Abstract
The treatment of older patients with acute myeloid leukemia that is secondary to previous myelodysplastic syndrome, myeloproliferative neoplasm, or prior cytotoxic exposure remains unsatisfactory. We compared 92 and 107 patients treated, respectively, with intensive chemotherapy or azacitidine within two centres. Diagnoses were 37.5% post-myelodysplastic syndrome, 17.4% post-myeloproliferative neoplasia, and 45.1% therapy-related acute myeloid leukemia. Patients treated by chemotherapy had less adverse cytogenetics, higher white blood-cell counts, and were younger: the latter two being independent factors entered into the multivariate analyses. Median overall-survival times with chemotherapy and azacitidine were 9.6 (IQR: 3.6−22.8) and 10.8 months (IQR: 4.8−26.4), respectively (p = 0.899). Adjusted time-dependent analyses showed that, before 1.6 years post-treatment, there were no differences in survival times between chemotherapy and azacitidine treatments whereas, after this time-point, patients that received chemotherapy had a lower risk of death compared to those that received azacitidine (adjusted HR 0.61, 95%CI: 0.38−0.99 at 1.6 years). There were no interactions between treatment arms and secondary acute myeloid leukemia subtypes in all multivariate analyses, indicating that the treatments had similar effects in all three subtypes. Although a comparison between chemotherapy and azacitidine remains challenging, azacitidine represents a valuable alternative to chemotherapy in older patients that have secondary acute myeloid leukemia because it provides similar midterm outcomes with less toxicity.
Keywords: therapy-related acute myeloid leukemia, secondary AML, azacitidine, intensive chemotherapy, older patients
INTRODUCTION
The subgroup of patients with non de novo acute myeloid leukemia (AML) is frequently and improperly named as having “secondary” AML. This group is heterogeneous and encompasses both therapy-related acute myeloid leukemia (t-AML), which occurs after prior exposure to cytotoxic chemotherapy and/or radiotherapy, and secondary AML (sAML), which occurs in the course of a previous myeloid disease such as myelodysplastic syndrome (MDS) or Philadelphia-negative myeloproliferative neoplasm (MPN) [1].
In most studies, both t-AML and sAML have been associated with a worse prognosis compared to de novo AML, although subgroups of patients with a better outcome have been reported [2-4]. Many well-known adverse factors are more frequently observed in patients with t-AML and sAML, which can explain the worse outcome: i.e., older age, comorbidities, multilineage dysplasia, and poor-risk cytogenetics [3]. Because both t-AML and sAML are often excluded from prospective trials, an optimal treatment remains to be established. In patients deemed fit for intensive therapy, which represents less than half of this older-patient population, therapeutic strategies differ little from those for de novo AML: these patients are offered induction chemotherapy and allogeneic stem-cell transplantation (HSCT).
Recent data from a Danish registry showed that MDS-sAML and tAML are independently associated with increased risk of death, although this effect was less pronounced in patients > 60 years and in patients with adverse or intermediate cytogenetics. However, AML secondary to MPN or chronic myelomonocytic leukemia (CMML) were associated with worse overall survival independent of cytogenetics and age [5]. The overall prognosis remains very poor, with median overall survival (OS) of less than six months, indicating that alternative treatments are needed [6].
Azacitidine has been recently approved in Europe for older AML patients and now offers a reasonable alternative to intensive chemotherapy, at least for a subset of patients [7]. We have previously shown in a series of 95 older AML patients, that most benefit was observed in patients that had a low white blood-cell count (WBC), a good performance status, and intermediate-risk cytogenetics [8]. In addition, when compared with conventional care regimen, azacitidine appeared better in patients of the adverse cytogenetic risk group [7]. It is noteworthy that in most studies that have focused on azacitidine treatment for AML, neither sAML nor t-AML has emerged as having worse risk factors [5-11]. Moreover, azacitidine has shown efficacy in subgroups that have features frequently encountered in t-AML or sAML, including multilineage dysplasia and adverse-risk cytogenetics [7, 8]. In this study, we compared the outcomes of patients aged > 60 years with t-AML or sAML and that had received intensive chemotherapy or azacitidine.
RESULTS
Patients and treatments
This study included 199 patients selected to receive intensive chemotherapy (n = 92) or azacitidine (n = 107). The median year of treatment was 2011 in the intensive arm and 2010 in the azacitidine arm. The patients’ characteristics are depicted in Table 1. There were 69 cases of post-MDS AML (37.5%), 32 cases of post-MPN AML (17.4%), and 83 cases of t-AML (45.1%). There were no clinical differences between the chemotherapy and azacitidine groups in terms of performance status and the Charlson comorbidity index. In the azacitidine group, patients were older, had lower WBC counts, and more frequently had post-MDS AML and adverse cytogenetics, including a monosomal karyotype, compared to the chemotherapy group.
Table 1. Patients characteristics.
| All patients N=199 (100%) |
Azacitidine N=107 (53.8%) |
Intensive chemotherapy N=92 (46.2%) |
p | |
|---|---|---|---|---|
|
Age (years) Median (IQR) Range |
72.0 (65.9-77.5) 60.6, 87.8 |
76.5 (71.4-80.6) 60.9, 87.8 |
66.6 (63.5-71.4) 60.6, 83.1 |
p<0.0001 |
|
Age (years) <70 ≥70 |
85 (42.7) 114 (57.3) |
20 (18.7) 87 (81.3) |
65 (70.7) 27 (29.3) |
p<0.0001 |
| Male gender-n (%) | 117 (58.8) | 62 (57.9) | 55 (59.8) | p=0.80 |
|
sAML subtype-n (%) Post-MDS Post-MPN Therapy- related |
69 (37.5) 32 (17.4) 83 (45.1) |
52 (50.5) 18 (17.5) 33 (32.0) |
17 (21.0) 14 (17.3) 50 (61.7) |
p<0.0001 |
|
Performance status-n (%) 0-1 2-3 |
123 (71.5) 49 (28.5) |
59 (67.8) 28 (32.2) |
64 (75.3) 21 (24.7) |
p=0.28 |
|
Charlson comorbidity index-n (%) 0 ≥1 |
59 (33.7) 116 (66.3) |
32 (36.8) 55 (63.2) |
27 (30.7) 61 (69.3) |
p=0.40 |
|
Infection at diagnosis-n (%) Yes No |
27 (15.1) 152 (84.9) |
18 (20.5) 70 (79.5) |
9 (9.9) 82 (90.1) |
p=0.048 |
|
WBC (G/L) N Median [IQR] Range |
197 4.2 [ 1.8-18.3] 0.5,433.0 |
105 2.4 [ 1.4- 5.8] 0.6,91.4 |
92 14.1 [ 3.6-62.7] 0.5,433.0 |
p<0.0001 |
|
WBC ≥ 15 G/L-n (%) Yes No |
54 (27.4) 143 (72.6) |
9 (8.6) 96 (91.4) |
45 (48.9) 47 (51.1) |
p<0.0001 |
|
Bone marrow blasts (%) Median [IQR] Range |
35.0 [25.0-62.0] 5.0,98.0 |
30.0 [21.0-46.0] 9.0,79.0 |
50.0 [27.5-82.0] 5.0,98.0 |
p<0.0001 |
|
Cytogenetics - n (%) Favorable Intermediate Unfavorable /non monosomal Monosomal karyotype |
0 126 (64.0) 46 (23.4) 25 (12.6) |
0 61 (58.1) 24 (22.9) 20 (19.0) |
0 65 (70.7) 22 (23.9) 5 (5.4) |
p=0.005 |
|
Serum ferritin (µgrams/L) N Median [IQR] Range |
108 747 [411-1384] 41,38950 |
47 537 [285-1204] 41,4015 |
61 1045 [494-1598] 49,38950 |
p=0.023 |
|
Serum albumin (grams/L) N Median [IQR] Range |
150 39.0 [35.4-42.0] 19.0,57.3 |
72 40.0 [36.9-43.0] 25.0,46.0 |
78 37.2 [34.4-41.0] 19.0,57.3 |
p=0.036 |
|
LDH (UI/L) N Median [IQR] Range |
168 574.5 [369-998] 136,12806 |
80 481 [346-641] 170,3525 |
88 738.5 [419.5-1509] 136,12806 |
p=0.002 |
Abbreviations: IQR: interquartile range, sAML: secondary AML; MDS: Myelodysplastic syndrome, MPN: Myeloproliferative neoplasm, WBC: white blood cell count.
The median times from diagnosis to initiation of treatment were 0.7 month (interquartile range (IQR): 0.4-1.5) for azacitidine and 0.2 (IQR: 0.1-0.5) for chemotherapy (p < 0.0001). In the azacitidine group, patients received a median number of seven cycles (IQR, 2-15), using the standard 7-day scheme in 83% of cases. In the chemotherapy group, 60% of patients received a three-drug schedule that combined idarubicin, standard-dose cytarabine, and lomustine (Supplementary Table 1). Among the 92 patients who received intensive chemotherapy, 47 achieved complete remission (CR): 43 after a single induction course and four after a second induction regimen with high-dose cytarabine. Twenty patients were refractory to intensive chemotherapy and were then managed by best supportive care without receiving hypomethylating agents. One patient underwent HSCT in a refractory situation. Thirty-four CR patients were treated during the post-remission phase: 14 with at least one course of intermediate-dose cytarabine (≥1 g/m2) followed by HSCT given to 5 patients, 18 patients received a low-intensity regimen of chemotherapy, and 2 patients received HSCT without any consolidation.
Among the 107 patients who received azacitidine, only one underwent HSCT. This patient is still alive at 49 months (Table 2). The median time between response to chemotherapy and the end of post-remission-treatment was 4.4 months (IQR: 2.1-8.4). None of the patient that received azacitidine as first treatment in our cohort received intensive chemotherapy as a second-line treatment, whereas only three patients in the chemotherapy group received a hypomethylating agent at relapse.
Table 2. Response and outcome according to treatment arm.
| Azacitidine N=107 |
Intensive chemotherapy N=92 |
p | |
|---|---|---|---|
| Overall response (CR+CRi)-n (%) | 21 (19.6%) | 58 (63.0%) | <0.0001 |
| CR-n (%) | 12 (11.2%) | 47 (51.1%) | <0.0001 |
| CRi-n (%) | 9 (8.4%) | 11 (12.0%) | 0.4070 |
| PR-n (%) | 7 (6.5%) | NA | |
| HI-n (%) -Overall -1 lineage -2 lineages -3 lineages |
21 (19.6%) 10 (9.4%) 8 (7.5%) 3 (2.8%) |
NA | |
| Day-30 deaths-n (%) Day-60 deaths-n (%) |
6 (5.6%) 19 (18.3%) |
10 (10.9%) 16 (17.4%) |
0.1930 0.8727 |
| Causes of early deaths a -n (%) -AML progression -Infection -Cardiac event -Hemorrhage -Unknown |
6 (5.6%) 1 (0.9%) 0 0 0 |
5 (5.4%) 4 (4.3%) 0 0 2 (2.2%) |
|
| AlloSCT-n (%) | 1 (1.1%) | 8 (8.7%) | 0.0348 |
Abbreviations: CR: complete response, CRi: complete response with incomplete blood recovery, PR: partial response, HI: Hematological improvement. a. Early death within 30 days from treatment (several causes are possible by patient), NA: Not applicable, AlloSCT: allogeneic stem-cell transplantation.
Assessment of choice criteria between azacitidine and chemotherapy
Univariate analyses showed that the main factors significantly associated with the choice of whether patients received chemotherapy or azacitidine were age, WBC count, and cytogenetic risk, especially if there was a monosomal karyotype (Table 3). In the multivariate analyses, the two mains factors regarding choice of treatment were age and WBC count, meaning that the probability of receiving chemotherapy was significantly decreased with age (adjusted OR 0.26, 95%CI: 0.18-0.39 for each increase of 5 years of age, p < 0.001), and an increased WBC count (adjusted OR 17.54, 95%CI: 6.13-50.2 for patients with a WBC count ≥15 G/L vs. < 15 G/L; p < 0.001) (Table 3).
Table 3. Univariate and multivariate analyses of factors associated with the choice of treatment.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| N | N Events | OR | 95% CI | p* | aOR | 95% CI | p* | |
|
Age as continuous variable Interval: 5 years |
199 | 92 | 0.31 | [0.22;0.43] | <0.001 | 0.26 | [0.18;0.39] | <0.001 |
|
Age (years) <70 ≥70 |
85 114 |
65 27 |
1 0.10 |
- [0.05;0.19] |
- <0.001 |
|||
|
Performance status 0-1 2-3 |
123 49 |
64 21 |
1 0.69 |
- [0.35;1.35] |
- 0.278 |
|||
|
Charlson comorbidity index 0 >0 |
59 116 |
27 61 |
1 1.31 |
- [0.70;2.46] |
- 0.394 |
|||
|
WBC (G/L) <15 ≥15 |
143 54 |
47 45 |
1 10.21 |
- [4.61;22.64] |
<0.001 | 1 17.54 |
- [6.13;50.21] |
<0.001 |
|
Cytogenetics Intermediate Unfavorable /non monosomal Monosomal karyotype |
126 46 25 |
65 22 5 |
1 0.86 0.23 |
- [0.44;1.69] [0.08;0.66] |
- 0.662 0.006 |
|||
Abbreviations: WBC: white blood cell count, OR: Odds ratio, CI: confidence interval, *p value for factors assessed in the decision criteria for chemotherapy (versus azacitidine).
Safety
There were 10 (10.9%) early deaths in patients treated by chemotherapy and 6 (5.8%) in patients that received azacitidine (p = 0.193) (Table 2). Univariate analyses of the factors that influenced early death are shown in Supplementary Table 2. In the multivariate analyses, only AML subtype and type of treatment remained significantly predictive of early death, meaning that t-AML was associated with a lower risk of death (vs. post-MDS AML, adjusted OR 0.08, 95%CI: 0.02-0.43; p = 0.003) and chemotherapy was associated with a higher risk (vs. azacitidine, adjusted OR 3.80, 95%CI: 1.17-12.4; p = 0.026) (Table 4).
Table 4. Multivariate analyses for early death, response and overall survival.
| N | N Events | aOR/aHR | 95% CI | p | |
|---|---|---|---|---|---|
| Early deaths | |||||
|
Subtype of sAML MDS-related MPN-related Therapy-related |
67 32 82 |
10 2 2 |
1 0.28 0.08 |
- [0.05;1.43] [0.02;0.43] |
- 0.126 0.003 |
|
Treatment arm Azacitidine Intensive chemotherapy |
104 92 |
6 10 |
1 3.80 |
- [1.17;12.36] |
- 0.026 |
| Response to treatment | |||||
|
Age as continuous variable Interval of 5 years |
196 | 78 | 0.67 | [0.50;0.90] | 0.008 |
|
Treatment arm Azacitidine Intensive chemotherapy |
104 92 |
20 58 |
1 4.09 |
- [1.94;8.63] |
- <0.001 |
| Overall survival | |||||
|
Ferritinemia (µg/L) <400 400-750 750-1400 ≥1400 |
25 29 26 27 |
15 23 20 25 |
1 1.57 1.46 3.70 |
- [0.80;3.05] [0.73;2.94] [1.89;7.22] |
- 0.188 0.287 <0.001 |
|
LDH (U/L) Normal Elevated a |
48 120 |
37 100 |
1 1.87 |
- [1.25;2.82] |
- 0.003 |
|
Cytogenetics Intermediate Unfavorable /non monosomal Monosomal karyotype |
126 46 25 |
98 43 22 |
1 2.50 4.20 |
- [1.71;3.67] [2.51;7.02] |
- <0.001 <0.001 |
|
Treatment arm Azacitidine Intensive chemotherapy |
105 92 |
92 71 |
See Figure 1C | ||
Abbreviations: sAML: secondary AML, MDS: Myelodysplastic syndrome, MPN: Myeloproliferative neoplasm, aOR: adjusted odds ratio, aHR: adjusted hazard ratio, CI: confidence interval, a. rate above the benchmark.
Efficacy
Overall response (CR+CRi) was documented in 58 patients (63%) that received chemotherapy and in 21 patients (19.6%) that received azacitidine (p < 0.0001) (Table 2). In the azacitidine group, seven additional patients (6.5%) achieved a partial response (PR) and 21 patients (19.6%) that were classified as failure with the IWG-AML criteria achieved a major hematological improvement. The median delay before the best response to azacitidine was 5.7 months (IQR: 5.2-8.9). Univariate analyses on the factors that influenced response to treatment are shown in Supplementary Table 3.
In the multivariate analyses, only age and type of treatment remained significantly associated with response, meaning that the probability of response decreased with age (adjusted OR 0.67, 95%CI: 0.50-0.90 for each increase in 5 years of age, p = 0.008) and increased in patients treated with chemotherapy (adjusted OR 4.09, 95%CI: 1.94-8.63; p < 0.001) (Table 4).
Overall survival
With a median follow-up period of 3.4 years (IQR: 2.1-5.4), the median OS time for the entire cohort was 10.8 months (IQR: 4.8-26.4). Median OS with chemotherapy was 9.6 months (IQR: 3.6-22.8) and with azacitidine it was 10.8 months (IQR: 4.8-26.4, p = 0.899). Univariate analyses of the factors associated with OS are shown in Supplementary Table 4.
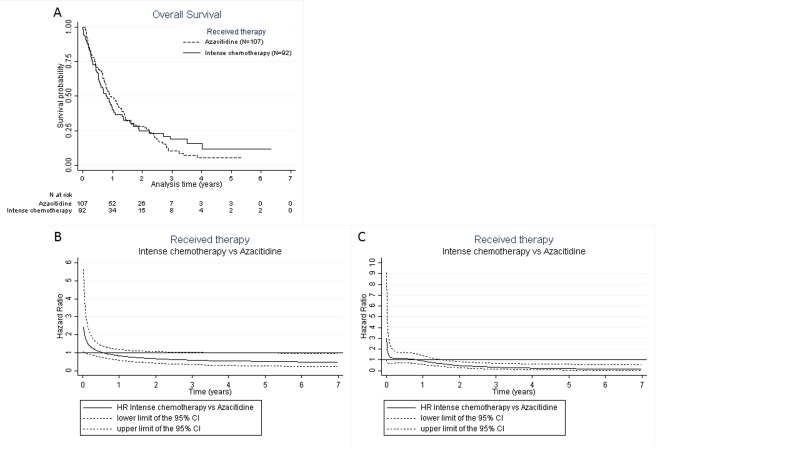
The Kaplan-Meier curve showed a time-dependent effect according to treatment arm, indicating that the log-rank test could not adequately compare the treatments (Figure 1A). Thus, we used the Royston and Parmar model, which took into account the interactions between time and treatment effect, and allowed graphical representation of the unadjusted risk of death, as shown in Figure 1B. Multivariate analysis of the factors associated with survival according to this model showed that serum ferritin ≥1400 µg/L (adjusted OR 3.70, 95%CI: 1.89-7.22 vs. < 400 µg/L; p < 0.001), elevated lactate dehydrogenase (adjusted OR 1.87, 95%CI: 1.25-2.82; p = 0.003), and cytogenetic risk (adjusted OR 2.50, 95%CI: 1.71-3.67; p < 0.001 for adverse non-monosomal, and adjusted OR 4.20, 95%CI: 2.51-7.02; p < 0.001 for monosomal karyotype, both vs. intermediate karyotype) were significantly associated with worse OS (Table 4). Changes in the HR for death with chemotherapy vs. azacitidine, and adjusted for the covariables in the multivariate model, are shown in Figure 1C: this indicates that before 1.6 years, there were no difference in survival between chemotherapy and azacitidine treatments whereas, after this time-point, patients that received chemotherapy had a lower risk of death compared to those that received azacitidine (adjusted HR 0.61, 95%CI: 0.38-0.99 at 1.6 years).
Figure 1. A.
Kaplan-Meier curve for overall survival according to treatment. B. Royston and Parmar non-adjusted hazard ratio for overall survival after treatment with azacitidine vs. chemotherapy for each year after diagnosis. Before 15 days of follow-up, patients treated with intensive chemotherapy had a significantly higher risk of death compared to those that received azacitidine. At day 15, the risk of death was higher in the intensive-chemotherapy group compared to the azacitidine group (HR 2.22, 95%CI: 1.03-4.75). Beyond 15 days of follow-up, there was no significant difference in survival between the two groups. C. Royston and Parmar adjusted hazard ratio for overall survival after treatment with azacitidine vs. chemotherapy for each year after diagnosis. Before 1.6 years of follow-up, there was no significant difference in survival between the two groups. After 1.6 years, patients treated with intensive chemotherapy had a significantly reduced risk of death compared to those that received azacitidine (aHR 0.61, 95%CI: 0.38-0.99). Interaction between azacitidine vs. chemotherapy and the AML subtypes (t-AML, post-MDS, or post-MPN AML) was not significant, showing that the effect of azacitidine vs. chemotherapy was not significantly different according to AML subtypes. So, there is no indication to stratify the analysis on AML subtypes (Figure C was the same for t-AML, post-MDS, or post-MPN AML).
Characteristics and outcomes of patients with t-AML, post-MDS, or post-MPN AML
The characteristics of patients according to AML subtype are shown in Table 5. Patients with post MDS-AML were older and more often received azacitidine, whereas patients with post-MPN had a higher-risk cytogenetics, including the monosomal karyotypes. There was no interaction between the treatment arms and AML subtypes in all the multivariate analyses, indicating that the effects of treatment were similar for all three groups of AML.
Table 5. Characteristics of patients according to AML subtypes.
| Post-MDS AML N=69 (37.5%) |
Post-MPN AML N=32 (17.4%) |
tAML N=83 (45.1%) |
P | |
|---|---|---|---|---|
|
Age (years) Median (IQR) Range |
75.2 (68.4-79.9) 61.0,86.6 |
70.5 (65.0-75.3) 61.2,83.8 |
70.1 (64.5-74.9) 60.6,87.8 |
0.0018 |
|
Age (years) <70 ≥70 |
21 (30.4) 48 (69.6) |
16 (50.0) 16 (50.0) |
40 (48.2) 43 (51.8) |
0.0510 |
| Male gender-n (%) | 47 (68.1) | 17 (53.1) | 42 (50.6) | 0.0800 |
|
Performance status-n (%) 0-1 2-3 |
42 (76.4) 13 (23.6) |
15 (53.6) 13 (46.4) |
59 (75.6) 19 (24.4) |
0.0560 |
|
Charlson comorbidity index-n (%) 0 >0 |
23 (37.1) 39 (62.9) |
14 (53.9) 12 (46.2) |
17 (22.1) 60 (77.9) |
0.0080 |
|
Infection at diagnosis-n (%) Yes No |
9 (14.7) 52 (85.3) |
5 (19.2) 21 (80.8) |
12 (15.0) 68 (85.0) |
0.8790 |
|
WBC (G/L) N Median (IQR) Range |
68 3.1 (1.7-8.8) 0.6,168.4 |
32 5.0 (3.0-13.0) 0.9,80.1 |
83 3.8 (1.5-27.2) 0.5,122.8 |
0.3071 |
|
WBC ≥ 15 G/L-n (%) Yes No |
12 (17.7) 56 (82.4) |
6 (18.8) 26 (81.3) |
25 (30.1) 58 (69.9) |
0.1560 |
|
Bone marrow blasts (%) Median (IQR) Range |
29 (22-45) 20,95 |
32 (21-40) 12,85 |
52 (29-76) 5,98 |
0.0001 |
|
Cytogenetics - n (%) Favorable Intermediate Unfavorable /non monosomal Monosomal karyotype |
0 (0.0) 50 (74.6) 13 (19.4) 4 (6.0) |
0 (0.0) 13 (40.6) 11 (34.4) 8 (25.0) |
0 (0.0) 51 (61.5) 20 (24.1) 12 (14.5) |
0.0140 |
|
Serum ferritin (µgrams/L) N Median (IQR) Range |
42 547 (336-1310) 72,38950 |
14 1103 (597-1538) 41,9750 |
42 768 (438-1304) 49,3252 |
0.3397 |
|
Serum albumin (grams/L) N Median (IQR) Range |
54 40 (36-42) 19,46 |
20 37 (34-41) 22,48 |
65 38 (35-42) 21,57 |
0.2551 |
|
LDH (UI/L) N Median (IQR) Range |
59 572 (402-792) 170,12806 |
23 836 (448-1735) 229,3525 |
75 494 (332-774) 136,7258 |
0.0512 |
|
Treatment Intensive Chemotherapy Azacitidine |
17 (24.6) 52 (75.4) |
14 (43.8) 18 (56.2) |
50 (60.2) 33 (39.8) |
<0.0001 |
|
Response – n (%) All Chemotherapy Azacitidine |
19 (27.5) 9 (52.9) 10 (19.2) |
10 (31.3) 7 (50.0) 3 (16.7) |
45 (54.2) 38 (76.0) 7 (21.2) |
0.0019 0.0790 1.0000 |
|
Early death -n (%) All Chemotherapy Azacitidine |
10 (14.9) 4 (23.5) 6 (12.0) |
2 (6.3) 2 (14.3) 0 (0.0) |
2 (2.4) 2 (4.0) 0 (0.0) |
0.0126 0.0350 0.0430 |
|
Median OS-months (IQR) All Chemotherapy Azacitidine |
13.2 (3.6-33.6) 7.2 (2.4-26.4) 13.2 (3.6-33.6) |
8.4 (4.8-16.8) 7.2 (3.6-19.2) 8.4 (4.8-13.2) |
10.8 (6.0-22.8) 10.8 (6.0-22.8) 13.2 (7.2-25.2) |
0.1230 0.8425 0.0690 |
Abbreviations: MDS: Myelodysplastic syndrome, MPN: Myeloproliferative neoplasm, tAML: therapy related AML, IQR: interquartile range, WBC: white blood cell count.
DISCUSSION
In this study, we have described one of the largest cohorts of t-AML and sAML patients aged ≥60 years and selected from daily practice for two different treatment approaches over a 7-year period. We show that patients that received intensive chemotherapy had a better complete response rate, a higher early death rate, and similar median OS times compared to patients that received azacitidine. However, as the risk of death varied over time according to treatment, we compared the survival of patients treated by azacitidine versus intensive chemotherapy using time-dependent analyses and showed that, after adjustment for the main prognostic factors, patients that received intensive chemotherapy had better survival times after 1.6 years post-diagnosis.
The AZA-AML-001 randomized trial compared azacitidine versus conventional-care regimen in AML patients aged ≥65 years and with a WBC count of < 15 G/L. The investigators chose between intensive chemotherapy, low-dose cytarabine, and best supportive care [7]. This international multicenter trial included 488 patients, of whom only 88 were selected for randomization versus chemotherapy, indicating that only a minority of physicians considered this issue of relevance. This suggests that patients are primarily selected to receive intensive chemotherapy and only thereafter for other strategies if patients are deemed unfit for chemotherapy because of the comorbidities, adverse cytogenetics, or the patient’s choice. Based on the subjective criteria used to select treatment in routine practice, we have previously identified three distinct groups of AML patients aged > 60 years [8]: i.e., (i) the intensive-chemotherapy group included the “youngest” patients with proliferative, de novo AML, or non-adverse cytogenetics, (ii) the group that received a hypomethylating agent included patients with a low WBC count, secondary AML, and adverse cytogenetics, and (iii) the best supportive-care group included the “oldest” patients that also had proliferative AML. In the present study, it seems that this same selection was applied to patients with t-AML and sAML, at least regarding the choice between chemotherapy and azacitidine, as patients selected for chemotherapy had less adverse cytogenetics, higher WBC counts, and were younger, with the latter two factors being the independent factors of choice in our multivariate analyses.
Reflecting the difficulty in including sufficient numbers of cases to make a thorough comparison between chemotherapy and azacitidine, we could only match 18 patients from each group based on the propensity score method, which precluded any relevant analysis in these matched subgroups. However, our adjusted analyses were robust enough to support our findings. Indeed, although we cannot avoid the biases inherent in this observational (non-randomized) study, we used multivariate analyses adjusted for all parameters known to influence the outcomes in AML. Finally, we can at least infer that the midterm prognosis of older patients with higher risk disease treated by azacitidine is similar to that of younger patients selected for intensive chemotherapy.
Overall, azacitidine appeared to be a reasonable alternative to chemotherapy as it provided similar midterm outcomes with less toxicity when compared to chemotherapy in patients aged ≥60 years that had t-AML/sAML, similar de novo AML [5, 7, 8]. Finally, we acknowledge that the impact of both therapeutic strategies on the quality of life (QOL) is a key point. Although we cannot provide data on QOL because of our study’s design, no clear difference in QOL was found between azacitidine and conventional care in the AZA-AML-001 trial.
In our series, ∼80% of patients had a high level of serum ferritin, and hyperferritinemia was independently associated with lower OS. As a marker of red blood cell transfusion burden, increased serum ferritin levels have been associated with worse outcomes in MDS and sAML because of the negative impact of iron overload [12,13]. We have previously shown that hyperferritemia also impacts on the OS of younger patients with de novo AML who do not have post-transfusion iron-overload at diagnosis [14]. Serum ferritin could thus affect the prognosis of de novo and secondary AML through multiple mechanisms, including resistance to chemotherapy and likely also azacitidine.
In conclusion, the prognosis of secondary AML remains very poor, with future therapies and progress urgently needed. New therapeutic strategies that include hypomethylating agents or chemotherapy combined with novel drugs are being intensively trialed [15,16]. Such therapies should hopefully improve the outcomes for this difficult-to-treat AML population [17].
MATERIALS AND METHODS
Patients and treatments
The selection criteria for this retrospective study were the following: diagnosis of AML according to WHO criteria [1] (excluding acute promyelocytic leukemia and core-binding factor AML) that were made between January 1st, 2007 and December 31st 2013 in Toulouse or Bordeaux University Hospitals. Patients were aged ≥60 years and had no previous treatment, except for hydroxyurea, secondary to (i) MDS/CMML diagnosed more than 3 months before AML (post MDS-AML), (ii) Philadelphia-negative MPN (post-MPN AML), or (iii) prior exposure to chemotherapy or radiotherapy (t-AML). All patients with post MDS-AML that had previously received azacitidine for MDS were excluded from the study.
The center’s policies slightly differed: in Toulouse center, an adaptive approach was applied in routine practice based on initial characteristics such as white blood cell count, cytogenetics, age, secondary AML, performance status and comorbidities. Briefly, the first issue was to judge if patients could benefit from intensive chemotherapy (i.e < 75years, favorable/intermediate cytogenetic risk, de novo AML). If not, the second issue was to determine if patients could benefit from azacitidine (regardless of the BM blast percentage) with a special attention paid to proliferative AML since high WBC was already described as a poor prognostic factor in patients treated by azacitidine, as previously described [8]. In Bordeaux center, same evaluation based on initial characteristics such as white blood cell count, cytogenetics, age, secondary AML, performance status and comorbidities was also applied but azacitidine was performed only for patients unfit with < 30% BM blasts.
Written informed consent was obtained in accordance with the Declaration of Helsinki, to allow the collection of clinical data from an anonymized database, registered at the Commission Nationale de l’Informatique et des Libertés (CNIL) under access No. 1778920. These data were retrospectively collected until November 2011 from Toulouse and until November 2012 from Bordeaux, and were then prospectively collected thereafter. Classification of cytogenetic risk was defined according to the MRC classification [18]. Data on comorbidities were collected according to the Charlson comorbidity index [19]. The regimens of intensive induction chemotherapy and azacitidine are detailed in Supplementary Table 1.
Assessment of safety and efficacy
See Supplementary File.
Statistical analyses
Before doing any analysis, we assessed the power of the study: 165 deaths (94 treated by azacitidine and 71 by chemotherapy) provided a power of > 80% to detect a hazard ratio (HR) of death of ≥1.6 (for azacitidine vs. chemotherapy), with a two-sided type-1 error rate of 5% (α = 0.05), for the comparison of two exponential survival distributions [20]. Statistical analyses were performed using STATA statistical software, release 11.2 (STATA Corp., College Station, TX). We described the patients’ characteristics using numbers and frequencies for qualitative data, and medians, inter-quartile ranges (IQR), and ranges (minimum−maximum) for quantitative data. Comparisons between the patients’ characteristics were assessed using Student’s t-test or ANOVA (Mann-Whitney or Kruskall-Wallis test when the distribution departed from normality or when homoscedasticity was rejected) for continuous variables, and the χ2-test (or Fisher’s exact test when there were small expected numbers) for categorical variables. Assessment of independent-choice criteria between azacitidine and chemotherapy was based on a logistic regression model. Comparison of OS after azacitidine vs. chemotherapy was assessed using Kaplan-Meier curves and the log-rank test in the univariate analyses. Because the proportional-hazards assumption was not respected for treatments (azacitidine vs. chemotherapy), we used a Royston and Parmar survival model [21]. Differences in early death and response rate were compared between treatments using a logistic regression model. Included in the multivariate analyses were variables (particularly differences between azacitidine and chemotherapy groups) that had a p-value of < 0.20 in the univariate analyses and remained significantly and independently associated with OS, early death, or response rate (p-value < 0.05), after backward analysis. Allogeneic stem-cell transplantation was evaluated as a time-dependent potential confounder. Interactions between azacitidine vs. chemotherapy and the independent covariates (particularly the AML subtypes, t-AML, post-MDS, or post-MPN AML) were tested in the final models. None were significant. All reported p-values were two-sided and the significance threshold was < 0.05.
Authorship
PYD, SB, CM, ST, TL, FH, EF, AS, MS, PB, and CR performed the research; PYD, SB, EB, NM, AP, and CR designed the research study; PYD, SB, EY, and EB analyzed the data; PYD, SB, EB, and CR wrote the paper.
SUPPLEMENTARY MATERIALS TABLES
Acknowledgments
We thank all the members of the G.A.E.L (Gaël Adolescent Espoir Leucémie) and A.G.M.O.M.P (Association des Greffés de Moelle Osseuse de Midi-Pyrénées) associations for their kind support regarding the patients. We also thank Sir Jean Delpouy, his family, and friends for their generous support. We also thank the data-management unit at Toulouse University Hospital for enabling e-CRF.
Footnotes
CONFLICTS OF INTEREST
C.R., N.M., and A.P. received honoraria from Celgene.
GRANT SUPPORT
This work has been approved by the National Research Agency (ANR), granted by the French government under the «Investissement d’avenir “ program (ANR-11-PHUC-001) and by the Groupement Interrégional de Recherche Clinique et d’Innovation-Sud Ouest Outre Mer (APITHEM 2014).
REFERENCES
- 1.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Ornstein MC, Mukherjee S, Mohan S, Elson P, Tiu RV, Saunthararajah Y, Kendeigh C, Advani A, Kalaycio M, Maciejewski JP, Sekeres MA. Predictive factors for latency period and a prognostic model for survival in patients with therapy-related acute myeloid leukemia. Am J Hematol. 2014;89:168–73. doi: 10.1002/ajh.23605. [DOI] [PubMed] [Google Scholar]
- 3.Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20:29–37. doi: 10.1016/j.beha.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A, Götze K, Rummel M, Nachbaur D, Schlegelberger B, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–45. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 5.Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, Friis LS, Kjeldsen E, Marcher CW, Preiss B, Severinsen M, Nørgaard JM. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:3641–9. doi: 10.1200/JCO.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 6.Ostgård LSG, Kjeldsen E, Holm MS, Brown PDN, Pedersen BB, Bendix K, Johansen P, Kristensen JS, Nørgaard JM. Reasons for treating secondary AML as de novo AML. Eur J Haematol. 2010;85:217–26. doi: 10.1111/j.1600-0609.2010.01464.x. [DOI] [PubMed] [Google Scholar]
- 7.Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, Récher C, Sandhu I, Bernal del Castillo T, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with > 30% blasts. Blood. 2015;126:291–9. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bories P, Bertoli S, Bérard E, Laurent J, Duchayne E, Sarry A, Delabesse E, Beyne-Rauzy O, Huguet F, Récher C. Intensive chemotherapy, azacitidine, or supportive care in older acute myeloid leukemia patients: an analysis from a regional healthcare network. Am J Hematol. 2014;89:E244–252. doi: 10.1002/ajh.23848. [DOI] [PubMed] [Google Scholar]
- 9.Ramos F, Thépot S, Pleyer L, Maurillo L, Itzykson R, Bargay J, Stauder R, Venditti A, Seegers V, Martínez-Robles V, Burgstaller S, Récher C, Debén G, et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: clinical use and outcome prediction. Leuk Res. 2015;39:296–306. doi: 10.1016/j.leukres.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Thépot S, Itzykson R, Seegers V, Recher C, Raffoux E, Quesnel B, Delaunay J, Cluzeau T, Marfaing Koka A, Stamatoullas A, Chaury MP, Dartigeas C, Cheze S, et al. Azacitidine in untreated acute myeloid leukemia: a report on 149 patients. Am J Hematol. 2014;89:410–6. doi: 10.1002/ajh.23654. [DOI] [PubMed] [Google Scholar]
- 11.Pleyer L, Burgstaller S, Girschikofsky M, Linkesch W, Stauder R, Pfeilstocker M, Schreder M, Tinchon C, Sliwa T, Lang A, Sperr WR, Krippl P, Geissler D, et al. Azacitidine in 302 patients with WHO-defined acute myeloid leukemia: results from the Austrian Azacitidine Registry of the AGMT-Study Group. Ann Hematol. 2014;93:1825–38. doi: 10.1007/s00277-014-2126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazzola M, Della Porta MG, Malcovati L. Clinical relevance of anemia and transfusion iron overload in myelodysplastic syndromes. Hematol Educ Program Am Soc Hematol Am Soc Hematol Educ Program. 2008;2008:166–75. doi: 10.1182/asheducation-2008.1.166. [DOI] [PubMed] [Google Scholar]
- 13.Lim ZY, Fiaccadori V, Gandhi S, Hayden J, Kenyon M, Ireland R, Marsh J, Ho AYL, Mufti GJ, Pagliuca A. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010;34:723–7. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Lebon D, Vergez F, Bertoli S, Harrivel V, De Botton S, Micol JB, Marolleau JP, Récher C. Hyperferritinemia at diagnosis predicts relapse and overall survival in younger AML patients with intermediate-risk cytogenetics. Leuk Res. 2015;39:818–21. doi: 10.1016/j.leukres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Montalban-Bravo G, Garcia-Manero G. Novel drugs for older patients with acute myeloid leukemia. Leukemia. 2015;29:760–9. doi: 10.1038/leu.2014.244. [DOI] [PubMed] [Google Scholar]
- 16.Feldman EJ. Novel Therapeutics for Therapy-Related Acute Myeloid Leukemia: 2014. Clin Lymphoma Myeloma Leuk. 2015;(15 Suppl):S91–93. doi: 10.1016/j.clml.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros B, Lancet J, Cortes J, Newell L, Lin T, Ritchie E. Analysis of Efficacy By Age for Patients Aged 60-75 with Untreated Secondary Acute Myeloid Leukemia (AML) Treated with CPX-351 Liposome Injection Versus Conventional Cytarabine and Daunorubicin in a Phase III Trial. Abstract 902, American Society of Hematology; 2016.
- 18.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK. National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Machin D. Sample size tables for clinical studies. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 21.Royston P. Parametric Survival Analysis Using Stata: Beyond the Cox Model. StataCorp LP: Stata Press books; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.