of positive feedback loops are critical to create a bistability-destined to live or die (Deppmann et al., 2008). In the first feedback loop, NGF signaling regulates the expression of its own receptor, TrkA. This affects the robustness of neurotrophin signaling. The second feedback loop found to be critical for competition for survival was one whereby NGF regulated its own signal duration. This was attributed to an increase in signaling endosome stability and Coronin-1a upregulation (Suo et al., 2014).
4. CORONIN-1A AS A SIGNALING EFFECTOR PROTEIN
A growing body of work suggests that Coronin-1a is required for a range of receptor-mediated signaling pathways. This section will focus primarily on how Coronin-1a mediates signaling from GPCRs (b2-adrenergic receptor) and RTKs (TrkA). The extent to which Coronin paralog functions overlap remains unclear; however, the phenotypes from knockout mice described later suggest that, at least in some cell types, Coronin-1a is not functionally redundant with other family members.
4.1 Coronin-1a in Pathogen–Host Signaling
The role of Coronin-1a as a signaling effector protein was first described in the context of pathogen–host interaction. Using M. tuberculosis infection of macrophages as a model, Pieters and colleagues have elucidated several facets of Coronin-1a signaling that are required for Coronin-1a function and which are also conserved in neural signaling.
Upon engulfment of M. tuberculosis by macrophages, pathogenic phagosomes recruit Coronin-1a to their membrane, allowing them to evade lysosomal fusion. Coronin-1a then mediates calcium release and calcineurin activation, and this pathway is required for protection from the lysosome. Loss of Coronin-1a, calcium, or calcineurin activity is sufficient to drive pathogenic phagosomes toward lysosomal fusion (Jayachandran & Pieters, 2015; Jayachandran et al., 2007). Interestingly, Coronin-1a must be oligomerized into a trimer for this protection to occur. Disruption of trimerization using point mutants that either mimic constitutive phosphorylation or coiled-coil deletions render Coronin-1a unable to activate Ca2+/calcineurin and protect the pathogenic phagosome from lysosomal fusion (BoseDasgupta & Pieters, 2014a, 2014b). It is unclear whether these mutants are capable of binding F-actin or membranes more generally. Because many Coronin functions require membrane association, this suggests that trimerization may be required for functions beyond cytoskeletal rearrangement.
4.2 Coronin-1a as a Regulator of Internalization Mechanisms
In the context of pathogen–host interaction, Coronin-1a not only prevents lysosomal fusion but is also an important regulator in the switch from phagocytosis to macropinocytosis (BoseDasgupta & Pieters, 2014a, 2014b). During the initial stages of an infection by M. tuberculosis, macrophages primarily internalize cargo, including mycobacterium, via phagocytosis. However, phagocytosis is an ineffective mechanism for clearing bacterial infections because of the ability of mycobacteria to evade phagosome/lysosomal fusion and because of its reliance on receptor-mediated internalization. Macropinocytosis, however, presents an efficient mechanism to clear infectious material as it is nonsaturatable and independent of receptor binding. Indeed, after prolonged exposure to infection the immune system mobilizes cytokine signaling which subsequently reprograms the major internalization mechanism from phagocytosis to macropinocytosis by stimulating PKC activity (BoseDasgupta & Pieters, 2014a, 2014b). PKC activity increases PI3K activity, and PI3K promotes macropinocytosis by generating PIP3 at the membrane. This helps create the membrane ruffles necessary for macropinocytosis and shifts internalization away from phagocytosis. Macropinosomes containing mycobacteria are able to fuse with the lysosome such that macrophages can effectively clear the infection.
Coronin-1a has emerged as a potentially key component in the switch to macropinocytosis. A phosphomimetic form of Coronin-1a, in the absence of cytokine signaling, is sufficient to initiate PI3K signaling and switch the internalization mechanism to macropinocytosis (BoseDasgupta & Pieters, 2014a, 2014b). As mentioned previously, PKC phosphorylates Coronin-1a, thereby disrupting its trimerization and preventing Ca2+/calcineurin signaling (BoseDasgupta & Pieters, 2014a, 2014b). Additionally, PI3K, which is necessary for this switch to macropinocytosis, is known to be inhibited by Ca2+/calcineurin signaling. At this time, it is unclear whether decreased Ca2+/calcineurin signaling following Coronin-1a inactivation lifts inhibition of PI3K activity, or whether Coronin-1a interacts directly with PI3K to activate it after monomerizing and redistributing to the cytosol (BoseDasgupta et al., 2015). This relationship between PI3K and Coronin-1a has been observed in other systems, i.e., axon growth, which will be elaborated upon in Section 4; however, the mechanism by which this interaction occurs is equally elusive (Suo, Park, Young, Makita, & Deppmann, 2015).
4.3 Coronin-1a Is Required for GPCR Activation in Excitatory Neurons
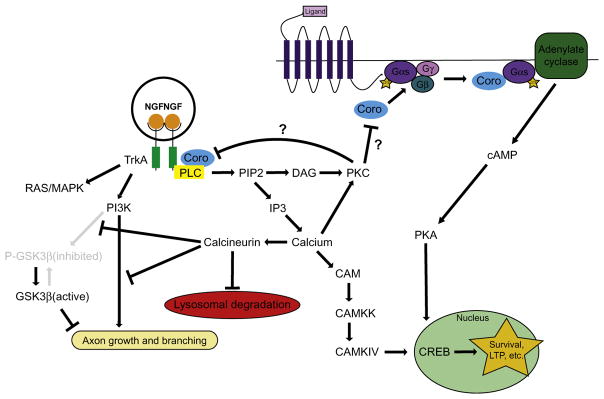
Coronin-1a has recently been shown to impact the development of excitatory synaptic connections in the central nervous system. Coronin-1a knockout mice display a dramatic loss of excitatory connections resulting in decreased anxiety and social interactions, increased aggression, and learning deficits (Jayachandran et al., 2014). This effect is attributed to a requirement of Coronin-1a for activation of cAMP/PKA signaling after activation of G protein-coupled receptors (GPCRs). Without Coronin-1a, both cAMP/PKA signaling and downstream signaling pathways, such as CREB phosphorylation, were lost (Jayachandran et al., 2014). A deficit in CREB phosphorylation has also been observed in peripheral neurons isolated from Coronin-1a knockout mice, but a link to GPCR signaling was not investigated in those studies (Suo et al., 2014). Because Coronin-1a shares homology with the Gβ subunit, Pieters and colleagues suggest that Coronin-1a may activate Gα-s and adenylate cyclase by displacing Gβ after GPCR activation (Jayachandran et al., 2014) (Fig. 2). This claim is supported by coimmunoprecipitation of Coronin-1a with Gα-s but not Gα-i (Jayachandran et al., 2014). Notably, the behavioral phenotypes observed in the Coronin-1a knockout mouse could be rescued by infusing these animals with the cAMP analog, 8-Br-cAMP (Jayachandran et al., 2014).
Fig. 2.
Schematic of the role of Coronin-1a in NGF–TrkA and beta-adrenergic GPCR signaling pathways in neurons. It is not known whether activation of PKC by Coronin-1a results in a negative feedback loop in which PKC downregulates Coronin-1a activity.
4.4 Coronin-1a in NGF–TrkA Signaling
Coronin-1a has also been shown to be important for RTK signaling. In particular, we recently demonstrated that Coronin-1a is a novel effector for NGF–TrkA signaling (Fig. 2). This type of signaling is well established as being critical for several aspects of peripheral nervous system (PNS) development including survival, synapse formation, and axon growth (Ascaño, Richmond, Borden, & Kuruvilla, 2009; Levi-Montalcini & Booker, 1960; Sharma et al., 2010). Like other RTKs, NGF binding induces TrkA dimerization and autophosphorylation, which in turn elicits classic downstream pathways, including Ras-MAPK, PI3K, and PLC-γ/calcium signaling (Harrington & Ginty, 2013). Importantly, these pathways are persistently activated on the signaling endosome where they direct trafficking, maturation, and stability. We found that Coronin-1a binds to the signaling endosome once it arrives at the cell body from the distal axon (Suo et al., 2014). This recruitment was necessary for the signaling endosome to evade lysosomal fusion, which would extinguish TrkA signaling (Fig. 3). This is reminiscent of the role for Coronin-1a in protecting pathogenic phagosomes from lysosomal fusion (BoseDasgupta & Pieters, 2014a, 2014b). Indeed, the signaling mechanisms allowing M. tuberculosis to persist in macrophages are similarly involved in protecting the NGF–TrkA signaling endosome. For instance, Coronin-1a is required for NGF–TrkA-dependent calcium release and calcineurin activation (Suo et al., 2014). There is also a severe deficit in several NGF–TrkA specific pathways that rely on calcium signaling including phosphorylation of CREB, which is a critical mediator of peripheral neuron survival (Lonze & Ginty, 2002; Riccio, Pierchala, Ciarallo, & Ginty, 1997). The implications of this are discussed later.
Fig. 3.
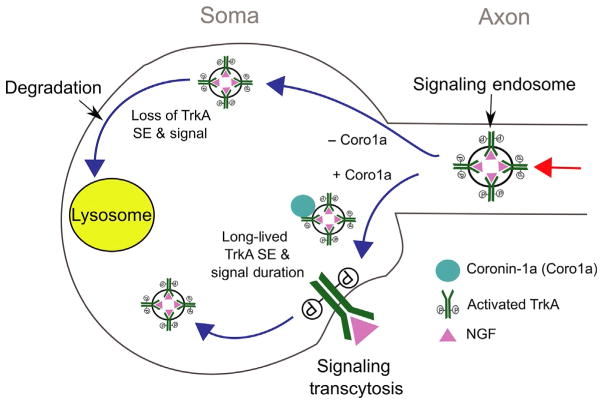
Coronin-1a endows the NGF–TrkA signaling endosome with enhanced signaling and trafficking capabilities. By associating with Coronin-1a, the NGF–TrkA signaling endosome can undergo transcytosis and prolong NGF–TrkA signaling.
4.5 Coronin-1a in Signaling Endosome Maturation
The Coronin-1a-dependent signaling events endow the signaling endosome with unique maturation capabilities. For example, in Coronin-1a knockout mice the NGF–TrkA signaling endosome degrades via lysosomal fusion 6–10 times faster than in wild-type neurons, in which signaling endosomes successfully evade lysosomal fusion for up to 24 h (Suo et al., 2014). This loss of protection can be phenocopied by manipulating downstream signals of Coronin-1a, including the addition of antagonists of PLC-γ, calcium, or calcineurin. Likewise, the Coronin-1a knockout phenotype can be partially rescued by calcium ionophores. Based on the previous findings that PKC activity can cause Coronin-1a to become monomeric and dissociate from actin and phagosomes, it will be interesting to determine whether these treatments also change the association of Coronin-1a with TrkA and the signaling endosome (Fig. 2). Importantly, Coronin-1a does not influence the degradation rate of another RTK, EGF receptor. This suggests that Coronin-1a may be selectively recruited to signaling endosomes that require long-lived signaling, such as those that undergo long distance transport.
4.6 Coronin-1a in Signaling Endosome Trafficking
Coronin-1a provides the NGF–TrkA signaling endosome with unique trafficking capabilities (Fig. 3). Upon reaching the soma, Coronin-1a is recruited to the signaling endosome. This is required not only for prolonging the signal but also for enabling a newly described trafficking event that we refer to as signaling transcytosis. During signaling transcytosis, retrograde TrkA receptors are exocytosed onto somal or dendritic membranes and later reinternalized. This phenomenon was observed using a modified version of the TrkA receptor from mice in which a Flag epitope was fused to the extracellular domain and knocked into the endogenous locus (Sharma et al., 2010). Applying a mouse anti-Flag antibody to live cells allows for tracking of the TrkA receptor following internalization. Using a modified version of this assay in which a second Cy3-coupled antimouse secondary antibody was applied to the cell body compartment after the initial anti-Flag antibody feeding at the distal axon, we were able to observe that retrogradely transported TrkA (decorated with anti-FLAG antibody) recycles to the cell body plasma membrane and reinternalizes (decorated with anti-FLAG antibody and Cy3-antimouse antibody). Coronin-1a KO neurons are unable to undergo this recycling event (Suo et al., 2014). These experiments confirmed that Coronin-1a is a necessary component for TrkA signaling transcytosis at the soma.
The necessity of Coronin-1a in TrkA signaling and recycling suggests that it may be a critical component of the signaling endosome with an array of functions allowing for proper neural development. Shuttling of retrograde NGF–TrkA signaling endosomes into dendrites is an essential event during the formation of postsynaptic densities in the PNS that results in the clustering of postsynaptic density components (Sharma et al., 2010). So far, it can be said that Coronin-1a assists in this process by prolonging the signaling capabilities of the NGF–TrkA signaling endosome and enabling transcytosis to occur, but whether it may play a larger role in mediating actin dynamics or protein clustering, or whether other Coronin family members expressed in the nervous system are involved, is yet to be known. It is also possible that the NGF–TrkA signaling endosome may not directly undergo recycling but will instead form a multivesicular body (MVB). MVBs have the potential to recycle TrkA to dendritic or somal surfaces or release vesicles into the extracellular environment as exosomes. Currently, it remains an open question as to how presynaptic neurons are guided to postsynaptic neurons, and how postsynaptic neurons receiving comparable levels of NGF undergo different levels of survival signaling. Whether the stability engendered by Coronin-1a to NGF–TrkA endosomes allows for the trafficking necessary to produce paracrine messengers warrants future study.
It is worth noting that the divergent fates of the NGF–TrkA signaling endosome likely give rise to distinct signaling endosomes with unique molecular profiles and functions. For instance, a TrkA receptor that has undergone signaling transcytosis might very well be decorated with a different set of actin modifiers, second messengers, and Rabs than an NGF–TrkA signaling endosome recently trafficked from the distal axon. It also stands to reason that the NGF-dependent processes elicited by these different pools of signaling endosomes likely bear different and significant consequences on survival, growth, and morphology of developing neurons.
4.7 Coronin-1a in Axon Guidance and Target Innervation
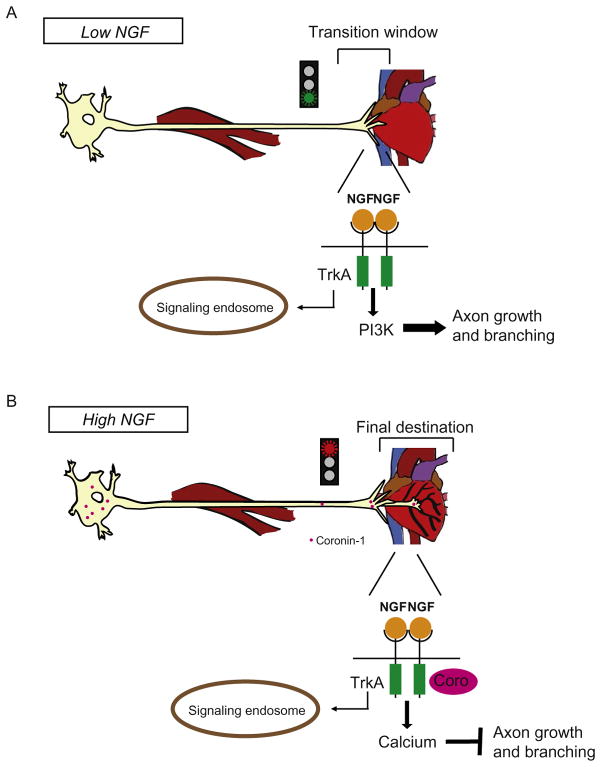
Coronin-1a is also involved in NGF–TrkA-dependent axon growth cone dynamics of neurons approaching their target. In Coronin-1a knockout mice, growth cones show a fourfold decrease in area (Suo et al., 2015). This is significant because smaller growth cones are associated with accelerated axon growth, whereas larger growth cones result in decelerated growth but increased capacity for turning and branching (Carmeliet, 2003). Consistent with this, loss of Coronin-1a resulted in reduced NGF-dependent branching and overshooting of final targets (Suo et al., 2015). These phenotypes agree with the previously stated role of Coronin-1a in mediating actin reorganization and promoting lamellipodial protrusions. Because Coronin-1a expression is induced by final target innervation and exposure to NGF, this represents a critical molecular switch in axon patterning of targets like the heart (Suo et al., 2014). When sympathetic axons initially reach their target prior to Coronin-1a expression, NGF-dependent PI3K signaling promotes rapid axon extension (Fig. 4A). Upon Coronin-1a upregulation, exuberant axon growth and branching is suppressed downstream of NGF-PI3K signaling at the level of GSK3beta activation (Figs. 2 and 4B). We speculate that this throttling of axon growth and branching allows for appropriate arborization of the final target tissue by innervating axons. We have previously demonstrated that Coronin-1a does not associate with the signaling endosome until it reaches the cell body; however, the mechanism for this spatial selectivity is unknown. Therefore, we suggest that NGF–TrkA-Coronin-1a-mediated axon growth and branching phenotypes are likely to be driven by axonal plasma membrane signaling and perhaps directly effecting cytoskeletal dynamics.
Fig. 4.
(A) Upon initially encountering a target organ, NGF concentrations are low and NGF-induced genes are not yet expressed. This marks a “transition window,” in which low NGF availability enhances axon growth and branching via PI3K signaling. (B) High NGF induces Coronin-1a expression, and PI3K signaling is dampened. Axon growth and branching slows.
5. CONCLUSIONS AND FUTURE DIRECTIONS
Despite the unveiling of Coronins as highly conserved actin modifiers and signaling mediators nearly 10 years ago, our understanding of their involvement in these processes across cell lineages remains limited. Structurally, the Coronin family is interesting, as the structural variations in each subtype suggest distinct roles, while the conserved WD repeats allow for potentially intersecting functions across a diverse array of signaling effectors. The details of Coronin subtype interplay as well as differences in its activity depending on oligomeric assembly are not fully understood. Indeed, Coronin appears to be a mediator of several receptor pathways involved in immune and nervous system development. The multifaceted roles of Coronin-1a in actin assembly and signal modulation suggest that it may act as a key mediator of neuron development and function. Already there are examples of its function in axon growth, branching, and growth cone morphology, but our understanding of the role for Coronin family members within nervous system development remains limited and many more functions are likely to be discovered. Understanding when and where Coronin participates in this diversity of functions provides an important substrate for future investigation.
Acknowledgments
C.D.D. was supported by NIH-NINDS (1R01NS072388) and NSF-IOS-1453242. B.W. was supported by NIH-NINDS (R01NS083378). We are grateful to Dorothy Schafer for helpful discussion. The authors declare no competing financial interests.
References
- Appleton BA, Wu P, Wiesmann C. The crystal structure of murine coronin-1: A regulator of actin cytoskeletal dynamics in lymphocytes. Structure. 2006;14(1):87–96. doi: 10.1016/j.str.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ascaño M, Richmond A, Borden P, Kuruvilla RJ. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. The Journal of Neuroscience. 2009;29(37):11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoseDasgupta S, Pieters J. Coronin 1 trimerization is essential to protect pathogenic mycobacteria within macrophages from lysosomal delivery. FEBS Letters. 2014a;588(21):3898–3905. doi: 10.1016/j.febslet.2014.08.036. [DOI] [PubMed] [Google Scholar]
- BoseDasgupta S, Pieters J. Inflammatory stimuli reprogram macrophage phagocytosis to macropinocytosis for the rapid elimination of pathogens. PLoS Pathogens. 2014b;10(1):e1003879. doi: 10.1371/journal.ppat.1003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoseDasgupta S, Suzette M, Jenoe P, Pieters J. Cytokine-induced macropinocytosis in macrophages is regulated by 14-3-3f through its interaction with serine-phosphorylated coronin 1. FEBS Journal. 2015;282(7):1167–1181. doi: 10.1111/febs.13214. [DOI] [PubMed] [Google Scholar]
- Cai L, Holowecky N, Schaller MD, Bear JE. Phosphorylation of coronin 1B by protein kinase C regulates interaction with Arp2/3 and cell motility. The Journal of Biological Chemistry. 2005;280(36):31913–31923. doi: 10.1074/jbc.M504146200. [DOI] [PubMed] [Google Scholar]
- Cai L, Makhov AM, Bear JE. F-actin binding is essential for coronin 1B function in vivo. Journal of Cell Science. 2007;120(Pt. 10):1779–1790. doi: 10.1242/jcs.007641. [DOI] [PubMed] [Google Scholar]
- Cai L, Makhov AM, Schafer DA, Bear JE. Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell. 2008;134:828–842. doi: 10.1016/j.cell.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: Common signals, pathways and diseases. Nature Reviews Genetics. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Chan KT, Creed SJ, Bear JE. Unraveling the enigma: Progress towards understanding the Coronin family of actin regulators. Trends in Cell Biology. 2011;21(8):481–488. doi: 10.1016/j.tcb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM. The cell: A molecular approach. 2. Sunderland (MA): Sinauer Associates; 2000. [Google Scholar]
- de Hostos EL. The coronin family of actin-associated proteins. Trends in Cell Biology. 1999;9(9):345–350. doi: 10.1016/s0962-8924(99)01620-7. [DOI] [PubMed] [Google Scholar]
- de Hostos EL, Bradike B, Lottspeich F, Guggenheim R, Gerisch G. Coronin, an actin-binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein/3 subunits. The EMBO Journal. 1991;10:4097–4104. doi: 10.1002/j.1460-2075.1991.tb04986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hostos EL, Rehfuess C, Bradtke B, Waddell DR, Albrecht R, Murphy J, et al. Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. The Journal of Cell Biology. 1993;120(1):163–173. doi: 10.1083/jcb.120.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD. A model for neuronal competition during development. Science. 2008;320(5874):369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson A, Williams MJ, Voisin S, Hansson I, Krishnan A, Philippot G, et al. Implication of coronin 7 in body weight regulation in humans, mice and flies. BMC Neuroscience. 2015;16:13. doi: 10.1186/s12868-015-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97(4):435–447. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Achard V, Blanchoin L, Goode BL. Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Molecular Cell. 2009;34(3):364–374. doi: 10.1016/j.molcel.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Jangi M, Goode BL. Functional surfaces on the actin-binding protein coronin revealed by systematic mutagenesis. The Journal of Biological Chemistry. 2010;285(45):34899–34908. doi: 10.1074/jbc.M110.171496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nature Reviews Neuroscience. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- Huang W, Ghisletti S, Saijo K, Gandhi M, Aouadi M, Tesz GJ, et al. Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature. 2011;470(7334):414–418. doi: 10.1038/nature09703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran R, Liu X, BoseDasgupta S, Müller P, Zhang CL, Moshous D, et al. Coronin 1 regulates cognition and behavior through modulation of cAMP/protein kinase A signaling. PLoS Biology. 2014;12(3):e1001820. doi: 10.1371/journal.pbio.1001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran R, Pieters J. Regulation of immune cell homeostasis and function by coronin 1. International Immunopharmacology. 2015;28(2):825–828. doi: 10.1016/j.intimp.2015.03.045. [DOI] [PubMed] [Google Scholar]
- Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130(1):37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Booker B. Destruction of the sympathetic ganglia in mammals by an antiserum to a nerve-growth protein. Proceedings of the National Academy of Sciences of the United States of America. 1960;46(3):384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Needham KM, May JR, Nolen BJ. Mechanism of a concentration-dependent switch between activation and inhibition of Arp2/3 complex by coronin. The Journal of Biological Chemistry. 2011;286:17039–17046. doi: 10.1074/jbc.M111.219964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Marshall TW, Aloor HL, Bear JE. Coronin 2A regulates a subset of focal-adhesion-turnover events through the cofilin pathway. Journal of Cell Science. 2009;122:3061–3069. doi: 10.1242/jcs.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Breitsprecher D, Jansen S, Reisler E, Goode BL. Coronin enhances actin filament severing by recruiting cofilin to filament sides and altering F-actin conformation. Journal of Molecular Biology. 2015;427(19):3137–3147. doi: 10.1016/j.jmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshous D, de Villartay JP. The expanding spectrum of human coronin 1A deficiency. Current Allergy and Asthma Reports. 2014;14(12):481. doi: 10.1007/s11882-014-0481-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takeuchi K, Muraoka S, Takezoe N, Mori N. A neurally enriched coronin-like protein, ClipinC, is a novel candidate for an actin cytoskeleton-cortical membrane-linking protein. Journal of Biological Chemistry. 1999;274(19):13322–13327. doi: 10.1074/jbc.274.19.13322. [DOI] [PubMed] [Google Scholar]
- Oku T, Nakano M, Kaneko Y, Ando Y, Kenmotsu H, Itoh S, et al. Constitutive turnover of phosphorylation at Thr-412 of human p57/coronin-1 regulates the interaction with actin. The Journal of Biological Chemistry. 2012;287(51):42910–42920. doi: 10.1074/jbc.M112.349829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277(5329):1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Rogers SL, Vale RD, Jan LY, Jan Y. Drosophila Pod-1 crosslinks both actin and microtubules and controls the targeting of axons. Neuron. 2003;39(5):779–791. doi: 10.1016/s0896-6273(03)00508-7. http://dx.doi.org/10.1016/s0896-6273(03)00508-7. [DOI] [PubMed] [Google Scholar]
- Rottner K, Stradal TEB. How distinct Arp2/3 complex variants regulate actin filament assembly. Nature Cell Biology. 2016;18:1–3. doi: 10.1038/ncb3293. [DOI] [PubMed] [Google Scholar]
- Rybakin V, Stumpf M, Schulze A, Majoul I, Noegel A, Hasse A. Coronin 7, the mammalian POD-1 homologue, localizes to the Golgi apparatus. FEBS Letters. 2004;573(1–3):161–167. doi: 10.1016/j.febslet.2004.07.066. [DOI] [PubMed] [Google Scholar]
- Sharma N, Deppmann CD, Harrington AW, St Hillaire C, Chen ZY, Lee FS, et al. Long-distance control of synapse assembly by target-derived NGF. Neuron. 2010;67(3):422–434. doi: 10.1016/j.neuron.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, et al. The actin regulator coronin-1A is mutated in a thymic egress deficient mouse strain and in a T–B+NK+ SCID patient. Nature Immunology. 2008;9(11):1307–1315. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo D, Park J, Harrington AW, Zweifel LS, Mihalas S, Deppmann CD. Coronin-1 is a neurotrophin endosomal effector required for developmental competition for survival. Nature Neuroscience. 2014;17(1):36–45. doi: 10.1038/nn.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo D, Park J, Young S, Makita T, Deppmann CD. Coronin-1 and calcium signaling governs sympathetic final target innervation. Journal of Neuroscience. 2015;35(9):3893–3902. doi: 10.1523/JNEUROSCI.4402-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi YK, Kocaefe YC, Ayter S. Coronin 1A inhibits neurite outgrowth in PC12 cells. Neuroscience Letters. 2014;582:38–42. doi: 10.1016/j.neulet.2014.08.044. [DOI] [PubMed] [Google Scholar]
- Yan M, Di Ciano-Oliveira C, Grinstein S, Trimble WS. Coronin function is required for chemotaxis and phagocytosis in human neutrophils. The Journal of Immunology. 2007;178:5769–5778. doi: 10.4049/jimmunol.178.9.5769. [DOI] [PubMed] [Google Scholar]
- Yee CSK, Ozden S, Chou JS, Geha RS, Ayvaz D, Aytekin C, et al. Coronin-1A oligomerization is critical for host defense against viral pathogens. Journal of Allergy and Clinical Immunology. 2014;133(2):AB94. [Google Scholar]