Abstract
Intradialytic hypotension (IDH) is a common and often distressful complication of hemodialysis. However, despite its clinical significance, there is no consensus, evidence-based medical definition for the condition. Over the years, numerous definitions have been implemented in both the clinical and research settings. Definition inconsistencies have hindered data synthesis and the development of evidence-based guidelines for the prevention and treatment of IDH as well as prevented accurate estimation of population burden and patient risk assessment. Most existing IDH definitions are comprised of one or more of the following components: 1) intradialytic BP criteria (requisite BP declines or minimum BP thresholds), 2) the provision of interventions aimed at restoring effective arterial volume, and/or 3) patient-reported symptoms. Remarkably, there are insufficient data to inform IDH definition construction, and it remains unknown if a single, universal definition can adequately capture the condition across patient subgroups and clinical and research settings.
Keywords: Definition, hemodialysis, intradialytic hypotension, blood pressure
“Define: To state precisely or determinately; to specify.”1
-The Oxford English Dictionary
Well-appointed medical definitions facilitate accurate identification of clinical conditions across patient populations, providers and settings. Dependence on consensus medical definitions to precisely and reliably identify abnormal clinical phenomena is a cornerstone of medical practice. IDH is a widely-recognized treatment-related complication that has plagued patients and providers since the inception of hemodialysis. Advances in hemodialysis technology such as real-time hemodynamic monitoring, volumetric control, bicarbonate-based buffer, and biocompatible membranes have generally reduced the occurrence of IDH over the years.2 However, some of these same advances, along with the advent of high-flux, high-efficiency dialyzers and increased emphasis on clinic efficiency, have led to shorter dialysis treatment times which may paradoxically increase hemodynamic instability. IDH remains a common and distressful complication in the 21st century.
IDH complicates 10 to 70% of dialysis treatments depending on the definition used.3 Associated patient and clinical characteristics include older age, female sex, longer dialysis vintage, diabetes, lower pre-dialysis blood pressure (BP), and lower albumin.3,4 IDH has been associated with a range of clinical and pathogenic consequences including patient-reported symptoms, inadequate dialysis dose, vascular access thrombosis, end-organ ischemia and increased mortality.3,5–16 Despite its clinical significance, we lack a consensus medical definition for the condition. Over the years, numerous definitions have been implemented in both the clinical and research settings. Unfortunately, definition inconsistencies have hindered data synthesis and the development of evidence-based guidelines for the prevention and treatment of IDH as well as prevented accurate estimation of population burden and patient risk assessment.
In hemodialysis practice, we observe two types of hypotension: episodic, precipitous BP drops during treatment and chronically low BP. The latter afflicts approximately 5% of individuals with end-stage kidney disease and is typically characterized by a low pre-dialysis BP. Chronic hypotension is more prevalent among individuals with advanced heart failure, severe vascular disease and malnutrition and associates with higher mortality rates.7,17,18 While chronic hypotension is an important clinical phenomenon, for the remainder of this review, we will focus on the more common and, perhaps, more preventable, episodic IDH. Herein, we: 1) describe the challenges associated with defining IDH, 2) review existing definitions and their components, 3) discuss additional definition considerations, and 4) briefly review the associations between different IDH definitions and mortality.
Medical definition properties and the challenge of defining IDH
The English word ‘definition’ originates from the Latin term ‘dēfīnīre’, to limit or bound.19 Understanding the bounds that differentiate normal clinical states from aberrant clinical states is necessary to formulate accurate medical definitions. Ideally, such boundaries are universal, objective, informed by underlying disease pathophysiology and easily ascertained in clinical practice. To differentiate normal from abnormal clinical states, medical definitions typically rely on singular laboratory or diagnostic test result thresholds or a combination of such thresholds along with physical exam findings, and/or symptoms. However, numerous challenges render the development of universal, consensus-based medical definitions difficult and, in some cases, impossible to generate. Challenges include: 1) poorly defined normal (i.e. target) ranges, 2) differences in clinical phenomena across sub-populations, 3) under-elucidated disease pathophysiology, and 4) limited high quality empiric evidence linking aspects of the aberrant state to adverse outcomes.
Many of the aforementioned challenges arise when trying to define IDH. First, concretely defining abnormal BP phenomena, either too low of a BP (hypotension) or too high of a BP (hypertension), hinges upon the existence of an established “normal” BP range. However, there is no broadly accepted definition of “normal” BP for individuals with end-stage kidney disease. Observational studies have demonstrated a “U-shaped” or “reverse J-shaped” relationship between BP and mortality among hemodialysis patients. 17,20–23 The highest mortality risk occurs among patients with the lowest peri-dialytic BPs (including those BPs considered normal in the general population),17,20–23 leaving many providers hesitant to target BPs accepted as “normal” among other populations.
Related, existing evidence suggest that the optimal BP target may differ across time and patient subpopulations. For example, the association between BP and mortality appears modified by time with lower BP-associated risk abating over time.18 Similarly, associations between BP and outcomes vary across clinical characteristics. For example, in a cohort of >16,000 patients, Myers at al. found that lower pre-dialysis systolic BP (<140 mmHg) was associated with increased mortality. However, in subgroup analyses stratified by age, the association between lower BP and increased mortality lost significance among younger individuals (<50 years). The authors also found that the lower BP—mortality association was stronger among individuals with diabetes compared to those without.24 These results, along with others, suggest that a universally applicable BP target may not be appropriate. Absence of an accepted, “normal” BP range for dialysis patients is a major barrier to developing definitions for BP-related clinical phenomena including IDH.
Further complicating efforts to develop BP-related medical definitions are gaps in knowledge regarding “normal” BP behavior during hemodialysis and, related, the ideal intradialytic BP thresholds at which therapeutic interventions for BP restoration are indicated. During uncomplicated hemodialysis treatments, most patients experience a gradual BP decline that is largely driven by fluid removal. In fact, such an intradialytic BP decline is associated with better outcomes. In an observational cohort study of over 110,000 hemodialysis patients, Park et al. reported peak survival among patients with a pre- to post-dialysis systolic BP decline of 14 mmHg.23
However, consideration of pre- to post-dialysis BP change disregards intradialytic BP behavior. In a study evaluating intradialytic BPs in 218 patients across >2,100 hemodialysis treatments, Dinesh et al. characterized the typical pattern of intradialytic systolic BP decline. The authors described a rapid decrease in BP during the first quarter of treatment (slope of −25.5 ± 1.5 (standard error) mmHg) followed by a more gradual BP decline in the latter 75% of treatment (slope of −5.8 ± 0.5 mmHg).25 Deviations from this expected course, both small (intradialytic BP variability)26 and large (episodes of IDH and hypertension)3,8,14–16,23,27,28 have been associated with increased mortality. However, clear thresholds for intervention across abnormal intradialytic BP phenomena, including IDH, remain unestablished.
Ideally, the optimal medical definition for IDH would identify a BP threshold below which individuals sustain end-organ pathologic insults linked to adverse clinical outcomes. Clinicians have historically thought of IDH as a “know it when you see it” phenomenon, recognizing that sudden, substantial BP declines with accompanying dramatic clinical presentations such as vomiting and syncope are indicative of physiologic harm and necessitate swift intervention to restore hemodynamic stability. As reviewed below, growing evidence suggests that less-dramatic, asymptomatic intradialytic BP drops may be clinically significant, underscoring the need for an IDH definition that relies on more precise diagnostic criteria than clinical instinct alone.
Physiologically, an episode of IDH occurs when the rate of fluid removal during dialysis outpaces the rate of plasma refill and associated cardiovascular and neurohormonal compensatory responses. Decreased effective arterial blood volume results in reduced cardiac filling, diminished cardiac output and ultimately, frank hypotension. Intradialytic physiologic imaging reveals ischemia to numerous organs including the heart, brain, gut, liver and kidneys during hemodialysis.9–13 Repeat episodes of such ischemic injury are hypothesized to lead to long-term organ damage and associated morbidity and mortality.29 The ability to maintain hemodynamic stability during hemodialysis is dependent, in part, on treatment-related factors such as ultrafiltration volume, treatment time and dialysate composition. However, preservation of adequate circulating blood volume and, thus, end-organ perfusion is also dependent on multiple patient-related factors, including co-morbid cardiovascular disease (heart failure, peripheral vascular disease), plasma osmolarity (nutritional status), and autonomic dysfunction. Thus, it is plausible that the BP threshold, below which individuals sustain pathologic end-organ insults during hemodialysis, may vary from patient-to-patient and/or by pre-dialysis BP levels. Confirmatory evidence in this regard is needed.
In summary, there are many challenges to identifying the optimal medical definition for IDH. Existing data suggest that the bounds of “normal” BP and “normal” intradialytic BP behavior may differ across patients and time. Additionally, the intradialytic BP at which pathophysiologic harm occurs is not known, leaving the optimal threshold for intervention unclear. Despite these limitations, a medical definition for IDH (either universal or subgroup-specific) that adequately delineates the bounds of “normal” and “low” intradialytic BP is needed to guide clinical decision-making.
Guideline body-defined IDH
Numerous IDH definitions, likely of varying validity, have been used over time. Table 1 provides an overview of IDH definitions used by various international guideline bodies. In 2005, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) became the first clinical practice guideline body to formally issue a specific clinical definition for IDH. The K/DOQI definition, a decrease in systolic BP by ≥20 mmHg or a drop in mean arterial pressure (MAP) ≥10 mmHg associated with symptoms, was presented in a special section of the guidelines focused on key clinical topics without sufficient evidence to generate formally graded recommendations.30 Notably, the definition has not been updated in over a decade, highlighting the persistent paucity of outcomes-based evidence in this area. Subsequently, other guideline bodies have published their own IDH definitions, but all acknowledge the relatively sparse data underlying definition specification.31–33
Table 1.
Clinical practice guideline definitions for intradialytic hypotension.
| Guideline (Year) | Intradialytic hypotension definition |
|---|---|
| K/DOQI Clinical Practice Guidelines (2002)30 | A decrease in systolic BP ≥20 mmHg or a decrease in MAP ≥10 mmHg associated with symptoms that include: abdominal discomfort; yawning; sighing; nausea; vomiting; muscle cramps; restlessness; dizziness or fainting; and anxiety |
| European Best Practice Guidelines (2007)32 | A decrease in systolic BP ≥20 mmHg or a decrease in MAP ≥10 mmHg associated with clinical events and need for nursing interventions |
| UK Renal Association Guidelines (2009)33 | An acute symptomatic fall in BP during dialysis requiring immediate intervention to prevent syncope |
| Japanese Society for Dialysis Therapy Guidelines (2012)31 | Symptomatic sudden drop systolic BP ≥30 mmHg during dialysis or a decrease in the mean BP by ≥10 mmHg |
Abbreviations: BP, blood pressure; K/DOQI, Kidney Disease Outcomes Quality Initiative; MAP, mean arterial pressure; UK, United Kingdom
IDH definition components
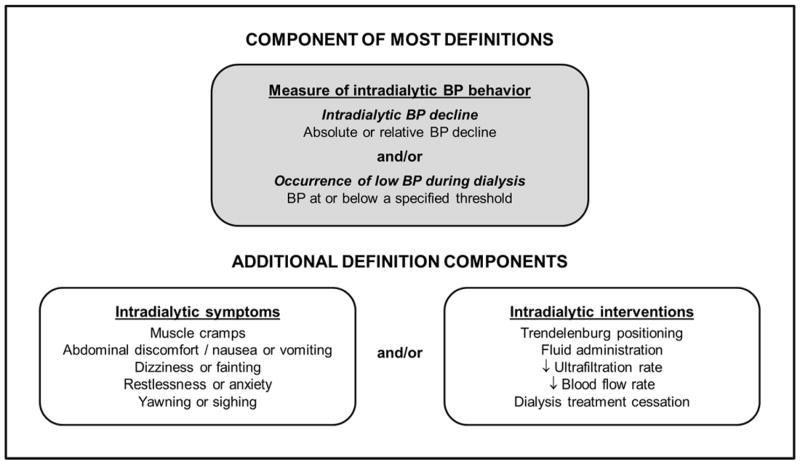
Lack of a consensus IDH definition has led to a wide range of accepted criteria for the condition in both the clinical and research realms. Table 2 provides an overview of representative intradialytic hypotension definitions. Definitions typically consider at least one of the following components: 1) a measure of intradialytic BP behavior (a requisite decline or a minimum threshold); 2) the need for a clinical intervention aimed at restoring circulating volume; and/or 3) patient-reported symptoms (Figure 1).
Table 2.
Representative intradialytic hypotension definitions used in clinical research studiesa
| Author (Year) Country | Definition of intradialytic hypotension |
|---|---|
| Raja (1979)46 United States |
Systolic BP <90 mmHg during dialysis |
| HEMO Study (1999)51 United States |
An affirmative response to the following question during monitored dialysis treatments: “was there hypotension requiring saline infusion, lowering of the UF rate, or reduced blood flow?” |
| Santoro (2002)56 Italy |
Meet any of the following criteria:
|
| Kyriazis (2002)45 Greece |
Asymptomatic: Intradialytic systolic BP <95 mmHg without symptoms Symptomatic (meet either criteria):
|
| Tislér (2003)16 Hungary |
Sudden systolic BP fall to <90 mmHg or a sudden absolute drop in systolic BP >30 mmHg, associated with symptoms of hypotension not responding to the supine position but necessitating resuscitation with normo- or hypertonic fluid administration |
| Chesterton (2009)36 United Kingdom |
Systolic BP ≤100 mmHg during dialysis (even in the absence of symptoms) or a fall in systolic BP >10% of the pre-dialysis BP in association with symptoms (e.g. headaches, cramps, light-headedness) |
| Locatelli (2010)53 Italy |
Rapid symptomatic systolic BP fall ≥30 mmHg or any fall in BP that required nursing and/or medical intervention |
| Caplin (2011)5 United Kingdom |
Dizziness due to low BP or intradialytic nursing interventions for hypotension |
| Dubin (2011)34 United States |
Systolic BP fall ≥ 20 mmHg |
| Munoz Mendoza (2011)57 United States |
Systolic BP fall >20 mmHg requiring the administration of normal or hypertonic saline solution with or without the presence of symptoms |
| Sands (2014)4 United States |
Systolic BP fall >30 mmHg to a level of <90 mmHg |
| Kotanko (2015)48 United States/Canada |
Event when hypotensive symptoms led to either lowering of the ultrafiltration rate or saline administration |
| Mc Causland (2015)40 United States |
Systolic BP fall ≥35 mmHg during dialysis or any intradialytic systolic BP <90 mmHg |
Note: Numerous studies have defined intradialytic hypotension using the various clinical practice guideline definitions. The clinical research studies listed in this table were selected to illustrate the broad range of intradialytic hypotension definitions that have been employed. Additional definitions for intradialytic hypotension exist.
Abbreviations: BP, blood pressure; HEMO, hemodialysis.
Figure 1.
Potential intradialytic hypotension definition components.
Abbreviations: BP, blood pressure
Blood pressure decline
An element of many IDH definitions is the presence of either an absolute or relative BP decline during dialysis. An absolute BP decline is a subtraction; a relative blood pressure decline is a ratio. Studies employing BP decline-based definitions have used a range of requisite absolute (e.g. ≥20, >30, or ≥40 mmHg)14,34,35 and relative (e.g. >10%, or >25%)36,37 declines to signify IDH. For both absolute and relative declines, systolic BP has the predominant parameter considered. However, a few definitions have used diastolic BP and MAP.38,39
An absolute intradialytic BP change is calculated as the difference between two discrete BP measurements during a single treatment. The timing of the BP readings selected for evaluation has differed across studies. In the majority of reports, a BP decline is identified by subtracting the minimum intradialytic BP from the pre-dialysis BP measurement. For example, in a study examining the association between serum osmolarity and BP change, a ≥35 mmHg decline in systolic BP from pre-dialysis to intradialytic minimum BP was defined as an episode of IDH.40 Under this paradigm, there is no requisite time period over which the BP decline must occur. Without acuity indicators, such definitions conflate rapid BP declines with more gradual BP declines. In particular, definitions encompassing small requisite BP declines without consideration of intervening time (e.g. ≥20 mmHg) may classify BP changes that are expected and commonplace as IDH. To limit such misclassification, some definitions specify that the requisite fall must occur between consecutive intradialytic BP measurements. For example, Arrameddy et al. used a >30 mmHg decline between any two consecutive BP measurements to define IDH.41 Another way to account for event acuity is to specify a time period over which the requisite decline must occur. Dheenan et al. defined IDH as an abrupt drop of systolic BP >40 mmHg or diastolic BP >20 mmHg occurring over 10 to 15 minutes.38 In rare cases, peri-dialytic BP measurements (pre- and post-dialysis BPs) have been used to define IDH.42 However, this approach fails to capture both acuity and intradialytic BP behavior.
Relative BP change considers the percent change in BP during dialysis. The referent BP measure, the BP to which intradialytic BPs are compared, is typically pre-dialysis BP. For instance, a drop in systolic BP by ≥25% from pre-dialysis levels was considered an episode of IDH by Ie et al.43 One potential advantage of relative decline-based definitions is the individualization of the decline to the starting BP. Like absolute declines, relative decline-based definitions require timeframe specification to capture event acuity. An additional disadvantage of relative BP metrics is that they are more challenging to implement in clinical practice than subtraction-based absolute decline definitions.
Minimum blood pressure threshold
Reliance on the minimum (nadir) systolic BP is another method for defining IDH with BP measurements. Past studies considering nadir-based definitions have used a range of minimum systolic BP thresholds including <90, <95 and <100 mmHg.3,44–46 For instance, in a study examining the association between intradialytic food consumption and BP, Benaroia et al. defined hypotension as a systolic BP <100 mmHg at any time during treatment.44 Threshold-based definitions do not account for the magnitude of BP decline and, in fact, may identify IDH in instances where BP did not, in fact, decline during treatment (effectively conflating between constructs of: chronic low BP and precipitous BP drops). In cases where authors seek to measure episodic, precipitous BP declines, it may be optimal to combine both nadir-based and decline-based criteria. However, in cases where authors seek to measure overall clinical burden from “low BP,” single component, nadir-based definitions may be appropriate.
Clinical interventions
A criterion required by many IDH definitions is the provision of a clinical intervention aimed at restoring blood volume such as administration of saline or other intravenous fluids, Trendelenburg positioning, reduction in blood flow, reduction or cessation of ultrafiltration or, in severe cases, cessation of the dialysis treatment. Often, definitions with intervention components require that the corrective action be taken in response to an intradialytic BP change or symptom occurrence. For example, IDH in the Hemodialysis (HEMO) Study was defined as an affirmative response to the following question: “was there hypotension requiring saline infusion, lowering of the ultrafiltration rate or reduced blood flow?”47 In a secondary analysis of the Frequent Hemodialysis Network (FHN) daily and nocturnal trials, Kotanko et al. defined IDH as an event where hypotensive symptoms led to ultrafiltration rate reduction or saline administration.48 Addition of an intervention requirement to symptoms or a specified BP criterion may be one way of capturing event acuity. However, in some cases, interventions may not correlate with hemodynamic instability. Intravenous fluid boluses can be given for electrolyte-mediated cramping, and ultrafiltration may be slowed or stopped in response to nausea or vomiting not related to intravascular depletion.
Patient symptoms
Some IDH definitions consider patient-reported symptoms. BP drops associated with intravascular volume depletion may cause abdominal pain, chest pain, heart palpitations, nausea/vomiting, cramping, feelings of restlessness, lightheadedness, and syncope. Symptom-containing definitions usually consider symptoms in conjunction with other IDH definition components. For example, Knoll et al. defined IDH as a fall in the systolic BP below 100 mmHg accompanied by at least one of the following: diaphoresis, nausea, vomiting, cramps, headache, or dizziness.49
While symptoms, particularly cramping, are undeniably distressful to patients and staff, many symptoms often attributed to hemodynamic instability can be caused by other conditions such as electrolyte imbalances. In fact, Meredith et al. found that symptoms, particularly nausea, did not reliably correlate with intradialytic BP change.6 This weak relationship may be attributed to differences in patient characteristics that impact the physiologic thresholds at which volume-related symptoms manifest or differences in patient reporting or provider documentation. Symptom prevalence varies across studies. In a survey of maintenance hemodialysis patients, Caplin et al. found that 74% of patients reported cramping, 63% of patients reported dizziness and 54% of patients reported headache.5 However, other studies have reported much lower symptom frequencies: roughly 20% for cramping, 12 – 23% for dizziness and 21% for headache.6,50 Use of symptom-based IDH definitions in retrospective studies has been hindered by limited symptom data in large administrative databases. Most studies with IDH definitions relying on symptom criteria have been small, single-center observational studies6 or randomized trials that prospectively collected dialysis treatment symptom data.49,51
IDH definition construction
Single component definitions
IDH definitions containing a single definition component are most often comprised of a measure of intradialytic BP behavior, a decline and/or minimum threshold. Examples include systolic BP decline of ≥20 mmHg34 and a nadir systolic BP <90 mmHg46. Such definitions are common in large observational studies as electronic medical records and administrative datasets do not reliably include symptom or intervention information. As discussed, without specified time intervals for requisite BP declines or the addition of symptom or intervention criteria to designate event acuity, decline-based definitions may conflate a number of distinct clinical phenomena, including acute episodic IDH, chronically low BP, and expected intradialytic BP decline. Additionally, single component decline-based definitions do not require achievement of a minimum BP. Thus, patients with high pre-dialysis BP may meet the decline-based definition but have an intradialytic nadir BP that is elevated. The degree of IDH-related end-organ damage may not be consistent across strata of pre-dialysis BPs. It is thus plausible that single component, nadir-based definitions may better identify organ hypo-perfusion. However, like decline-based definitions, nadir-based definitions do not account for condition acuity.
Multicomponent definitions
Multicomponent IDH definitions, definitions that contain two or more criteria, are common. More complex definitions may better distinguish episodic hypotension events from gradual intradialytic BP declines and chronic hypotensive conditions. For example, symptom and/or intervention requirements are often added to fall- or nadir-based BP criteria to detect event acuity and grade clinical severity. Examples include a fall in MAP >20% with associated signs and symptoms of dizziness, nausea, sweating, or pallor;52 and a rapid symptomatic systolic BP decline ≥30 mmHg or any BP decline requiring nursing and/or medical intervention.53 However, as reviewed above, symptom occurrence and intervention administration may not always correlate with hemodynamic instability or end-organ damage, introducing potential misclassification.
Other definition specification considerations
Episodic versus pattern identification
The intended use of the IDH definition also factors into definition component selection. Definitions designed to inform clinical intervention thresholds may differ from definitions designed to characterize longitudinal BP patterns, or phenotypes. For example, a decline- or nadir-based definition may be appropriate for clinical protocols intended to reduce hypotension-driven pathologic consequences via prompt nursing intervention. In other settings, clinicians or investigators may desire to characterize an individual’s BP phenotype. Such definitions are aimed at identifying patients who are IDH-prone in order to measure associated long-term risk or identify patients for clinical trials of preventative therapies.
Mean versus frequency-based definitions for pattern identification
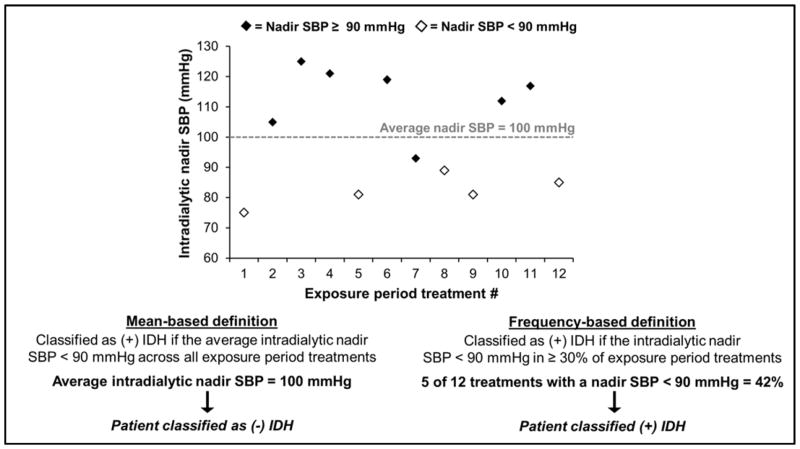
To define the presence (or absence) of an intradialytic hypotensive phenotype, researchers evaluate the mean of BPs or the frequency of hypotensive events during a given time period (Figure 2). This time period of interest is often termed the “exposure assessment period” and is the time period during which (+) vs. (−) IDH status is designated. Mean-based approaches are only possible in decline- or nadir-based IDH definitions. Examples of mean-based definition calculations are: 1) the average change in systolic BP during dialysis (pre-dialysis BP - minimum intradialytic BP) across exposure period treatments and 2) the average nadir intradialytic systolic BP during the exposure assessment period. However, arithmetic means are sensitive to extreme values, and outliers may over-influence results. Such an effect is magnified when fewer opportunities for BP assessment (i.e. shorter exposure assessment periods) are used for mean calculations.
Figure 2.
Differences in mean- and frequency-based intradialytic hypotension definitions.
The figure depicts the application of mean and frequency-based intradialytic hypotension definitions in a 30-day exposure assessment period (12 hemodialysis treatments) in a single, hypothetical individual.
Abbreviations: IDH, intradialytic hypotension; SBP, systolic blood pressure
On the other hand, frequency-based definitions identify the proportion of exposure period dialysis treatments complicated by IDH. Under this paradigm, multicomponent definitions that consider symptoms or interventions may be used. Frequency-based definitions are growing in use as their construction may better align with the theory that repetitive episodes of IDH are pathogenic. The frequency threshold used to delineate patients prone to IDH (versus not), has differed across investigations, ranging from >25% to ≥75% of hemodialysis treatments affected.54,55 For example, in an analysis of various IDH definitions and mortality, Flythe et al. defined IDH-prone as meeting the specified IDH definition in ≥30% of exposure period treatments (versus not).3
Time period considerations
When characterizing intradialytic BP patterns across time, consideration should also be given to the length of time over which dialysis treatments are assessed (i.e. the duration of the exposure assessment period). Time-fixed exposures are assessed over a single, finite period of time, whereas time-varying exposures are assessed and updated longitudinally. The exposure period selected for time-fixed approaches is often intended to reflect a “window” into long-term risk.
Time-fixed exposure periods vary widely across studies, ranging from one to over 100 hemodialysis treatments.14,16 Use of BP behavior over a single dialysis treatment has obvious downsides as numerous clinical factors (e.g. acute illness, ultrafiltration volume, dialysate temperature, and antihypertensive use) may influence BP. When selecting among longer time-fixed exposure periods (30, 90, 180 days, etc.), issues related to generalizability may arise. In research studies, follow-up time begins immediately after the exposure period ends. Study inclusion is contingent on survival throughout the exposure period and into the start of follow-up. Thus, the exposure period length may alter the cohort case-mix. Individuals included in studies with longer exposure periods may be healthier than patients included in studies with shorter exposure periods since they had to live longer to survive to the follow-up period. To assess whether study findings are influenced by exposure period length, researchers may conduct sensitivity analyses to determine if varied exposure period durations impact study results. Furthermore, it is also plausible that BP pattern assessment over time-limited periods may not accurately depict long-term BP behavior and thus may not fully capture IDH-associated risk. It may be prudent for investigators to conduct time-updated analyses in addition to time-fixed analyses to ensure that analyses of a single time window are robust.
IDH and mortality
Another consideration in selecting a medical definition for IDH is the association of the definition with important clinical outcomes. Likely related to the above-discussed analytical issues and the use of varied definition criteria, observational studies examining IDH and mortality have yielded mixed results.16,14,15 Flythe et al. investigated the association of various IDH definitions and mortality using a frequency-based exposure characterization approach. Authors considered 8 commonly used IDH definitions: decline-based (systolic BP decline ≥20 and ≥30 mmHg), nadir-based (minimum intradialytic systolic BP <90 and <100 mmHg), the HEMO Study definition (systolic BP decline resulting in an intervention), the K/DOQI definition (systolic BP decline ≥20 mmHg with the presence of symptoms), and composite BP definitions that combined BP decline and nadir criteria. Individuals with ≥30% exposure period dialysis treatments meeting the individually specified definitions were classified as (+) IDH (prone to IDH), and patients not meeting the IDH definition in ≥30% of treatments were classified as (−) IDH (not prone to IDH). In analyses of a large, nationally representative hemodialysis patient cohort (and confirmed in a post-hoc analysis of the HEMO Study cohort), the presence of systolic BP <90 mmHg in >30% of exposure HD treatments (versus not) was associated with an increased odds of all-cause mortality. Other IDH definitions were not associated with mortality. Neither the addition of symptoms nor interventions to BP decline- or nadir-based definitions strengthened mortality associations.3 Findings suggest that a nadir-based IDH definition may best reflect mortality risk. Patient-centered outcomes such as hospitalizations and symptoms were not evaluated, and the optimal definitions for capturing these important outcomes are unknown.
Conclusion
In summary, lack of consensus regarding clinically significant BP thresholds and variation in data availability have led to the use of a wide range of IDH definitions in practice and research. Ideally, IDH definition specification should be guided by the intended use of the definition and an understanding of the pathophysiologic relevant thresholds for outcomes of interest. Additional investigations of such thresholds as well as differences in these thresholds across patient and pre-dialysis BP subgroups are needed. It is plausible and, perhaps, likely that a “one-size fits all” medical definition for IDH may not exist. However, accumulating data suggesting long-term harm from asymptomatic BP drops render the “know when you see it” definition imprecise and make the case for ongoing efforts “to state precisely or determinately” the clinical state known as IDH.
Acknowledgments
Funding sources: Dr. Assimon is supported by grant F32 DK109561 and Dr. Flythe by grant K23 DK109401, both awarded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
Disclosures: Dr. Flythe has received speaking honoraria from Dialysis Clinic, Incorporated, Renal Ventures, American Renal Associates, American Society of Nephrology, Baxter, and multiple universities. Drs. Flythe and Assimon have received investigator initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America.
References
- 1.Oxford English Dictionary. define, v. Oxford University Press; 2016. [Google Scholar]
- 2.Drukker W, Parsons FM, Maher JF. Replacement of renal function by dialysis : a textbook of dialysis. 2. Boston Hingham, MA: M. Nijhoff ; distributors for the U.S. and Canada, Kluwer Boston, Inc; 1983. [Google Scholar]
- 3.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26(3):724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sands JJ, Usvyat LA, Sullivan T, et al. Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int. 2014;18(2):415–422. doi: 10.1111/hdi.12138. [DOI] [PubMed] [Google Scholar]
- 5.Caplin B, Kumar S, Davenport A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant. 2011;26(8):2656–2663. doi: 10.1093/ndt/gfq763. [DOI] [PubMed] [Google Scholar]
- 6.Meredith DJ, Pugh CW, Sutherland S, Tarassenko L, Birks J. The relationship between symptoms and blood pressure during maintenance hemodialysis. Hemodial Int. 2015;19(4):543–552. doi: 10.1111/hdi.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henrich WL. Principles and practice of dialysis. 4. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 8.Chang TI, Paik J, Greene T, et al. Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol. 2011;22(8):1526–1533. doi: 10.1681/ASN.2010101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4(12):1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26(4):957–965. doi: 10.1681/ASN.2013101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 13.McIntyre CW, Harrison LE, Eldehni MT, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3):1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 15.Stefánsson BV, Brunelli SM, Cabrera C, et al. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol. 2014;9(12):2124–2132. doi: 10.2215/CJN.02680314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tislér A, Akocsi K, Borbas B, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant. 2003;18(12):2601–2605. doi: 10.1093/ndt/gfg450. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Lacson E, Jr, Lowrie EG, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48(4):606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Stidley CA, Hunt WC, Tentori F, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol. 2006;17(2):513–520. doi: 10.1681/ASN.2004110921. [DOI] [PubMed] [Google Scholar]
- 19.Oxford English Dictionary. definition, n. Oxford University Press; 2016. [Google Scholar]
- 20.Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33(3):507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 21.Tozawa M, Iseki K, Iseki C, Takishita S. Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int. 2002;61(2):717–726. doi: 10.1046/j.1523-1755.2002.00173.x. [DOI] [PubMed] [Google Scholar]
- 22.Zager PG, Nikolic J, Brown RH, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54(2):561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Rhee CM, Sim JJ, et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 2013;84(4):795–802. doi: 10.1038/ki.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers OB, Adams C, Rohrscheib MR, et al. Age, race, diabetes, blood pressure, and mortality among hemodialysis patients. J Am Soc Nephrol. 2010;21(11):1970–1978. doi: 10.1681/ASN.2010010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinesh K, Kunaparaju S, Cape K, Flythe JE, Feldman HI, Brunelli SM. A model of systolic blood pressure during the course of dialysis and clinical factors associated with various blood pressure behaviors. Am J Kidney Dis. 2011;58(5):794–803. doi: 10.1053/j.ajkd.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Flythe JE, Inrig JK, Shafi T, et al. Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am J Kidney Dis. 2013;61(6):966–974. doi: 10.1053/j.ajkd.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inrig JK, Oddone EZ, Hasselblad V, et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71(5):454–461. doi: 10.1038/sj.ki.5002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inrig JK, Patel UD, Toto RD, Szczech LA. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis. 2009;54(5):881–890. doi: 10.1053/j.ajkd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIntyre CW, Odudu A. Hemodialysis-associated cardiomyopathy: a newly defined disease entity. Semin Dial. 2014;27(2):87–97. doi: 10.1111/sdi.12197. [DOI] [PubMed] [Google Scholar]
- 30.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 31.Hirakata H, Nitta K, Inaba M, et al. Japanese Society for Dialysis Therapy guidelines for management of cardiovascular diseases in patients on chronic hemodialysis. Ther Apher Dial. 2012;16(5):387–435. doi: 10.1111/j.1744-9987.2012.01088.x. [DOI] [PubMed] [Google Scholar]
- 32.Kooman J, Basci A, Pizzarelli F, et al. EBPG guideline on haemodynamic instability. Nephrol Dial Transplant. 2007;22(Suppl 2):ii22–44. doi: 10.1093/ndt/gfm019. [DOI] [PubMed] [Google Scholar]
- 33.Mactier R, Hoenich N, Breen C. [Accessed 23 Dec 2016];UK Renal Association clinical practice guidelines: haemodialysis. 2009 doi: 10.1159/000328072. http://www.renal.org/guidelines/modules/haemodialysis#sthash.eBdbSrRk.dpbs. [DOI] [PubMed]
- 34.Dubin R, Owens C, Gasper W, Ganz P, Johansen K. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension. Hemodial Int. 2011;15(3):350–358. doi: 10.1111/j.1542-4758.2011.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song JH, Park GH, Lee SY, Lee SW, Lee SW, Kim MJ. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. J Am Soc Nephrol. 2005;16(1):237–246. doi: 10.1681/ASN.2004070581. [DOI] [PubMed] [Google Scholar]
- 36.Chesterton LJ, Selby NM, Burton JO, McIntyre CW. Cool dialysate reduces asymptomatic intradialytic hypotension and increases baroreflex variability. Hemodial Int. 2009;13(2):189–196. doi: 10.1111/j.1542-4758.2009.00355.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Wang M, Li H, et al. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am J Nephrol. 2014;40(2):140–150. doi: 10.1159/000365819. [DOI] [PubMed] [Google Scholar]
- 38.Dheenan S, Henrich WL. Preventing dialysis hypotension: a comparison of usual protective maneuvers. Kidney Int. 2001;59(3):1175–1181. doi: 10.1046/j.1523-1755.2001.0590031175.x. [DOI] [PubMed] [Google Scholar]
- 39.Sherman RA, Casale P, Cody R, Horton MW. Effect of predialysis verapamil on intradialytic blood pressure in chronic hemodialysis patients. ASAIO Trans. 1990;36(2):67–69. doi: 10.1097/00002480-199004000-00005. [DOI] [PubMed] [Google Scholar]
- 40.McCausland FR, Waikar SS. Association of Predialysis Calculated Plasma Osmolarity With Intradialytic Blood Pressure Decline. Am J Kidney Dis. 2015;66(3):499–506. doi: 10.1053/j.ajkd.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arramreddy R, Sun SJ, Munoz Mendoza J, Chertow GM, Schiller B. Individualized reduction in dialysate sodium in conventional in-center hemodialysis. Hemodial Int. 2012;16(4):473–480. doi: 10.1111/j.1542-4758.2012.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalainy S, Reid R, Jindal K, Pannu N, Braam B. Fluid volume expansion and depletion in hemodialysis patients lack association with clinical parameters. Can J Kidney Health Dis. 2015;2:54. doi: 10.1186/s40697-015-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ie EH, Krams R, Vletter WB, Nette RW, Weimar W, Zietse R. Myocardial contractility does not determine the haemodynamic response during dialysis. Nephrol Dial Transplant. 2005;20(11):2465–2471. doi: 10.1093/ndt/gfi088. [DOI] [PubMed] [Google Scholar]
- 44.Benaroia M, Iliescu EA. Oral intake during hemodialysis: is there an association with intradialytic hypotension? Hemodial Int. 2008;12(1):62–65. doi: 10.1111/j.1542-4758.2008.00242.x. [DOI] [PubMed] [Google Scholar]
- 45.Kyriazis J, Glotsos J, Bilirakis L, et al. Dialysate calcium profiling during hemodialysis: use and clinical implications. Kidney Int. 2002;61(1):276–287. doi: 10.1046/j.1523-1755.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 46.Raja R, Henriquez M, Kramer M, Rosenbaum JL. Intradialytic hypotension - role of osmolar changes and acetate influx. Trans Am Soc Artif Intern Organs. 1979;25:419–421. doi: 10.1097/00002480-197902500-00080. [DOI] [PubMed] [Google Scholar]
- 47.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 48.Kotanko P, Garg AX, Depner T, et al. Effects of frequent hemodialysis on blood pressure: Results from the randomized frequent hemodialysis network trials. Hemodial Int. 2015;19(3):386–401. doi: 10.1111/hdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knoll GA, Grabowski JA, Dervin GF, O’Rourke K. A randomized, controlled trial of albumin versus saline for the treatment of intradialytic hypotension. J Am Soc Nephrol. 2004;15(2):487–492. doi: 10.1097/01.asn.0000108971.98071.f2. [DOI] [PubMed] [Google Scholar]
- 50.Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960–967. doi: 10.2215/CJN.00990207. [DOI] [PubMed] [Google Scholar]
- 51.HEMO Study protocol. Bethesd: NIH/NIDDK; Jun, 1999. [Google Scholar]
- 52.Pelosi G, Emdin M, Carpeggiani C, et al. Impaired sympathetic response before intradialytic hypotension: a study based on spectral analysis of heart rate and pressure variability. Clin Sci (Lond) 1999;96(1):23–31. [PubMed] [Google Scholar]
- 53.Locatelli F, Altieri P, Andrulli S, et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol. 2010;21(10):1798–1807. doi: 10.1681/ASN.2010030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnas MG, Boer WH, Koomans HA. Hemodynamic patterns and spectral analysis of heart rate variability during dialysis hypotension. J Am Soc Nephrol. 1999;10(12):2577–2584. doi: 10.1681/ASN.V10122577. [DOI] [PubMed] [Google Scholar]
- 55.van der Sande FM, Wystrychowski G, Kooman JP, et al. Control of core temperature and blood pressure stability during hemodialysis. Clin J Am Soc Nephrol. 2009;4(1):93–98. doi: 10.2215/CJN.01800408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santoro A, Mancini E, Basile C, et al. Blood volume controlled hemodialysis in hypotension-prone patients: a randomized, multicenter controlled trial. Kidney Int. 2002;62(3):1034–1045. doi: 10.1046/j.1523-1755.2002.00511.x. [DOI] [PubMed] [Google Scholar]
- 57.Munoz Mendoza J, Bayes LY, Sun S, Doss S, Schiller B. Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice-weekly in-center nocturnal hemodialysis: a quality improvement study. Am J Kidney Dis. 2011;58(6):956–963. doi: 10.1053/j.ajkd.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]