Abstract
Tumor necrosis (TN) is associated with worse prognosis in several solid cancers. Whether TN predicts poor outcome in natural killer cell / T cell lymphoma (NKTCL) is unclear. We investigated the clinical impact of TN on survival and other novel prognostic parameters in upper aero-digestive tract (UAT) NKTCL of 100 patients with limited stage. TN was significantly associated with poor performance status (p = 0.049), high Korean Prognostic Index score (p = 0.024), high C-reactive protein/albumin ratio (p = 0.003), higher maximum standard uptake value on positron emission tomography/computed tomography (PET/CT) (p = 0.008) and higher metabolic tumor volume (MTV) on PET/CT (p < 0.001). In univariate and multivariate analyses, progression-free survival and overall survival were independently associated with High MTV status (p = 0.001, p = 0.032), TN (p = 0.018, p = 0.009), local tumor invasiveness (p = 0.007, p = 0.035), complete resection (p = 0.020, p = 0.028) and regional lymph node involvement (p < 0.001, p < 0.001). TN and complete resection are concluded to be novel independent prognostic factors in patients with UAT NKTCL.
Keywords: extranodal natural killer/T-cell lymphoma, tumor necrosis, complete resection, prognosis
INTRODUCTION
Extranodal (EN) natural killer cell/T cell lymphoma (NKTCL) is a clinicopathologic disease entity with an aggressive clinical course [1, 2]. NKTCL usually involves the nasal cavity, nasopharynx and other upper aerodigestive tract (UAT) sites, although it can arise in any EN site [3-5].
Radiotherapy (RTx) is an important therapeutic modality in NKTCL, reflecting the radiosensitivity of the lymphoma. RT alone in early stage NKTCL can yield excellent overall and complete response rates [6]. However, systemic relapse after RTx alone occurs in up to 40 % of patients. Therefore, chemotherapy (CTx) combined with RTx has been used to improve clinical outcomes. Two recent prospective trials studied whether concurrent chemoradiotherapy can improve the clinical benefits of radio-sensitization in NKTCL [5, 7, 8]. The current guidelines of the National Comprehensive Cancer Network are equivocal regarding the optimal therapy for limited stage nasal NKTCL and include RTx alone, sequential CTx or concurrent chemoradiotherapy. Selection of an optimal therapy according to proper risk stratification in NKTCL is necessary to further improve the clinical outcome.
Several prognostic models for NKTCL including the International Prognostic Index (IPI) and Korean Prognostic Index (KPI), and positron emission tomography/ computed tomography (PET/CT) related parameters such as maximum standard uptake (SUVmax) have been proposed to accurately predict the clinical outcome [9, 10]. Furthermore, recent study reported that combined analysis of both circulating EBV DNA and post-treatment PET-CT response could predict recurrence of NKTCL in patients treated with current chemoradiotherapy or non-anthracycline-based chemotherapy [11]. However, their predictive values for the survival remain controversial. Other prognostic models studied for NKTCL include the Prognostic Index for T-cell lymphoma (PIT). This new model combined the KPI, Glasgow Prognostic Score (GPS) and metabolic tumor volume (MTV) on PET/CT [12-14].
Tumor necrosis (TN) is a recognized consequence of chronic hypoxic injury of cancer cells due to rapid tumor growth. Increased cellular hypoxia in cancer is associated with increased potential of distant metastasis and resistance to RTx and CTx [15, 16]. Because NKTCL is an angiocentric angio-destructive lymphoproliferative neoplasm, TN is a common clinical feature in NKTCL. However, the clinical importance of TN on survival is unknown.
Examination of TN as a meaningful negative prognostic factor interfering with RTx or CTx in NKTCL is necessary. This retrospective study investigated the clinical impact of TN on survival in patients with nasal NKTCL, as well as the importance of other clinical prognostic parameters.
RESULTS
Patient characteristics
The clinical characteristics of the 100 patients with limited stage nasal NKTCL are summarized in Table 1. Seventy-seven patients (77.0 %) were male and 45 patients (45.0 %) were > 60 years of age. Thirty-one patients (31.0 %) have regional nodal involvement and poor Eastern Cooperative Oncology Group (ECOG) performance status (PS) above grade 2 was present in 12 patients (12.0 %). Elevated lactate dehydrogenase (LDH) level was present in 29 patients (29.0 %) and B symptoms was present in 21 patients (21.0 %). Local tumor invasiveness was present in 22 patients (22.0 %).
Table 1. Baseline characteristics in patients with ENKTL.
| Characteristics | Total (n = 100) |
TN group (n = 41) |
No TN group (n = 59) |
p-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 77 (77.0) | 29 (29.0) | 48 (48.0) | 0.541 |
| Female | 23 (23.0) | 12 (12.0) | 11 (11.0) | |
| Age (years) | ||||
| > 60 years | 45 (45.0) | 20 (20.0) | 25 (25.0) | 0.529 |
| ≤ 60 years | 55 (55.0) | 21 (21.0) | 34 (34.0) | |
| Regional nodal involvement | ||||
| Yes | 31 (31.0) | 16 (16.0) | 15 (15.0) | 0.150 |
| No | 69 (69.0) | 25 (25.0) | 44 (44.0) | |
| ECOG PS | ||||
| ≥ 2 | 12 (12.0) | 8 (8.0) | 4 (4.0) | 0.049 |
| 0-1 | 88 (88.0) | 33 (33.0) | 55 (55.0) | |
| Serum LDH level | ||||
| High than normal | 29 (29.0) | 15 (15.0) | 14 (14.0) | 0.166 |
| Normal | 71 (71.0) | 26 (26.0) | 45 (45.0) | |
| B symptoms | ||||
| Yes | 21 (21.0) | 12 (12.0) | 9 (9.0) | 0.092 |
| No | 79 (79.0) | 29 (29.0) | 50 (50.0) | |
| IPI score | ||||
| 0-2 | 91 (91.0) | 35 (35.0) | 56 (56.0) | 0.102 |
| ≥ 3 | 9 (9.0) | 6 (6.0) | 3 (3.0) | |
| Local tumor invasiveness | ||||
| Yes | 22 (22.0) | 10 (10.0) | 12 (12.0) | 0.630 |
| No | 78 (78.0) | 31 (31.0) | 47 (47.0) | |
| Korean prognostic Index (KPI) | ||||
| High KPI score ≥ 3 points | 11 (11.0) | 8 (8.0) | 3 (3.0) | 0.024 |
| Glasgow prognostic score (GPS) | ||||
| High GPS (2 points) | 31 (31.0) | 17 (17.0) | 14 (14.0) | 0.061 |
| CRP/albumin ratio (CAR) | ||||
| High CAR | 22 (22.0) | 15 (15.0) | 7 (7.0) | 0.003 |
| SUVmax on PET/CT | ||||
| Median (range) | 6.4 (2.9-22.3) | 8.4 (2.9-21.1) | 5.2 (3.1-22.3) | 0.008 |
| Metabolic tumor volume on PET/CT | ||||
| Median (range) | 36.2 (5.1-1164.9) | 84.5 (5.1-1164.9) | 32.8 (6.2-337.0) | <0.001 |
| Treatment | ||||
| Complete resection group | 37 (37.0) | 13 (13.0) | 24 (24.0) | 0.363 |
| CMT | 8 (8.0) | 3 (3.0) | 5 (5.0) | 0.399 |
| Chemotherapy only | 29 (29.0) | 10 (10.0) | 19 (19.0) | |
| Only biopsy group | 63 (63.0) | 28 (28.0) | 35 (25.0) | 0.363 |
| CMT | 56 (56.0) | 26 (26.0) | 30 (30.0) | 0.490 |
| Chemotherapy only | 7 (7.0) | 2 (2.0) | 5 (5.0) |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase; IPI, International Prognostic Index; SUVmax, maximum standard uptake value; PET/CT, positron emission tomography; CT, computed tomography, CMT, combined modality treatment (concurrent chemoradiotherapy with chemotherapy)
We also analyzed distribution of the patients based on famous prognostic scores such as KPI, Glasgow prognostic score (GPS) and serum C-reactive protein/albumin ratio (CAR). Eleven patients (11.0 %) have high KPI score (≥ 3 points) and 31 patients (31.0 %) have high GPS (2 points). Determination for cut-off value between High and low CAR were performed by Receiver operating characteristic (ROC) curve analysis. According to ideal cut off value, 0.21 by the analysis, 22 patients (22.0 %) have high CAR (CAR ≥ 0.21) (Table 1).
Clinical characteristics and clinical impact of TN and complete resection
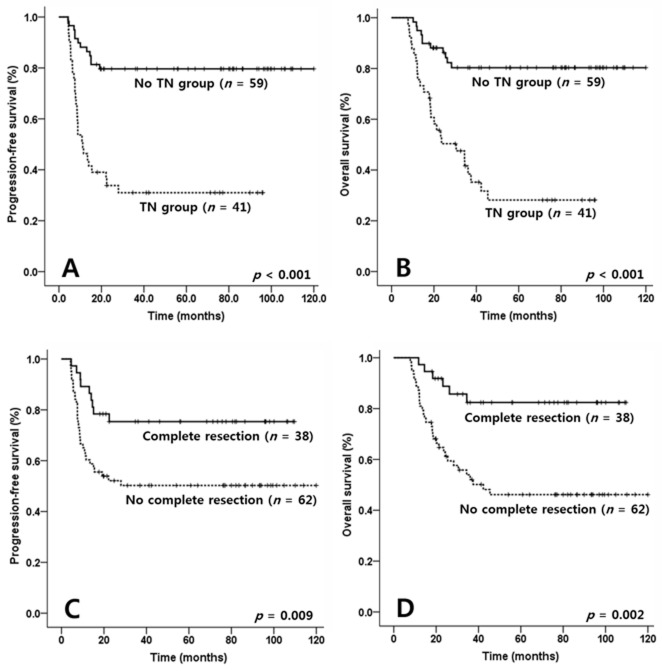
Forty-one patients displayed TN group and 59 patients did not. Adverse features that were significantly more prevalent in those with TN included poor ECOG PS above than grade 2 (p = 0.049), high KPI score (p = 0.024), high CAR (p = 0.003), higher SUVmax (p = 0.008) and higher MTV on PET/CT (p < 0.001) (Table 1). Survival was compared in the TN and no TN groups to ascertain the involvement of TN with worse survivals in addition to the aforementioned adverse features. Kaplan-Meier curves revealed shorter progression-free survival (PFS) and overall survival (OS) in the TN group (p < 0.001 and p < 0.001, respectively; Figures 1A and 1B).
Figure 1. Comparisons of progression-free survival (PFS) and overall survival (OS) according to tumor necrosis (TN) and complete resection in patients with NK/T cell lymphoma (NKTCL).
PFS and OS of TN group were inferior compared with no TN group in patients with NKTCL (p < 0.001, p < 0.001, Figure 1A & 1B). Meanwhile, survivals of complete resection group were superior compared with no complete resection group in the patients (p = 0.009, p = 0.002, Figure 1C & 1D).
Fifty-six (56.0 %) in only biopsy group and only 8 (8.0 %) in complete resection group received concurrent chemoradiotherapy. The complete resection group had relatively lower radiation exposure rate than only biopsy group (median 20.0 Gy in complete resection group versus median 45.0 Gy in only biopsy group, p < 0.001, data not shown). However, PFS and OS in complete resection group were superior compared with only biopsy group (p = 0.009 and p = 0.002, respectively; Figures 1C and 1D).
Clinical values of prognostic factors on survival
Clinical values of age > 60 years, male sex, regional LN involvement, poor ECOG PS ≥ grade 2, elevated LDH level, B symptoms, local tumor invasiveness, high KPI score ≥ 3 points, high GPS (2 points), high CAR (≥ 0.21), high SUVmax, high MTV status, TN and complete resection on survivals were analyzed. To determine ideal cut-off values of the SUVmax and MTV on survivals, the analysis ROC curve analysis was performed. The analysis indicated that ideal cut-off value of SUVmax and MTV was 8.5 and 94.2 cm3, respectively (sensitivity versus [vs.] specificity, 90.3% vs. 64.4% in SUVmax; 90.1% vs. 70.3% in MTV; data not shown).
To analyzed the clinical impact of the above prognostic factors on survivals, univariate analysis was performed. In the analysis, elevated LDH level (p = 0.034; p = 0.045), poor ECOG PS ≥ grade 2 (p = 0.047; p = 0.021), high SUV max ≥ 8.5 (p = 0.002; p = 0.007), high MTV ≥ 94.2 cm3 (p < 0.001; p < 0.001), TN (p < 0.001; p < 0.001), local tumor invasiveness (p < 0.001; p < 0.001), complete resection (p = 0.009; p = 0.002), regional LN involvement (p < 0.001; p < 0.001) and presence of B symptoms (p = 0.017; p = 0.018), high KPI score (p = 0.012; p = 0.033), high CAR (p < 0.001; p < 0.001) and high GPS (p = 0.009; p = 0.007) were significantly associated with PFS and OS in limited stage nasal NKTCL (Table 2).
Table 2. Univariate and multivariate analysis of prognostic factors in patients with ENKTL.
| Progression-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| P-value | HR (95% CI) | p-value | P-value | HR (95% CI) | p-value | |
| Age > 60 years | 0.515 | — | — | 0.605 | — | — |
| Male sex | 0.607 | — | — | 0.504 | — | — |
| Elevated LDH level | 0.034 | 2.093 (0.780-5.614) | 0.142 | 0.045 | 0.891 (0.354-2.239) | 0.805 |
| ECOG PS above grade 2 | 0.047 | 0.804 (0.274-2.354) | 0.690 | 0.021 | 0.693 (0.208-2.307) | 0.550 |
| High SUVmax on PET/CT | 0.002 | 1.796 (0.881-3.663) | 0.107 | 0.007 | 0.614 (0.257-1.468) | 0.273 |
| High MTV status | <0.001 | 3.810 (1.751-8.293) | 0.001 | <0.001 | 2.473 (1.492-6.162) | 0.032 |
| Tumor necrosis | <0.001 | 3.015 (1.210-7.508) | 0.018 | <0.001 | 3.482 (1.365-8.888) | 0.009 |
| Local tumor invasiveness | <0.001 | 2.962 (1.347-6.513) | 0.007 | <0.001 | 2.484 (1.067-5.787) | 0.035 |
| Complete resection | 0.009 | 0.299 (0.108-0.829) | 0.020 | 0.002 | 0.305 (0.106-0.879) | 0.028 |
| Regional LN involvement | <0.001 | 5.728 (2.428-13.513) | <0.001 | <0.001 | 5.037 (2.284-11.203) | <0.001 |
| B symptoms | 0.017 | 0.408 (0.148-1.122) | 0.082 | 0.018 | 1.301 (0.417-4.057) | 0.650 |
| High KPI score | 0.012 | 0.685 (0.187-2.510) | 0.568 | 0.033 | 0.407 (0.102-1.620) | 0.202 |
| High CAR | <0.001 | 2.038 (0.849-4.881) | 0.111 | <0.001 | 1.461 (0.591-3.611) | 0.411 |
| High GPS | 0.009 | 0.853 (0.393-1.848) | 0.686 | 0.007 | 1.758 (0.792-3.902) | 0.165 |
LDH, lactate dehydrogenase; ECOG, Eastern Cooperattive Group; PS, performance status; EN, extranodal; SUVmax, maximum standard uptake value; PET/CT, positron emission tomography/computed tomography; MTV, metabolic tumor volume; LN, lymph node; KPI, Korean Prognostic Index; CAR, C-reactive protein/albumin ratio; GPS, Glasgow prognostic score.
In order to measure independent clinical impacts of significant prognostic factors in univariate analysis on survivals, multivariate analysis was performed. High MTV status (PFS, HR = 3.810, 95% CI = 1.751 – 8.2935, p = 0.001; OS, HR = 2.473, 95% CI = 1.492 – 6.162, p = 0.032), TN (PFS, HR = 3.015, 95% CI = 1.210 – 7.508, p = 0.018; OS, HR = 3.482, 95% CI = 1.365 – 8.888, p = 0.009), local tumor invasiveness (PFS, HR = 2.962, 95% CI = 1.347 – 6.513, p = 0.007; OS, HR = 2.484, 95% CI = 1.067 – 5.787, p = 0.035), complete resection (PFS, HR = 0.299, 95% CI = 0.108 – 0.829, p = 0.020; OS, HR = 0.305, 95% CI = 0.106 – 0.879, p = 0.028) and regional LN involvement (PFS, HR = 5.728, 95% CI = 2.428 – 13.513, p < 0.001; OS, HR = 5.037, 95% CI = 2.284 – 11.203, p < 0.001) were independently associated with survival in patients with limited stage nasal NKTCL.
High volume TN and unresected TN predicts worse survival
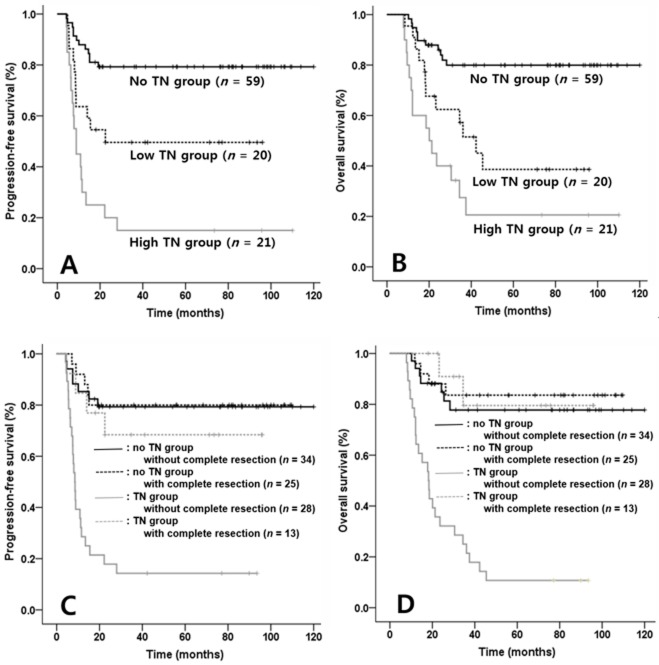
In order to identify the extent of TN that adversely affect survival, depending on median value of NTV as the cut-off value (median value, 97.1 cm3; range, 2.3 – 789.3 cm3; data not shown), the TN group was divided into high NTV (≥ 97.1 cm3, n = 21), low NTV (<97.2 cm3, n = 20) and no TN group (n = 59). PFS and OS among the three groups were compared and Kaplan-Meier survival curves were showed that PFSs were significantly different between no TN and low NTV group (p = 0.036) and between low and high NTV group (p = 0.002; Figure 2A). Similarly, OSs were different between no TN and low NTV group (p = 0.008) and between low and high NTV group (p = 0.010; Figure 2B).
Figure 2. Comparisons of progression-free survival (PFS) and overall survival (OS) according to extent of tumor necrosis (TN) and combined two factors such as TN and complete resection in patients with NK/T cell lymphoma (NKTCL).
Each PFS and OS among three groups divided according to extent of TN was significantly different in patients with NKTCL (PFS & OS between No TN & low NTV group, p = 0.027 & p = 0.007; PFS & OS between low NTV and high NTV group, p = 0.004 & p = 0.025, Figure 2A & 2B). Meanwhile, in comparisons according to combined two factors including TN and complete resection, TN group not received complete resection had lowest PFS and OS than other three groups (p < 0.001, p < 0.001, Figure 2C & 2D).
We also compared the combined clinical impacts of TN and complete resection as independent prognostic factors on survival. In the survival analysis, TN group without complete resection displayed inferior PFS and OS compared with other three groups (PFS, p < 0.001, Figure 2C; OS, p < 0.001, Figure 2D). Differences of survivals among other three groups (TN group with complete resection, no TN group with complete resection and no TN group without complete resection) were not present.
DISCUSSION
High MTV status, TN, local tumor invasiveness, complete resection and regional LN involvement were independently associated with PFS and OS in patients with nasal NKTCL. Local tumor invasiveness and regional LN involvement are clinical prognostic factors [12, 17]. Their clinical impact on survival was reaffirmed here. High MTV status as a measure of high tumor burden in NKTCL also had reliable prognostic values presently and in a recent clinical study [14].
The results implicate TN and complete resection as novel meaningful prognostic factors could significantly predict survival in NKTCL. TN is mainly caused by tissue hypoxia due to rapid tumor growth, insufficient neovascularization, compression and thrombotic obstruction of adjacent vessels of cancer. Recently, histopathologically-identified TN has been considered as an important prognostic factor for several solid cancer types including breast, lung, pancreas and kidney [15, 18-20]. We also found significant differences in survival between TN and no TN group in patients with (PFS, 79.7 % & OS, 81.4 % in no TN group versus 31.7 % and 34.1 % in TN group, respectively). Thus, TN could influence survival in UAT NKTCL.
FDG uptake of necrotic area is generally low due to cancer cell death. However, the present study showed that SUVmax in TN group was higher than non-TN group. The value of SUVmax at peri-necrotic area in many cases was high, even if the central necrotic area was low. TN is well-known to be significantly associated with increased angiogenesis, inflammation and cell proliferation which reflect tumor aggressiveness [21]. Therefore, we hypothesized that the excessive inflammation and angiogenesis stimulated by the tissue hypoxia could actively occur in peri-necrotic area, and ultimately increase metabolic activity of the area. Similarly, a previous laboratory study [22] demonstrated that newly formed granulation tissue and tumor-associated macrophages infiltrating the marginal areas surrounding the necrotic area of the tumor show a high uptake of 18F-FDG.
The extent of TN also affected prognosis in patients with NKTCL. Survival in the no TN group and the low and high necrotic tumor volume (NTV) subgroups based on median value of NTV were significantly different. Therefore, a higher extent of TN might be correlated with inferior survival in NKTCL. High NTV based on CT scan was reported to be lower in patients with recurrent head and neck cancer than control patients [23]. Tumors with less extensive necrosis (< 4 cm3) were associated with good prognosis regardless of the type of treatment. In several cancer states other than NKTCL, some studies have similarly described that larger TN volume reflects aggressive behavior and poor survival in patients [24, 25].
Although NKTCL is a radiosensitive lymphoma subtype, the optimal treatment strategy for nasal NKTCL is not clear. Hypoxic volume has been reported to be the strongest predictor of the response to RT and survival [26, 27]. TN could have a negative impact on survival by reducing radio-sensitivity. Necrotic components due to the discriminative angiocentric and angio-destructive propensity of NKTCL could diminish the efficacy of treatment. Therefore, a novel treatment strategy able to overcome the drawback of current treatment strategies is needed. Presently, the survival in complete resected patients with NKTCL was meaningfully superior compared with other patients not receiving complete resection, especially compared to the no TN group. In a prior study, only 3 of the cohort of 26 NKTCL patients received surgical resection [28]. Interestingly, these 3 patients were the only long-term survivors. Although clinical data about the prognostic value of surgical resection in NKTCL are limited, surgical resection in NKTCL may be linked with a favorable prognosis.
Concurrent chemoradiotherapy is one of optimal therapy in early stage NKTCL and has showed an excellent response rate and favorable survival benefits [7, 8]. However, malnutrition due to significant painful mucositis and xerostomia during or after concurrent chemoradiotherapy leads to adverse consequences including poor quality of life, decreased immune function and shorter survivals [29-31]. Nutritional deficiency in elderly patients is more frequent than young patients and previous data showed that older patients received little benefit from concurrent chemoradiotherapy and had lower tolerance and increased toxicity of the treatment [32-34]. Because median age at the time of diagnosis of UAT NKTCL was reported to be from 50 to 60 years, many of those would be included in elderly patients above than 60 years [35]. Therefore, the above adverse features from concurrent chemoradiotherapy might reduce response rate and survivals in UAT NKTCL, although it was reported be tolerable treatment modality.
Endoscopic resection and robotic surgery have shown excellent treatment results, quick recovery and very low sequelae in head and neck cancer [36, 37]. Therefore, surgical resection in nasal NKTCL could be an alternate treatment modality in patients with extensive TN not eligible for RTx or CTx.
Evaluation of the combined prognostic values of TN and complete resection as independent factors on survival showed that patients with TN without complete resection had inferior survival. Moreover, based on Figure 2C and 2D, the results showed that clinical impact of TN was maximized in patients who did not receive complete resections compared with those received complete resection. Therefore, the existence of unresected TN appears to have important prognostic significance on the survival in patients with nasal NKTCL. However, the present study has a limitation that TN is affirmed by only imaging modality, but not pathologic confirmation. Therefore, we regard that the pathologic identification for TN would be needed to confirm the clinical impact in NKTCL.
In conclusion, TN and complete resection are novel prognostic factors with clinical impact on survival in nasal NKTCL. Especially, TN contained lymphoma mass in the nasal cavity could counteract the clinical effect of RTx and CTx. TN should be eliminated completely to improve survival in nasal NKTCL. Further well designed clinical studies are warranted to confirm our results.
PATIENTS AND METHODS
Patient eligibility
From April 2006 to July 2015, 100 patients with limited stage nasal NKTCL were enrolled from four institutes. The patients were newly diagnosed as limited stage nasal NKTCL and had not been previously treated for the disease. The patients received concurrent chemoradiotherapy with or without non-anthracycline-based CTx or non-anthracycline-based CTx only. Patients with non-nasal NKTCL, those with other malignant diseases and those who received anthracycline-based CTx were excluded. Moreover, those who received autologous stem cell transplantation as a consolidation treatment were excluded in the present study.
All patients underwent staging procedures including physical examination, complete blood cell counts and blood chemistry, bilateral bone marrow biopsy, and imaging study including CT scan, magnetic resonance imaging and 18F-FDG-PET/CT scan. Nasal involvement was affirmed by the imaging studies. All pathologic specimens were classified based on strict morphologic criteria along with immunophenotypic findings (World Health Organization classification). Patient data were obtained by review of electric medical records by local doctors. Approval for the retrospective review of these records was obtained from the Institutional Review Boards of all participating medical centers.
Assessment of MTV on PET/CT
Dual-modality PET/CT tomography was performed using a biograph (Siemens Medical Solution, Hoffman Estates, IL, USA) according to the major guideline for standard oncological PET imaging [38]. Briefly, the patients fasted for at least 6 h prior to the intravenous administration of 18F-FDG (7.4 MBq per body weight) to ensure a serum glucose level below 7.2 mmol/L. At 60 min after 18F-FDG administration, transmission data were acquired using low-dose CT (120 kV, automated from 10 to 130 mA, a 512 × 512 matrix, a 50-cm field of view (FOV), 3.75-mm slice thickness, and a rotation time of 0.8 s), extending from the base of the skull to the proximal thighs. Immediately after CT acquisition, PET emission scans were acquired in the same anatomic locations with a 15.7-cm axial FOV acquired in the two-dimensional mode with a 128 × 128 matrix. The CT data were used for attenuation correction. The images were reconstructed using a conventional iterative algorithm (OSEM). A workstation (AW Volume Share™) providing multi-planar reformatted images was also used for image display and analysis. A target area having SUV ≥ 2.5 was defined as a lymphoma involvement area [39, 40]. Among all lesions detected on the PET/CT scan, highest FDG uptake valued area was defined as SUVmax. MTV regions on PET images were evaluated for nodal and EN area having the increased tracer uptake in patients with NKTCL. CT images of PET/CT were used for the attenuation correction. Corrected emission data images were reconstructed after Fourier transformation with AWOSEM software (2 iterations, 8 subsets, 5 mm Gaussian filter)
Definition and volumetric assessment of tumor necrosis in UAT NKTCL
The TN area on CT scans was defined as an area of diminished or non-enhancement of the mass lesion after intravenous administration of contrast material. TN in the lymph node was not considered in this measurement. The TN group comprised patients with TN detected on CT scan or MRI at the time of diagnosis. The volume of necrotic tumor area (NTV) was measured with manual segmentation (Voxar 3D ActiveX; Toshiba Medical Visualization Systems, Edinburgh, Scotland) [41]. All CT data were independently reviewed by two radiologists. The assessment of TN was decided by consensus agreement. Subsequently, the imaging data of patients with TN was centrally reviewed by expert radiologists.
Treatment modality
The treatment option was selected according to physician judgment at each medical center. The only biopsy group comprised 63 patients confirmed only by tissue biopsy at a pathologic lesion, such as a nasal mass or pathologic LN of NKTCL. The complete resection group included 37 patients who were confirmed by complete resection for the primary EN lymphoma mass within UAT, but not LN.
Fifty-six of the 63 only biopsy group received concurrent chemoradiotherapy followed by non-anthracycline-based CTx. Concurrent chemoradiotherapy was performed by weekly cisplatin administration. (RTx, median, 45.0 Gy; range, 38.0-50.0 Gy). The remaining 7 received only non-anthracycline based CTx. (median 6 cycles were performed in only biopsy group; range 5 - 8 cycles). Eight complete resection group patients received concurrent chemoradiotherapy for remnant lesions after discontented resection. (median, 20.0 Gy; range, 18.0-23.0 Gy). The remaining 29 complete resection patients received only non-anthracycline-based CTx (median 6 cycles were performed in complete resection group; range, 5 - 8 cycles). Non-anthracycline CTx regimens were ifosfamide, methotrexate, and etoposide (IMEP, 19 patients received); ifosfamide, etoposide, dexamethasone, and L-asparaginase (VIDL, 56 patients received); ifosfamide, etoposide, cisplatin and dexamethasone (VIPD, 25 patients received). The above 29 complete resection patients were treated with the CTx such as IMEP (6 patients), VIPD (6 patients), and VIDL (17 patients).
Statistical analyses
The Mann–Whitney U-test was used to compare differences in manifestations of prognostic factors in patients with nasal NKTCL. ROC curve estimated the accuracy in predicting the ideal cut-off value of CAR, SUVmax and MTV. Estimation of sensitivity and specificity was based on the cut-off value of MTV. PFS was calculated from the date of diagnosis to document disease progression. OS was calculated from the date of diagnosis until either death as a result of any cause, or the date last known to be alive. Kaplan-Meier method was used to evaluate PFS and OS. By log-rank test, differences of the survivals were compared. Statistical analysis was carried out with SPSS software version 18.0 (SPSS Inc., Chicago, IL). A probability value <0.05 was considered statistically significant.
Acknowledgments
All authors contributed significantly to this study, and all agree with the content of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO, Liang R. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, Abe M, Kinoshita T, Yoshino T, Iwatsuki K, Kagami Y, Tsuzuki T, Kurokawa M, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann oncol. 2010;21:1032–1040. doi: 10.1093/annonc/mdp418. [DOI] [PubMed] [Google Scholar]
- 3.Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, Wang SL, Liu XF, Zhou LQ, He XH, Lu N, Yu ZH. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24:181–189. doi: 10.1200/JCO.2005.03.2573. [DOI] [PubMed] [Google Scholar]
- 4.Liu QF, Wang WH, Wang SL, Liu YP, Huang WT, Lu N, Zhou LQ, Ouyang H, Jin J, Li YX. Immunophenotypic and clinical differences between the nasal and extranasal subtypes of upper aerodigestive tract natural killer/T-cell lymphoma. Int J Radiat Oncol Phys. 2014;88:806–813. doi: 10.1016/j.ijrobp.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Vazquez A, Khan MN, Blake DM, Sanghvi S, Baredes S, Eloy JA. Extranodal natural killer/T-Cell lymphoma: A population-based comparison of sinonasal and extranasal disease. Laryngoscope. 2014;124:888–895. doi: 10.1002/lary.24371. [DOI] [PubMed] [Google Scholar]
- 6.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121:4997–5005. doi: 10.1182/blood-2013-01-453233. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, Ishizawa K, Maseki N, Itoh K, Usui N, Wasada I, Kinoshita T, Ohshima K, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27:5594–5600. doi: 10.1200/JCO.2009.23.8295. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, Lee SW, Kim JS, Cho J, Lee GW, Kang KM, Eom HS, Pyo HR, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27:6027–6032. doi: 10.1200/JCO.2009.23.8592. [DOI] [PubMed] [Google Scholar]
- 9.Cai QQ, Luo XL, Zhang GR, Huang HQ, Huang H, Lin TY, Jiang WQ, Xia ZJ, Young KH. New prognostic model for extranodal natural killer/T cell lymphoma, nasal type. Ann Hematol. 2014;93:1541–1549. doi: 10.1007/s00277-014-2089-x. [DOI] [PubMed] [Google Scholar]
- 10.Suh C, Kang YK, Roh JL, Kim MR, Kim JS, Huh J, Lee JH, Jang YJ, Lee BJ. Prognostic Value of Tumor F-18-FDG Uptake in Patients with Untreated Extranodal Natural Killer/T-Cell Lymphomas of the Head and Neck. J Nucl Med. 2008;49:1783–1789. doi: 10.2967/jnumed.108.053355. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Choi JY, Hyun SH, Ki CS, Oh D, Ahn YC, Ko YH, Choi S, Jung SH, Khong PL, Tang T, Yan XX, Lim ST, et al. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol. 2015;2:E66–E74. doi: 10.1016/S2352-3026(15)00002-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, Lee DH, Huh J, Oh SY, Kwon HC, Kim HJ, Lee SI, Kim JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612–618. doi: 10.1200/JCO.2005.04.1384. [DOI] [PubMed] [Google Scholar]
- 13.Li YJ, Jiang WQ, Huang JJ, Xia ZJ, Huang HQ, Li ZM. The Glasgow Prognostic Score (GPS) as a novel and significant predictor of extranodal natural killer/T-cell lymphoma, nasal type. Am J Hematol. 2013;88:394–399. doi: 10.1002/ajh.23422. [DOI] [PubMed] [Google Scholar]
- 14.Song MK, Chung JS, Shin HJ, Moon JH, Ahn JS, Lee HS, Lee SM, Lee GW, Kim SJ, Lee SM. Clinical value of metabolic tumor volume by PET/CT in extranodal natural killer/T cell lymphoma. Leuk Res. 2013;37:58–63. doi: 10.1016/j.leukres.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Swinson DE, Jones JL, Richardson D, Cox G, Edwards JG, O’Byrne KJ. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: correlation with biological variables. Lung cancer. 2002;37:235–240. doi: 10.1016/s0169-5002(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 16.Langner C, Hutterer G, Chromecki T, Leibl S, Rehak P, Zigeuner R. Tumor necrosis as prognostic indicator in transitional cell carcinoma of the upper urinary tract. J Urol. 2006;176:910–913. doi: 10.1016/j.juro.2006.04.019. discussion 913–914. [DOI] [PubMed] [Google Scholar]
- 17.Kim TM, Park YH, Lee SY, Kim JH, Kim DW, Im SA, Kim TY, Kim CW, Heo DS, Bang YJ, Chang KH, Kim NK. Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage I(E)/II(E) extranodal NK/T-cell lymphoma, nasal type. Blood. 2005;106:3785–3790. doi: 10.1182/blood-2005-05-2056. [DOI] [PubMed] [Google Scholar]
- 18.Fisher ER, Palekar AS, Gregorio RM, Redmond C, Fisher B. Pathological findings from the national surgical adjuvant breast project (Protocol No. 4). IV. Significance of tumor necrosis. Hum Pathol. 1978;9:523–530. doi: 10.1016/s0046-8177(78)80133-6. [DOI] [PubMed] [Google Scholar]
- 19.Hiraoka N, Ino Y, Sekine S, Tsuda H, Shimada K, Kosuge T, Zavada J, Yoshida M, Yamada K, Koyama T, Kanai Y. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer. 2010;103:1057–1065. doi: 10.1038/sj.bjc.6605854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 21.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer metastasis Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 23.Kuhnt T, Mueller AC, Pelz T, Haensgen G, Bloching M, Koesling S, Schubert J, Dunst J. Impact of tumor control and presence of visible necrosis in head and neck cancer patients treated with radiotherapy or radiochemotherapy. J Cancer Res Clin Oncol. 2005;131:758–764. doi: 10.1007/s00432-005-0018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martel MK, Strawderman M, Hazuka MB, Turrisi AT, Fraass BA, Lichter AS. Volume and dose parameters for survival of non-small cell lung cancer patients. Radiother Oncol. 1997;44:23–29. doi: 10.1016/s0167-8140(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 25.Miller TR, Grigsby PW. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int J Radiot Oncol Biol Phys. 2002;53:353–359. doi: 10.1016/s0360-3016(02)02705-0. [DOI] [PubMed] [Google Scholar]
- 26.Dunst J, Stadler P, Becker A, Lautenschlager C, Pelz T, Hansgen G, Molls M, Kuhnt T. Tumor volume and tumor hypoxia in head and neck cancers. The amount of the hypoxic volume is important. Strahlenther Onkol. 2003;179:521–526. doi: 10.1007/s00066-003-1066-4. [DOI] [PubMed] [Google Scholar]
- 27.Stadler P, Becker A, Feldmann HJ, Hansgen G, Dunst J, Wurschmidt F, Molls M. Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiot Oncol Biol Phys. 1999;44:749–754. doi: 10.1016/s0360-3016(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 28.Pagano L, Gallamini A, Trape G, Fianchi L, Mattei D, Todeschini G, Spadea A, Cinieri S, Iannitto E, Martelli M, Nosari A, Bona ED, Tosti ME, et al. NK/T-cell lymphomas ‘nasal type’: an Italian multicentric retrospective survey. Ann Oncol. 2006;17:794–800. doi: 10.1093/annonc/mdl015. [DOI] [PubMed] [Google Scholar]
- 29.van Wayenburg CA, Rasmussen-Conrad EL, van den Berg MG, Merkx MA, van Staveren WA, van Weel C, van Binsbergen JJ. Weight loss in head and neck cancer patients little noticed in general practice. J Prim Health Care. 2010;2:16–21. [PubMed] [Google Scholar]
- 30.van Bokhorst-de van der S, van Leeuwen PA, Kuik DJ, Klop WM, Sauerwein HP, Snow GB, Quak JJ. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86:519–527. [PubMed] [Google Scholar]
- 31.Chang PH, Yeh KY, Huang JS, Lai CH, Wu TH, Lan YJ, Tsai JC, Chen EY, Yang SW, Wang CH. Pretreatment performance status and nutrition are associated with early mortality of locally advanced head and neck cancer patients undergoing concurrent chemoradiation. Eur Arch Otorhinolaryngol. 2013;270:1909–1915. doi: 10.1007/s00405-012-2290-2. [DOI] [PubMed] [Google Scholar]
- 32.Michal SA, Adelstein DJ, Rybicki LA, Rodriguez CP, Saxton JP, Wood BG, Scharpf J, Ives DI. Multi-agent concurrent chemoradiotherapy for locally advanced head and neck squamous cell cancer in the elderly. Head neck. 2012;34:1147–1152. doi: 10.1002/hed.21891. [DOI] [PubMed] [Google Scholar]
- 33.Merlano MC, Monteverde M, Colantonio I, Denaro N, Lo Nigro C, Natoli G, Giurlanda F, Numico G, Russi E. Impact of age on acute toxicity induced by bio- or chemo-radiotherapy in patients with head and neck cancer. Oral oncol. 2012;48:1051–1057. doi: 10.1016/j.oraloncology.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Boscolo-Rizzo P, Muzzi E, Trabalzini F, Gava A, Stellin M, Da Mosto MC. Functional organ preservation after chemoradiotherapy in elderly patients with loco-regionally advanced head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2011;268:1349–1355. doi: 10.1007/s00405-011-1489-y. [DOI] [PubMed] [Google Scholar]
- 35.Cheung MM, Chan JK, Lau WH, Foo W, Chan PT, Ng CS, Ngan RK. Primary non-Hodgkin’s lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998;16:70–77. doi: 10.1200/JCO.1998.16.1.70. [DOI] [PubMed] [Google Scholar]
- 36.Chan JY. Surgical management of recurrent nasopharyngeal carcinoma. Oral oncol. 2014;50:913–917. doi: 10.1016/j.oraloncology.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Ling DC, Chapman BV, Kim J, Choby GW, Kabolizadeh P, Clump DA, Ferris RL, Kim S, Beriwal S, Heron DE, Duvvuri U. Oncologic outcomes and patient-reported quality of life in patients with oropharyngeal squamous cell carcinoma treated with definitive transoral robotic surgery versus definitive chemoradiation. Oral oncol. 2016;61:41–46. doi: 10.1016/j.oraloncology.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lartizien C, Kinahan PE, Swensson R, Comtat C, Lin M, Villemagne V, Trebossen R. Evaluating image reconstruction methods for tumor detection in 3-dimensional whole-body PET oncology imaging. J Nucl Med. 2003;44:276–290. [PubMed] [Google Scholar]
- 39.Elstrom R, Guan L, Baker G, Nakhoda K, Vergilio JA, Zhuang H, Pitsilos S, Bagg A, Downs L, Mehrotra A, Kim S, Alavi A, Schuster SJ. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875–3876. doi: 10.1182/blood-2002-09-2778. [DOI] [PubMed] [Google Scholar]
- 40.Kako S, Izutsu K, Ota Y, Minatani Y, Sugaya M, Momose T, Ohtomo K, Kanda Y, Chiba S, Motokura T, Kurokawa M. FDG-PET in T-cell and NK-cell neoplasms. Ann Oncol. 2007;18:1685–1690. doi: 10.1093/annonc/mdm265. [DOI] [PubMed] [Google Scholar]
- 41.Meyrignac O, Lagarde S, Bournet B, Mokrane FZ, Buscail L, Rousseau H, Otal P. Acute Pancreatitis: Extrapancreatic Necrosis Volume as Early Predictor of Severity. Radiology. 2015;276:119–128. doi: 10.1148/radiol.15141494. [DOI] [PubMed] [Google Scholar]