Abstract
Background and Objectives
Epidemiological studies suggest a close association between periodontitis and prediabetes/insulin resistance but whether periodontitis causes prediabetes in humans is not known. Using various animal models, we have recently established that periodontitis can be an initiator of prediabetes which is characterized by glucose intolerance, hyperinsulinemia, and insulin resistance. In addition, our in vitro studies indicated that Porphyromonas gingivalis (Pg) induced insulin secretion in MIN6 β cells and this induction was in part SerpinE1 (plasminogen activator inhibitor 1, PAI1) dependent. However, the mechanism(s) by which periodontitis induces prediabetes is not known. Since α and β cells in pancreatic islets are the major modulators of glucose levels, we investigated whether experimental periodontitis by oral application of a periodontal pathogen caused molecular and/or cellular alterations in pancreatic islets and whether SerpinE1 was involved in this process.
Materials and Methods
We induced periodontitis in C57BL/6 mice by oral application of a periodontal pathogen, Pg, and determined changes that occurred in islets following 22 weeks of Pg application. Pancreatic islet architecture was determined by 2-D and 3-D immunofluorescence microscopy and SerpinE1 and its substrate uPA as well as insulin, glucagon and Pg/gingipain in islets was detected by immunofluorescence. The presence of apoptotic islet cells was determined by both histochemical and immunofluorescence TUNEL assays. To further investigate the direct effect of Pg on apoptosis and the involvement of SerpinE1 in this process, we used SerpinE1 knockdown and scrambled control clones of the MIN6 pancreatic β cell line.
Results
Pg/gingipain was detected in both the periodontium and pancreas in the experimental group. Islets from animals that were administered Pg orally (experimental group) developed significant changes in islet architecture, upregulation of SerpinE1, and increased β cell apoptosis compared with the control group. We also observed that exposure of MIN6 cells to Pg in vitro resulted in apoptosis. However, apoptosis was significantly reduced when SerpinE1 expression by MIN6 cells was knocked down.
Conclusion
Oral application of the periodontal pathogen Pg to C57BL/6 mice induces periodontitis, translocation of Pg/gingipain to the pancreas, and results in complex alterations in pancreatic islet morphology. SerpinE1 appears to be involved in this process.
Keywords: prediabetes, pancreatic islets, SerpinE1, Porphyromonas gingivalis
Introduction
Prediabetes, the precursor to Type 2 Diabetes (T2DM), has become a silent threat with 79 million individuals affected in the US - three times higher than the number of individuals with T2DM (1). Therefore, prevention of prediabetes is essential to reduce the number of individuals developing T2DM and its associated serious complications.
Periodontitis is a chronic inflammatory disease which destroys gingiva and alveolar bone over an extended period of time and is mediated by a host cell response to invading bacteria (2). These bacteria are disseminated to different sites of the body such as the liver (3), aorta (4), and atherosclerotic plaque (5,6) via the systemic circulation as well as via host cells such as myeloid dendritic cells (6,7). However, the mechanisms by which periodontal pathogens influence the development of systemic diseases such as insulin resistance and prediabetes have yet to be clarified.
Results from our previous studies indicated that hyperinsulinemia developed when periodontitis was induced in rats and mice (8–10). Since the pancreatic β cell is the only known source of circulating insulin, these data suggest that periodontitis influences insulin secretion by stimulating pancreatic β cells. To extend these findings, we investigated the effect of the periodontal pathogen, Porphyromonas gingivalis (Pg), on insulin secretion by the β cell line MIN6 (11) and demonstrated that upregulation of insulin secretion by these cells was mediated by SerpinE1, also known as Plasminogen Activator Inhibitor1 or PAI1 (12).
SerpinE1 is a serine protease inhibitor that inhibits tissue type and urokinase plasminogen activators (tPA and uPA respectively), thus its primary function is anti-fibrinolytic. However, SerpinE1 also regulates cell migration (13). Due to its anti-fibrinolytic activity, increased levels of SerpinE1 are thought to lead to the development of atherosclerosis and atherothrombosis (14,15). SerpinE1 is associated with cardiovascular diseases and Metabolic Syndrome (16), including insulin resistance (IR), obesity and diabetes (17). It has also been reported that subjects with periodontitis have higher concentrations of circulating SerpinE1 compared with periodontally healthy subjects (18). However, the functional role of this increase in SerpinE1 in subjects with periodontitis has yet to be determined.
Hyperinsulinemia occurs in prediabetes. It is generally accepted that hyperinsulinemia is caused by over-production of insulin by pancreatic β cells to compensate for insulin resistance in the insulin target organs. Stressed β cells eventually fatigue and die by apoptosis (19). Thus, pancreatic β cell apoptosis can ultimately lead to frank Type 2 Diabetes. This significant decrease in functional β cells is a cardinal feature of T2DM. However, the factors that initiate apoptosis are just beginning to be described (20).
Since SerpinE1 promotes insulin secretion in MIN6 cells when cells are exposed to Pg-LPS (12), we hypothesized that SerpinE1 may function in induction of hyperinsulinemia and/or β cell apoptosis. To test this hypothesis, we investigated SerpinE1 involvement in apoptosis in MIN6 cells exposed to Pg.
To understand the biological relevance of Pg on pancreatic islets in vivo, we induced chronic periodontitis by oral application of Pg in mice (10) and analyzed changes that occurred in islet morphology with respect to α and β cells. Understanding mechanisms by which these changes occur will help determine how to modulate the induction of the prediabetic condition triggered by periodontitis.
Materials and methods
MIN6 cell culture
MIN6 cells (passages 20–30) were grown in Dulbecco’s modified Eagle’s medium (DMEM, containing 25mM glucose, 10% fetal bovine serum, 4mM L-glutamine, 1mM sodium pyruvate, 0.1mg/ml penicillin/streptomycin and 2μl/liter of 2-mercaptoethanol). Cells were incubated at 37°C in a 5% CO2 humidified incubator. The culture medium was changed every 3–4 days and cells passaged once a week.
P. gingivalis culture
Pg (strain W83) was grown anaerobically (85% N2, 10% H2 and 5% CO2) in GasPak anaerobic containers (BD Bioscience, Franklin Lakes, NJ, USA) using GasPak EZ pouches (BD Bioscience) at 37 °C according to the methodology described by Bhat and Watanabe (12).
Western blotting
Western blot analysis was performed using the protocol described by Watanabe et al. (9). Briefly, cells were washed with ice-cold PBS and lysed with RIPA buffer (50mM Tis-HCl, pH7.5, 150mM NaCl, 1mM EDTA, 1% IGEPAL) supplemented with protease inhibitor tablet (Complete Mini) (Roche Diagnostics, Indianapolis, IN, USA), 10μm/ml of phosphatase inhibitor cocktail II (EMD Millipore, Billerica, MA. USA), 5μl /ml of 10mM NaVO4 and 20μl/ml of 50mM phenylmethyl sulfonyl fluoride). The lysates were briefly sonicated and centrifuged and the supernatants stored at −80°C in aliquots. The protein levels in extracts were quantitated by the BioRad (Hercules, CA, USA) method. 40μg of extracts were mixed with equal volume of 2X Laemmli Buffer, boiled for 3 min and proteins separated using 4–20% Mini-Protean TGX gels (BioRad) using Tris-glycine running buffer. Proteins were then transferred to PVDF membrane. Blots were blocked with 5% milk in TBST (140mM NaCl, 10mM Tris-Hcl, ph7.5, 0.1% Tween-20) for 1h at room temp and incubated with primary antibody for 1h at room temp (or overnight at 4°C). After washing 4 times in TBST buffer, blots were then incubated in HRP-conjugated anti Rabbit IgG (BioRad) and washed 4 times. The blots were developed in Super Signal West Dura substrate (Thermo Fisher Scientific, Waltham, MA, USA), photographed and bands were quantitated using the ChemiDoc XRS+ imaging system (BioRad). The primary antibodies used were: p-Akt (Cell Signaling, Danvers, MA, USA), Akt (pan) (Cell Signaling), PAI-1 (Cell Signaling and Abcam, Cambridge, MA, USA), GAPDH (Cell Signaling), α-tubulin (Abcam), cleaved caspase 3 (Cell Signaling), cleaved caspase 8 (Cell Signaling), cleaved caspase 9 (Cell Signaling).
TUNEL assay for in situ apoptosis detection
Mid-log MIN6 cells were plated at a density of 5×105 /ml/chamber (BD Bioscience) and incubated at 37°C with 5% CO2 for 24 h. Pg (MOI 1:200) was added to each well and slides re-incubated for 3, 8, 16 and 24 h. The media was removed from each well, cells were washed once with PBS and fixed with freshly prepared 3.7% buffered formaldehyde (pH 7.0) for 10 min at room temperature. The slides were washed in PBS and each well was covered with 50 μl of cytonin and stored at 4°C in a humidified container. The slides were then washed in PBS and exposed for 10 min in 70% ethanol. The slides were stained for histochemical analysis by using TACS 2 TdT DAB In Situ Apoptosis Detection kit (Trevigen Inc., Gaithersburg, MD, USA) according to the manufacturer’s protocol. Briefly, the slides were treated with proteinase K for 15 minutes, quenched for 5 minutes and labeled with TdT labeling reaction mix for 1 h and stop buffer for 5 min and then washed. The samples were treated with Strep-HRP solution for 10 min, washed, immersed in DAB solution for 4 min, then washed and immersed in 1% methyl green for 1 min. The slides were then dipped 10 times in 2 changes of deionized water, 95% ethanol, 100% ethanol, xylene and mounted with coverslips using paramount. After 24 h, apoptotic cells were counted (5 different fields for each sample, 4 independent experiments) using a Leica DM-750 microscope.
TUNEL detection of apoptosing cells in pancreatic islets from the experimental (E) and control (C) groups was performed by immunofluorescence microscopy using TdT In Situ Apoptosis Detection Kit (Roche Diagnostics) according to the manufacturer’s protocol. β cells were detected as described below.
Experimental periodontitis by oral application of P. gingivalis in mice
Experiments were carried out in strict accordance with the recommendations outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago (Protocol approval #12-152). Twenty 6-week old male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were housed at constant temperature (22 °C) and humidity (45–55%) in a 14-hr light/10-hr dark cycle. All mice were placed on a regular chow (Teklad LM-485, Taklad Diets, Madison, WI, USA) and food consumption and body weight were measured every week. Experimental periodontitis was performed by oral application of Pg. For detailed description of the study design and results from analyses of glucose intolerance, fasting insulin and glucose levels over time, see Ilievski et al. (10).
Immunofluorescence microscopy
Immunofluorescence microscopy was performed for detection of insulin (β cell marker), SerpinE1, glucagon (α cell marker), nuclei (DAPI), and Pg/gingipain using samples as follows.
Pancreas sections were permeabilized by incubating for 30 min with 0.25% Tween 20 (BioRad) in PBS. Sections were then incubated with anti-insulin antibody (Santa Cruz Biotechnology, Dallas, TX, USA), at a 1:100 dilution for 1.5 h at room temperature followed by donkey anti-goat IgG conjugated with Alexa Fluor 488 at a 1:800 dilution for 1.5 h at room temperature. To detect SerpinE1, anti-SerpinE1 antibody (Abcam) at a 1:100 dilution was added to sections for incubation at room temperature followed by donkey anti-Rabbit IgG secondary antibody (1:800 dilution) conjugated with Alexa Fluor 594 (Invitrogen) (1:800 dilution) as secondary antibody for 1.5 h incubation at room temperature. Glucagon was detected using anti-glucagon antibody (Abcam) at a 1:100 dilution to sections which were then incubated for 1.5 h at room temperature followed by donkey anti-mouse IgG conjugated with Alexa Fluor 647 as secondary antibody (1:800 dilution) for a 1.5 h incubation at room temperature. Specificity of the polyclonal antibody to SerpinE1 was determined by using a SerpinE1 specific competing peptide. Positive cells were counted in 5 random fields at 400 X magnification. Pg/gingipain was detected using monoclonal antibody 61BG1.3 (DSHB, Iowa City, Iowa, USA) at a 1:100 dilution. Sections were incubated for 1.5 h followed by incubation with donkey anti-mouse secondary antibody (Thermo Fisher Scientific) (1:800 dilution) conjugated with Alexa Flour 647 for 1 h.
3-D analysis of islets was performed using 5μm serial sections of pancreas and the images were analyzed after acquiring them by confocal microscopy. 3-D analysis of islet cell organization was performed by reconstruction of islet structure from z- sections with the software Imaris x64 version 7.7.2. The specific region in the reconstructed 3D islet was created using the Imaris masking and surface creation methods. The number of cells (staining positive for SerpinE1, glucagon, and insulin) within a newly created isosurface delimited volume was counted.
Statistical analysis
Statistical analysis was performed using a paired Student’s t-test with a significance level of p<0.05. 3-D analysis was performed by co-localization of staining determined with thresholded Mander’s coefficients (21).
Results
P. gingivalis and/or gingipains was detected in periodontitis sites as well as in the pancreas of the experimental group but not the control group
Pg produces cysteine proteases, gingipains, which are located on the outer membranes. These are known to degrade a variety of host proteins involved in host defense (22). The presence of the Pg/gingipain epitope was detected by immunofluorescence microscopy using a monoclonal antibody 61BG1.3 (DSHB) specific to an epitope in gingipain (23) in periodontal tissues (Fig. 1A b) and pancreata (Fig. 1B b) of the experimental group, but not in the control group (Fig. 1A a, B a). Pg and/or gingipain was present in both pancreatic islets and parenchyma of the experimental group, but not in the control group (Fig. 1B).
Figure 1.
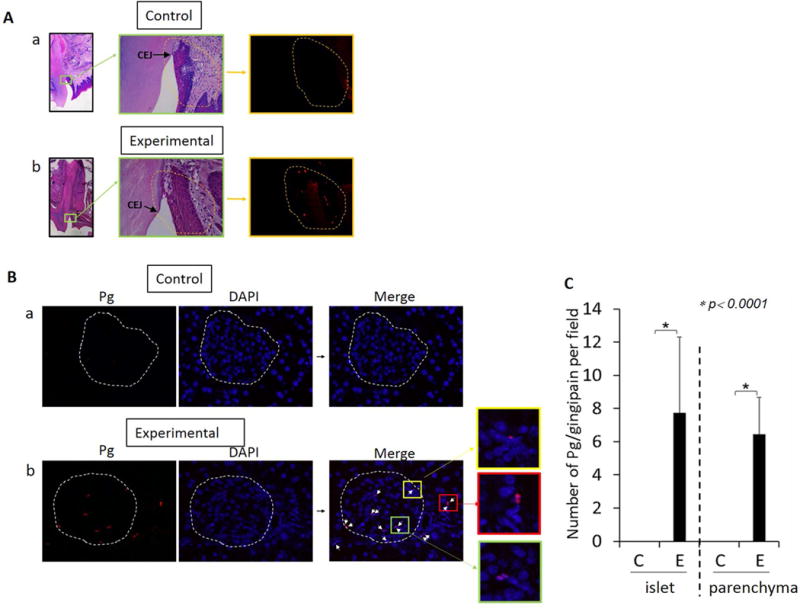
A. Pg/gingipain is present in the periodontium of experimental group but not in the control group. Left and middle panels: H&E staining of control (a) and experimental group (b) with magnification of 10X, 40X respectively. Right panel: Pg/gingipain immunofluorescence staining. Representative of N=5/group. Scale: 50μm. B. Pg/gingipain is present in the pancreas of the experimental group (E) (b) but not in the control group (C) (a). red: Pg, blue: DAPI. White arrows in the merged image indicate Pg/gingipain staining. Scale: 25μm. A Bar-graph shows the number of Pg/gingipain positive signals per field (c). Five random fields per sample were examined. X-axis: islet or parenchyma in control (C) and experimental (E) groups. Y-axis: number of Pg/gingipain positive signals/field. N=5 animals/group.
Oral application of Pg results in upregulation of SerpinE1 and changes in islet architecture
Results from immunofluorescence microscopy of islets indicated that the number of cells expressing SerpinE1 was significantly higher in the experimental group compared with the control group (Fig. 2A). Competing peptide analysis with SerpinE1-specific blocking peptide indicated that the primary polyclonal antibody to SerpinE1 was specific for SerpinE1 (data not shown). Furthermore, there was a significant alteration in the architecture of islets in the experimental group compared with controls. In control animals, α cells (defined by glucagon staining) were located in the mantle zone which surrounds the β- cell core (Fig. 2B a). However, in the experimental group, α cells were found inside the β-cell core as well as in the mantle zone (Fig. 2B b). SerpinE1 expression was minimal if any in the control group in the mantle zone as well as the β-cell core (Fig. 2C a, d). However, SerpinE1 expression was significantly higher in the α cells in the mantle zone (Fig. 2C b, c) and β cells in the β-cell core (Fig. 2C e, f) in islets from the experimental group compared with control group. This result is depicted by 3-D capture of glucagon, insulin and SerpinE1 staining (Fig. 2D).
Figure 2.
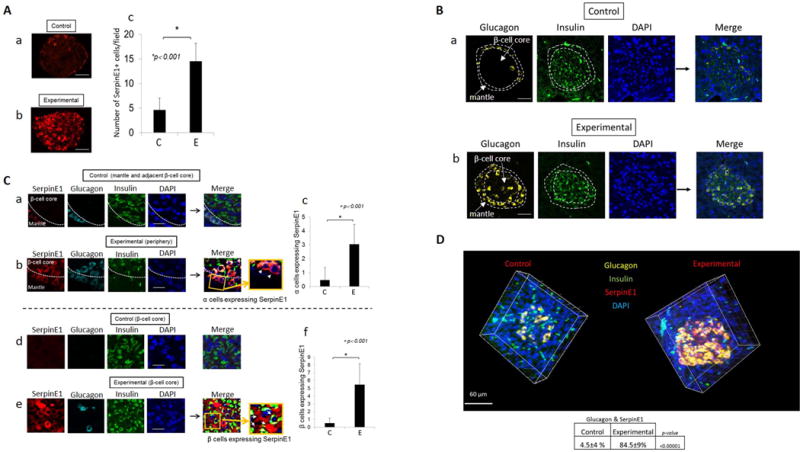
A. SerpinE1 expression in the pancreas is significantly greater in experimental group (b) compared with control group (a). SerpinE1 positive cells were counted in 5 random fields/sample (N=5 animals per group) (c). C: control, E: experimental. Scale: 40μm. B. Disorganization of α cells occurs during β cell compensatory phase/prediabetes in the experimental group. α cells (glucagon positive cells) are located in the mantle (periphery of β cell core) of the islet in control group while α cells are present in the mantle as well as dispersed in the β cell core in the experimental group. a: control animals, b: experimental group. Yellow: glucagon/alpha cells, Green: insulin/β-cells, blue: DAPI (nuclei). Representative of N=7 animals/group. Scale: 50μm. C. Confocal microscopy of representative pancreatic islets stained for α cells (glucagon), β cells (insulin), SerpinE1, and nuclei (DAPI). SerpinE1 expression is higher in the experimental group in both the mantle zone (b) and β cell core (e) compared with control group (a, d). Graphs show number of α cells (c) and β-cells (f) positive for SerpinE1. C: control, E: experimental. SerpinE1 positive cells were counted in 5 random fields (N=5 animals/group. Scale: 10μm. D. 3-D reconstruction of pancreatic islets from control and experimental groups. Glucagon (yellow), insulin (green), SerpinE1 (red), Nuclei were stained with DAPI (blue). Image acquired by Imaris X64. Number of islet cells (pixel) co-expressing glucagon and SerpinE1 is statistically higher in the experimental group compared with control group (P< 0.00001) (determined by t-test using Mander’s co-efficient). Representative of N=5 animals/group. Scale: 60μm.
Alpha cells but not beta cells express uPA, a substrate of SerpinE1, in animals with oral application of P. gingivalis
SerpinE1, when complexed with its substrate uPA, functions in cell migration (13). Thus, we investigated the pattern of uPA expression in islets. We observed that uPA was expressed in α cells (Fig. 3A a, b), but not β cells (Fig. 3A, β-cell core insets). There was a significantly higher number of cells co-expressing uPA and SerpinE1 in the experimental group (Fig. 3B b) compared with the control group (Fig. 3B a, c), and these co-expressing cells were α cells based on co-staining with glucagon (Fig. 3A c).
Figure 3.
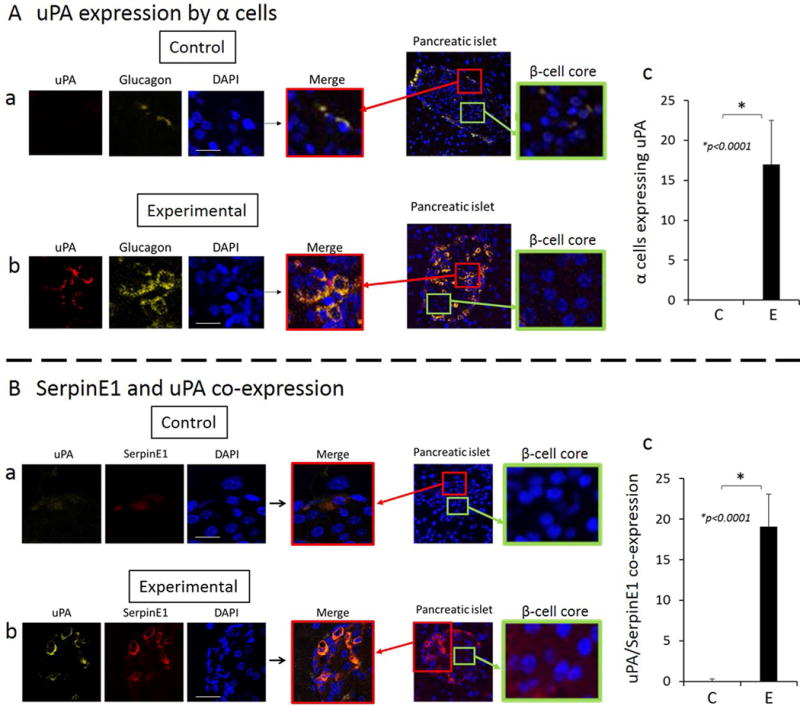
A. uPA is expressed in alpha cells (glucagon staining). Co-expression of uPA and glucagon is greater in the islets of experimental group compared with control (a–c). β cells do not express uPA (β-cell core insets). Scale: 10μm. B. uPA and SerpinE1 are co-expressed in pancreatic islets of experimental group and expression is significantly higher in the experimental group compared with the control group (a–c). Counts are positive cells at 40X magnification in 5 random fields per sample, and representative of N=5 animals/group. Scale: 10μm.
Beta cell apoptosis is evident in islets of animals with oral application of P. gingivalis.
To determine if cells in pancreatic islets of animals in the experimental group undergo apoptosis relative to control animals, we examined islets using immunofluorescence microscopy TUNEL assays (Fig. 4A a, b). We observed significant numbers of apoptotic cells in islets of the experimental group but not in the control group (Fig. 4A, B). These apoptotic cells were primarily β cells as indicated by insulin staining in the β-cell core (Fig. 4A b). The number of apoptotic β cells is significantly higher in the experimental group compared with the control group (Fig 4B).
Figure 4.
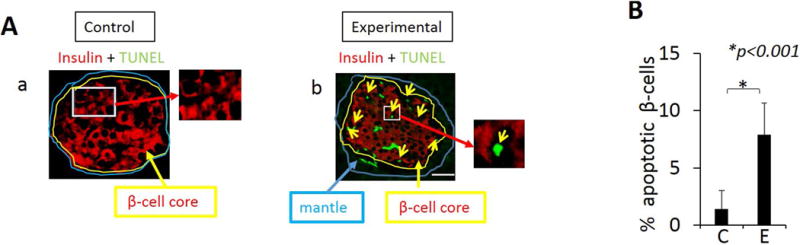
Detection of apoptotic cells in the pancreas of animals using TUNEL assay. A. Immunofluorescence images from TUNEL staining of β cells with merged images of islets from control group (a) and experimental group (b). TUNEL (green) positive cells are primarily β-cells (b). B. A significantly higher percentage of β-cells undergo apoptosis in tissue from experimental animals compared with controls. TUNEL positive cells were counted in 5 random fields. Representative of N=5 animals/group. Data are presented as mean ± SD. Scale: 25μm
P. gingivalis induces apoptosis of β cells via both intrinsic and extrinsic pathways in vitro and downregulation of SerpinE1 reduces MIN6 cell apoptosis
To determine the direct effects of Pg on apoptosis of β cells, we performed TUNEL assay using MIN6 cells. Pg at an MOI of 1:200 induced apoptosis by 8 h and, by 24 h over 90% of β cells were apoptosed (Fig. 5A). Western blot analyses indicated that caspase 8, 9 and downstream caspase 3 (Fig. 5B b, c, a respectively) were cleaved by 17 h after addition of Pg. Furthermore, addition of Pg to MIN6 cell cultures significantly reduced the phosphorylation of AKT, thereby limiting the ability of AKT to suppress apoptosis (Fig. 5C).
Figure 5.
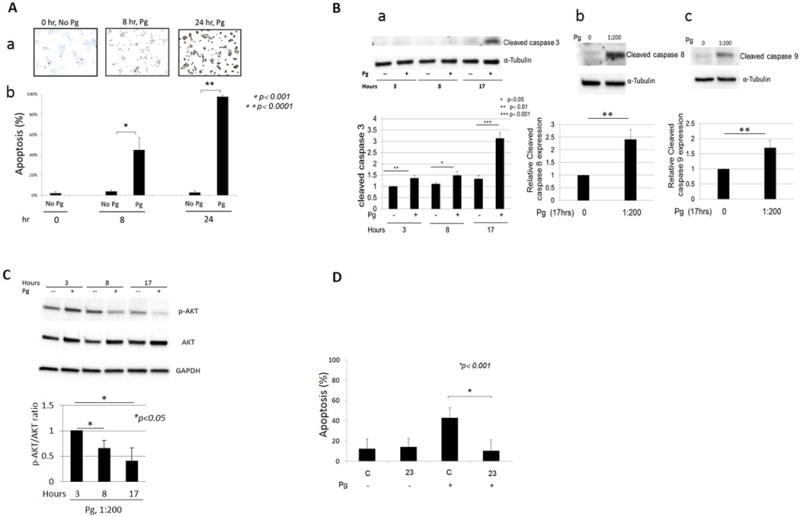
P. gingivalis induces apoptosis of MIN6 cells. A. a: TUNEL histochemical staining at time 0, 8 and 24 h after the addition of Pg (MOI of 1:200). B. % of TUNEL positive cells/total number of cells counted. Cells were counted in 5 random fields. Four independent experiments were performed. Data presented are mean ± SD. B. Results from Western blot analyses indicating cleaved caspase 3 (a), 8 (b) and 9 (c) in MIN6 cells in response to Pg. a. caspase 3 cleavage was monitored for 3, 8, and 17 h following addition of Pg (MOI 1:200). For b and c, caspase cleavage shown at 17 h post Pg addition. Four independent experiments were performed. Data are presented as mean ± SD. C. Pg suppresses AKT phosphorylation in MIN6 cells. Western blot probed for AKT and p-AKT. Four independent experiments were performed. Data are presented as mean ± SD. D. MIN6 cells with SerpinE1 knockdown are less susceptible to Pg induced apoptosis. Y-axis: % apoptosis determined by % TUNEL positive cells. C: control scrambled shRNA (clone C), 23: SerpinE1 shRNA transduced clone (clone 23). Cells were incubated with Pg or control media for 24 h. Four independent TUNEL experiments were performed. Data are presented as mean ± SD.
To determine whether SerpinE1 contributes to apoptosis in β cells, we used previously developed clones selected for reduction of SerpinE1 by transduction of SerpinE1 shRNA (clone 23) or control scrambled shRNA (clone C) MIN6 cells (12) and determined the extent of apoptosis following exposure to Pg (Fig. 5D). TUNEL staining of control clone C and clone 23 indicated that cells with SerpinE1 knockdown are less susceptible to Pg induced apoptosis 24 h after Pg addition.
Discussion
In our previous studies, we used application of LPS to the gingival sulci (24), placement of silk sutures with application of LPS (8), and oral application of Pg (10). In each of these model systems, we detected alveolar bone loss and all animals (experimental group) developed prediabetes. In the current study, we focused on the effect of oral application of Pg on the pancreas since this organ is the body’s only source of circulating insulin and is critical in maintaining normal glucose levels (19).
We previously reported that SerpinE1 was expressed in the MIN6 pancreatic β cell line in vitro and expression levels were significantly higher upon incubation of cells with Pg-LPS or live Pg (11,12). The results from the current in vivo study demonstrated that SerpinE1 was minimally expressed in the islets of the control group but was highly expressed in the experimental group, supporting the results from our in vitro study. As shown by 2-D and 3-D image analyses, SerpinE1 expression was upregulated not only in β cells but also in a majority of α cells in the experimental group.
One of the substrates of SerpinE1 is uPA and SerpinE1 bound to uPA is known to function in cell migration (13). We therefore examined uPA expression in pancreatic islets. uPA was co-expressed with SerpinE1 in α cells, but not in β cells, in the experimental group and was not expressed in the islets of the control group. This finding suggests that SerpinE1 in α cells may function in cell migration and contribute to disorganization of islet structure.
We observed that islet morphology was altered significantly in the experimental group animals. In this group, α cells were observed in the mantle zone and interdigitating between β cells in the β-cell core as evidenced by 2-D and 3-D immunofluorescence microscopy. In the normal pancreas, α cells are limited to the mantle zone, a belt like area that surrounds the β cell core. Our control group exhibited such morphology.
It is generally accepted that β cells proliferate in order to secrete more insulin to compensate for peripheral insulin resistance. Eventually these cells apoptose during the prediabetic, beta cell compensatory, phase (19). This loss of functional β cells leads to frank T2DM. However, this concept has been recently challenged (25) since near complete β cell specific destruction resulted in α cell proliferation followed by trans-differentiation of α to β cells (26). This showed a hitherto unknown plasticity of adult α cells. However, signals mediating this α to β cell conversion are still unknown. We determined that animals administered Pg developed hyperinsulinemia which could be explained by α to β cell transdifferentiation (26), exocytosis of insulin containing vesicles during apoptosis and/or hyperproduction of insulin by preapoptotic β cells.
To our knowledge, we are the first to report unique islet morphological changes characterized by increased expression of SerpinE1, increased number of α cells, invasion of α cells into β cell cores, and associated β cell apoptosis resulting from oral application of a periodontal pathogen.
Our in vitro data demonstrated that Pg-induced apoptosis of β cells occurred via both intrinsic (caspase 9 cleavage) and extrinsic (caspase 8 cleavage) pathways. The ratio of p-AKT/AKT decreased with addition of Pg, and thus reduced AKT signaling may contribute to the proapoptotic effects of Pg on β cells. TUNEL analysis in vivo revealed a significant number of apoptotic islet cells in the experimental group compared with control animals. Results from immunofluorescence microscopy indicated that the apoptotic cells in the experimental group were β cells and not α cells. It remains unclear why β cells and not α cells are susceptible to apoptosis. It was not unexpected that Pg would induce a significant amount of apoptosis in vitro within 24 h and yet it took many weeks to induce apoptosis in vivo. Cells in vitro are exposed to Pg directly and also the MIN6 cell generation time is short compared with β cells which are protected by matrices and host defense systems.
The effects of Pg on apoptosis have been studied by other investigators using primary human gingival epithelial cells (27, 28) and bovine primary coronary arterial endothelial cells (29). Pg 33277 and W83 induced apoptosis of primary gingival epithelial cells and bovine endothelial cells respectively via a gingipain-dependent mechanism (27,29). In contrast, Mao et al. (28) demonstrated that various strains of Pg inhibited chemically induced apoptosis of cultured gingival epithelial cells by blocking activation of caspase 3 (28) indicating an anti-apoptotic function of Pg. Thus, depending on the type of cells and the experimental conditions used in vitro, it appears that Pg can be pro or anti-apoptotic. Our results indicate that when Pg W83 is used to induce periodontitis by oral application, the apoptotic effect in pancreatic islets appears to be β cell specific. The effect of periodontitis (periodontal pathogens) in humans where a complex mixture of bacteria is present remains unknown. The presence of Pg/gingipain in both the islets and parenchyma of pancreas suggests, however, that both α and β cells were exposed to Pg/gingipain. Thus, susceptibility to apoptosis may be specifically inherent to β cells and/or α cells may possess an inherent apoptosis-resistance.
To investigate the possibility that SerpinE1 was involved in β cell apoptosis, we used clones developed by transducing SerpinE1 shRNA (clone 23, SerpinE1 knockdown) or scrambled shRNA (clone C, control clone) (12) and determined the extent of apoptosis following exposure to Pg by TUNEL assay. We demonstrated that clone 23 (SerpinE1 knockdown) cells were less susceptible to Pg induced apoptosis compared with control clone. We therefore conclude that SerpinE1, when expressed by β cells, is involved in promoting apoptosis. However, α cells expressed SerpinE1 in animals with periodontitis, but did not exhibit apoptosis as evidenced by TUNEL immunofluorescence microscopy. Thus, SerpinE1 appears to have different and distinct effects/functions on α vs β cells. Based on our finding that α cells co-express uPA and SerpinE1, we suggest that SerpinE1 plays a role in the migration of these cells. Whether uPA complexing with SerpinE1 in α cells but not β cells explains the protection of α cells from apoptosis needs further investigation.
There are variations in the experimental methodology in the oral application of Pg to induce periodontitis. It has been reported that bone loss varies depending on the strain of Pg used (30) and also the duration of Pg application (31,32). To study the effects of oral application of Pg on alveolar bone loss, short term application may be justified. However, to investigate the systemic effects of periodontitis/pathogen, a prolonged exposure to Pg to develop chronic periodontitis and/or repeated dosing of bacteria/bacteremia may be necessary (33).
The concept of a life-course chronicity/epidemiology is critical when diseases such as prediabetes and T2DM are studied. In contrast to acute bacterial infections which are resolved relatively quickly, the effects on target organs from repeated exposure to bacteria and their products, as occurs during periodontitis, may be cumulative and may have a critical role on the development of chronic disease in later life. The development of periodontitis and diabetes takes years or decades. Thus, the concept of chronic disease or life course chronicity/epidemiology involving repeated exposure to risk/etiological factors is an important consideration (34). One such example is smoking and the development of oral cancer which has a dose-response effect (frequency of exposure) (35). Another example is the effect of smoking on an individual’s health later in life (lifecourse study) as shown by North et al., (36). These studies showed that chronic repeated exposure to risk factors/etiological factors can cause disease in distant organs later in life and are relevant to our model in which repeated exposure to Pg was extended over 22 weeks.
Regular mastication, brushing and flossing expose the host to bacterial products repeatedly throughout life (37). The incidence and intensity of bacteremia associated with these processes increases with the severity of periodontitis and is via inflamed and ulcerated periodontal pockets next to the subgingival biofilm (38,39). This increase in bacterial numbers during periodontal infection collectively results in repeated exposure of distant organs such as the pancreas to bacteria and their (by)products. The pancreas receives blood via the coeliac and superior mesenteric arteries which are oxygenated but unfiltered by the liver. Thus, vital organs including the pancreas distant from periodontal tissues are repeatedly exposed to the periodontal microbiota and their products throughout life. This exposure provides a cogent mechanistic link between periodontal disease and the variety of systemic diseases with which periodontitis has been associated (40,41). Recent animal studies using ApoE−/− mice indicated that orally applied Pg can be translocated to different sites of the body including the liver, aorta, heart, and brain (31,33,42). In addition, a change in gut microbiota has been reported in C57BL mice following oral application of Pg (32). We here show that Pg and/or gingipain was detected in the pancreas of the experimental group and appeared to contribute to changes in both β cell survival and α cell migration resulting in islet disorganization.
In conclusion, this study has identified several significant changes that occur in the pancreas subsequent to oral application of Pg. These include alterations of pancreatic islet architecture, upregulation of SerpinE1 associated with islet cells (particularly α cells), hyperinsulinemia, and β cell apoptosis. All these events occurred while normoglycemia was maintained. A limitation of this study is that a single (albeit prototypical) pathogen was used to induce periodontitis, a disease characterized by a complex and not fully characterized microflora. Further studies will be necessary to determine if the use of more representative samples of periodontal microflora may hasten islet disorganization. In addition, it will be of significant clinical interest to determine if inhibition of SerpinE1 prevents or slows islet disorganization and subsequent development of prediabetes in the animal model system.
Acknowledgments
MIN6 cells were a kind gift from Dr. Donald Steiner at the University of Chicago. This study was supported by the NIH R01DE021405 (KW).
Footnotes
The authors report no conflicts of interest related to this study.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet. 2011 available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed December 2011.
- 2.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima M, Arimatsu K, Kato T, et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One. 2015;10:e0134234. doi: 10.1371/journal.pone.0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stelzel M, Conrads G, Pankuweit S, et al. Detection of Porphyromonas gingivalis DNA in aortic tissue by PCR. J Periodontol. 2002;73:868–870. doi: 10.1902/jop.2002.73.8.868. [DOI] [PubMed] [Google Scholar]
- 5.Figuero E, Sanchez-Beltran M, Cuesta-Frechoso S, et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82:1469–1477. doi: 10.1902/jop.2011.100719. [DOI] [PubMed] [Google Scholar]
- 6.Carrion J, Scisci E, Miles B, et al. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J Immunol. 2012;189:3178–87. doi: 10.4049/jimmunol.1201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frusho H, Miyauchi M, Hyogo H, et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J Gastroenterol. 2013;48:1259–1270. doi: 10.1007/s00535-012-0738-1. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Petro BJ, Shlimon AE, Unterman TG. Effect of periodontitis on insulin resistance and the onset of Type 2 Diabetes Mellitus in ZDF rats. J Periodontol. 2008;79:1208–1216. doi: 10.1902/jop.2008.070605. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Iizuka T, Adeleke A, et al. Involvement of toll-like receptor 4 in alveolar bone loss and glucose homeostasis in experimental periodontitis. J Periodont Res. 2011;46:21–30. doi: 10.1111/j.1600-0765.2010.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilievski V, Kinchen JM, Prabhu R, et al. Experimental periodontitis results in prediabetes and metabolic alterations in brain, liver and heart: Global untargeted metabolomic analyses. J Oral Biol (Northborough) 2016;3 doi: 10.13188/2377-987X.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat UG, Ilievski V, Unterman TG, Watanabe K. Porphyromonas gingivalis lipopolysaccharide upregulates insulin secretion from pancreatic beta cells line MIN6. J Periodontol. 2014;85:1629–1636. doi: 10.1902/jop.2014.140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat UG, Watanabe K. Serpine1 Mediates Porphyromonas gingivalis induced Insulin Secretion in the Pancreatic Beta Cell Line MIN6. J Oral Biol (Northborough) 2015;2:7–14. doi: 10.13188/2377-987X.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czekay RP, Wilkins-Port CE, Higgins SP, et al. PAI-1: An integrator of cell signaling and migration. Int J Cell Biol. 2011:562481. doi: 10.1155/2011/562481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 15.Sobel BE, Taatjes DJ, Schneider DJ. Intramural plasminogen activator inhibitor type-1 and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1979–1989. doi: 10.1161/01.ATV.0000091250.53231.4D. [DOI] [PubMed] [Google Scholar]
- 16.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–2207. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 17.Lyon CJ, Hsueh WA. Effect of plasminogen activator inhibitor-1 in diabetes mellitus and cardiovascular disease. Am J Med. 2003;115:62S–68S. doi: 10.1016/j.amjmed.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Bizzarro S, van der Velden U, ten Heggeler JM, et al. Periodontitis is characterized by elevated PAI-1 activity. J Clin Periodontol. 2007;34:574–580. doi: 10.1111/j.1600-051X.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 19.Rafacho A, Cestari TM, Taboga SR, Boschero AC, Bosqueiro JR. High doses of dexamethasone induce increased beta-cell proliferation in pancreatic rat islets. Am J Physiol Endocrinol Metab. 2009;296:E681–9. doi: 10.1152/ajpendo.90931.2008. [DOI] [PubMed] [Google Scholar]
- 20.Wali JA, Masters SL, Thomas HE. Linking metabolic abnormalities to apoptotic pathways in beta cells in Type 2 Diabetes. Cells. 2013;2:266–283. doi: 10.3390/cells2020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul JD, Coulombe KL, Toth PT, et al. SLIT3-ROBO4 activation promotes vascular network formation in human engineered tissue and angiogenesis in vivo. J Mol Cell Cardiol. 2013;64:124–131. doi: 10.1016/j.yjmcc.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Collyer CA. Gingipains from Porphyromonas gingivalis-complex domain structures confer diverse functions. Eur J Microbiol Immunol. 2011;1:41–58. doi: 10.1556/EuJMI.1.2011.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth V, Lehner T. Characterization of the Porphyromonas gingivalis antigen recognized by a monoclonal antibody which prevents colonization by the organism. J Periodontal Res. 1997;32:54–60. doi: 10.1111/j.1600-0765.1997.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 24.Ilievski V, Cho Y, Katwala P, et al. TLR4 expression by liver resident cells mediates the development of glucose intolerance and insulin resistance in experimental periodontitis. PLoS One. 2015;10:e0136502. doi: 10.1371/journal.pone.0136502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung CH, Levine F. Adult pancreatic alpha-cells: A new source of cells for beta-cell regeneration. Rev Diabet Stud. 2010;7(2):124–131. doi: 10.1900/RDS.2010.7.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28:1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 27.Stathopoulou PG, Galicia JC, Benakanakere MR, Garcia CA, Potempa J, Kinane DF. Porphyromonas gingivalis induce apoptosis in human gingival epithelial cells through a gingipain-dependent mechanism. BMC Microbiol. 2009;9:107. doi: 10.1186/1471-2180-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheets SM, Potempa J, Travis J, Fletcher HM, Casiano CA. Gingipains from Porphyromonas gingivalis W83 synergistically disrupt endothelial cell adhesion and can induce caspase-independent apoptosis. Infect Immun. 2006;74:5667–5678. doi: 10.1128/IAI.01140-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker PH, Dixon M, Evans RT, Roopenian DC. Heterogeneity of Porphyromonas gingivalis strains in the induction of alveolar bone loss in mice. Oral Microbiol Immunol. 2000;15:27–32. doi: 10.1034/j.1399-302x.2000.150105.x. [DOI] [PubMed] [Google Scholar]
- 31.Velsko IM, Chukkapalli SS, Rivera MF, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS ONE. 2014;9:e97811. doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arimatsu K, Yamada H, Miyazawa H, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poole S, Singhrao S, Chukkapalli S, et al. Active invasion of Porphyromonas gingivalis and infection–induced complement activation in ApoE−/− mice brains. J Alzheimers Dis. 2015;43:67–80. doi: 10.3233/JAD-140315. [DOI] [PubMed] [Google Scholar]
- 34.Nascimento GG, Leite FR, Correa MB, Horta BL, Peres MA, Demarco FF. Relationship between periodontal disease and obesity: the role of life-course events. Braz Dent J. 2014;25:87–89. doi: 10.1590/0103-6440201300019. [DOI] [PubMed] [Google Scholar]
- 35.Loyha K, Vatanasapt P, Promthet S, Parkin DM. Risk factors for oral cancer in northeast Thailand. Asian Pac J Cancer Prev. 2012;13:5087–5090. doi: 10.7314/apjcp.2012.13.10.5087. [DOI] [PubMed] [Google Scholar]
- 36.North TL, Palmer TM, Lewis SJ, et al. Effect of smoking on physical and cognitive capability in later life: a multicohort study using observational and genetic approaches. BMJ Open. 2015;5:e008393. doi: 10.1136/bmjopen-2015-008393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–25. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelkha SA, Freilich RW, Amar S. Periodontal innate immune mechanisms relevant to atherosclerosis and obesity. Periodontol 2000. 2010;54:207–221. doi: 10.1111/j.1600-0757.2010.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hujoel PP, White BA, García RI, Listgarten MA. The dentogingival epithelial surface area revisited. Periodontal Res. 2001;36:48–55. doi: 10.1034/j.1600-0765.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- 40.Kebschull M, Demmer RT, Papapanou PN. “Gum bug, leave my heart alone!”–epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chukkapalli SS, Velsko IM, Rivera-Kweh MF, Zheng D, Lucas AR, Kesavalu L. Polymicrobial oral infection with four periodontal bacteria orchestrates a distinct inflammatory response and atherosclerosis in ApoEnull mice. PlosOne. 2015;10:e0143291. doi: 10.1371/journal.pone.0143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera MF, Lee J, Aneja M, et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoEnull mice. PLoS One. 2013;8:e57178. doi: 10.1371/journal.pone.0057178. [DOI] [PMC free article] [PubMed] [Google Scholar]


