Abstract
Objectives
To evaluate the correlates of a clinically significant high score on the Social Responsiveness Scale (SRS) in 10-year-old children who were born extremely preterm and who did not meet criteria for Autism Spectrum Disorder (ASD).
Methods
After excluding 61 participants diagnosed with ASD, we grouped children by IQ < or ≥ 85 and then compared the prevalence of neurocognitive and other deficits between those who had SRS total and component scores ≥ 65 and their peers who had lower scores.
Results
Among children who had IQ ≥ 85, the prevalence of SRS total scores ≥ 65 was 16% (n=103/628), and among children who had IQ < 85 it was 27% (n=40/148), higher than the 4% prevalence expected based on normative population data. Among children who had IQ ≥ 85, those who had high SRS scores more often than their peers had deficits in attention and executive function, and language and communication, and they were more often rated by their parents and teachers as having behavioral (e.g., ADHD) and emotional (e.g., anxiety, depression) problems.
Conclusions
SRS-defined social impairment was much more common in our cohort of 10-year-old children born extremely preterm than was expected based on general population norms. High SRS scores were characteristic of children who had intellectual, neurocognitive, language and communication limitations, as well as deficits in behavior and emotion regulation.
Introduction
Compared to their term-born and normal-birth weight peers, preterm and low-birth-weight children are at higher risk of social deficits, including peer problems, fewer prosocial behaviors, difficulties making friends, and social withdrawal.1 Sometimes given the label “preterm behavioral phenotype,”2 these deficits are likely to contribute to affected children’s increased risk of emotional and behavior problems,3 anxiety or other psychiatric disorders.4 Interest in the topic of socialization after very preterm birth is further piqued by evidence that social skills are associated with executive function and academic achievement among children who were born very preterm or with low birth weight.5
Reports about the “preterm behavioral phenotype” have not used a validated instrument to estimate the prevalence of social impairment among school-age children who were born very preterm. Nor is it clear how often affected children have co-occurring limitations in other domains of neurocognitive and behavioral development. To enhance the care of children now and in the future, clinicians and researchers need information about the co-occurrence of dysfunctions in vulnerable children.6 Only one study has specifically assessed the relationship of neurocognitive deficits to social information processing and social adjustment in children born preterm.7
We used the Social Responsiveness Scale (SRS8) to characterize the constellation of social deficits considered typical of the “preterm behavioral phenotype” in a large, cohort of 10-year-old children born extremely preterm. The SRS is a brief, parent-completed questionnaire designed to evaluate a child’s social abilities, including social awareness, social cognition, social communication, social motivation, and autistic mannerisms. Though often used as an ASD screen or to assess related traits, the SRS has also been used to assess social-communicative limitations and ASD-like traits that do not meet standard clinical criteria,9 including those that may arise from other deficits; for example, in cognitive ability, language, executive function, attention, and behavior and emotion regulation.
We sought to describe the prevalence and correlates of SRS-defined social impairment in a large cohort of ten-year-olds who were born before the 28th week of gestation who did not meet diagnostic criteria for ASD.
Methods
Participants
We performed a multi-center prospective, observational study of the risk of structural and functional neurologic disorders in extremely preterm infants.10 A total of 1506 infants born before the 28th week of gestation were enrolled during the years 2002–2004 and 1200 survived to 2 years. Briefly, at age 10 years, 889 of 966 (92%) eligible children returned for an assessment of cognitive and executive function, language and communication, academic achievement, and social-emotional and social-communicative function. Of these, 874 (98%) completed an IQ test, 861 (97%) completed neurocognitive testing and 852 (96%) were evaluated by parents with the SRS. Children who survived to age ten years but who did not participate were more likely than participants to have indicators of social disadvantage (lower maternal education and receipt of public health insurance), but did not differ on sex, gestational age, or birth-weight Z-score (a measure of fetal growth restriction). Enrollment and consent procedures were approved by the institutional review boards of all participating institutions. This study did not include 61 children who met rigorous diagnostic assessments for ASD at the 10-year follow-up.11
Demographic and pregnancy variables
After delivery, a trained research nurse interviewed each mother in her native language using a structured data collection form and following procedures defined in a manual. Twenty percent of interviews were conducted with the assistance of native speaking translator.
Newborn variables
The gestational age estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), LMP without fetal ultrasound (7%), and gestational age recorded in the log of the neonatal intensive care unit (1%).
The birth weight Z-score is the number of standard deviations the infant’s birth weight is above or below the median weight of infants at the same gestational age in referent samples not delivered for preeclampsia or fetal indications.12
Assessment procedures at age 10 years
All families targeted for recruitment were contacted by mail and then by phone to invite them to participate in the 10-year follow up. Lost to follow-up families were searched for on state vaccination registries, and other openly-available websites, including Facebook.
Families willing to participate were scheduled for one visit during which all of the measures reported here were administered in 3 to 4 hours, including breaks. While the child was tested, the parent or caregiver completed questionnaires regarding the child’s medical and neurological status and behavior. The parent was also asked to complete the Kaufman Brief Intelligence Test – 2 (KBIT-2; Kaufman & Kaufman, 2004) nonverbal subscale.
Test measures were selected to provide comprehensive information about neurocognitive, academic, social-emotional, and social-communicative function in one testing session. All test measures were well-validated and provided recently normed standard scores allowing comparison to US population norms.
SOCIAL IMPAIRMENT
We used the Social Responsiveness Scale (SRS)8 to assess a constellation of social deficits considered typical of the “preterm behavioral phenotype”, and to assess its severity. The participant’s parent completed this 65-item instrument, which provides a total score reflecting severity of social deficits in the autism spectrum, as well as in five subdomains of social reciprocity: social awareness, social cognition, social communication, social motivation, and autistic mannerisms. Among the individual items are: “13. Is awkward in turn-taking interactions with peers (doesn’t seem to understand the give-an-take of conversations),” “Does not join group activities unless told to do so,” “27. Avoids starting social interactions with peers or adults,” “29. Is regarded by other children as odd or weird.” We dichotomized scores at 65 (i.e., the 96th percentile in the general population), as have others, which is higher than the published threshold of 60 (i.e., the 84th percentile) used to indicate moderate deficiencies in reciprocal social behavior that are “clinically significant” in the general population.
By choosing this higher criterion, we are making it clear that we want to identify children who have social limitations that are likely to interfere with, or limit, their daily activities. The SRS has high reliability and good accuracy.13 Confirmatory factor analysis in three US samples combined (N > 9,000) identified three factors among items encompassing social communication impairment (emotion recognition, social avoidance, and interpersonal relatedness) and two factors among items encompassing insistence on sameness and repetitive mannerisms.14
We used the Social Communication Questionnaire (SCQ) as a complementary measure of social impairment. The SCQ is a 40-item parent-report screening instrument that assesses social-communicative deficits and other ASD-related behaviors. Children receiving an SCQ score of 15 or higher are typically considered at risk for ASD. We report the item-specific results from the SCQ of those children who did not meet study criteria for ASD as a secondary measure of the prevalence of subclinical social impairment and ASD-like features.
NEUROCOGNITIVE AND OTHER ASSESSMENT MEASURES
We assessed a broad number of neuropsychological and behavioral functions, including intelligence, executive function, language, academic achievement, and social-emotional status. Details about the assessments of cognition and executive function (Differential Ability Scales–II; DAS-II), NEuroPSYchological Assessment-II; NEPSY-II), and language (Oral and Written Language Scales; OWLS) are provided in previous publications.11,15
While the child was tested, the parent or caregiver also completed the Child Symptom Inventory-4 Parent Checklist (CSI-4). The child’s current teacher was also asked to complete the Child Symptom Inventory-4 Teacher Checklist. CSI-4 diagnostic classifications were assigned based on CSI-4 DSM-5-keyed criteria. We also assessed parent report of communication function using the Children’s Communication Checklist-2 (CCC-2), which has 70 items that assess structural language (speech, syntax, semantics and coherence) and social language skills and language pragmatics (initiation, stereotyped language, use of context, nonverbal communication, social relations, and interests). The CCC-2 allows an assessment of social-communication impairment by contrasting a child’s pragmatic language to structural language score. We calculated Z-scores using normative data.
Data Analyses
We describe the prevalence of SRS total and component scores ≥ 65 according to summary and item specific measures of neurocognitive and executive function (DAS-II, NEPSY-II, OWLS), CSI-4 psychiatric classifications of social and emotional function, individual items of the SCQ, and communication function on the CCC-2. To avoid misattributing features of intellectual deficit to social impairment, we divided our cohort of eligible children into two groups: IQ < 85 (i.e., one standard deviation below the normative mean; N=148) or IQ ≥ 85 (N=628), respectively). In each of these two IQ groups, we determined the prevalence of SRS-defined social impairment (SRS total score ≥ 65), and calculated 95% confidence intervals. Confidence intervals that do not include the general population norm of 4% prevalence are statistically significantly different, and the width of each interval is an indication of precision. With 628 children who had an IQ >85, we had 99% power to detect a doubling of the prevalence rate, while with 148 children who had IQ<85 (n=148), we had 99% power to detect a tripling of prevalence the prevalence rate.
To describe the characteristics and scores of children who had SRS total scores ≥ 65 relative to those of their peers whose SRS total scores were <65, we calculated Z-scores using the norms from each standardized assessment. In our sample, the scores on most functional assessments were skewed towards the low end of the distributions, prompting us to focus on scores one or more standard deviations below the expected mean.
Results
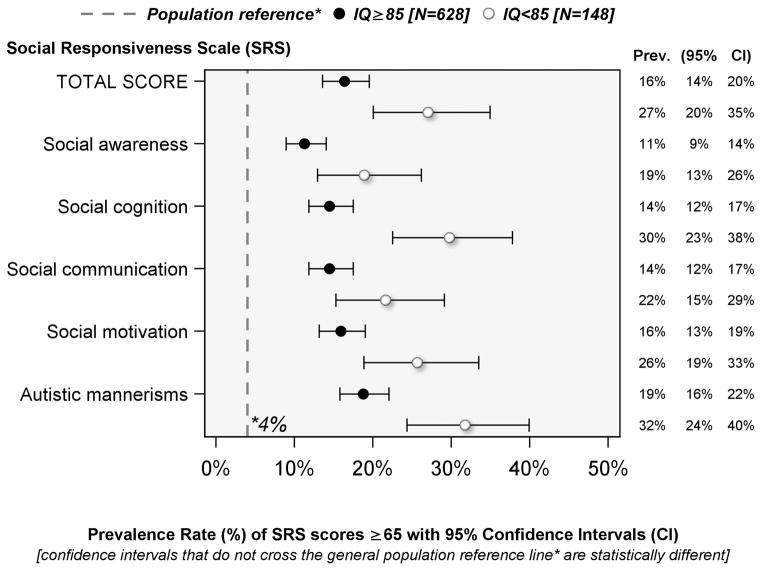
Prevalence of SRS-defined social impairment at age 10 years among children born before the 28th week of gestation [Figure 1]
Figure 1.
Prevalence of Social Impairment (SRS Total score ≥ 65) among children whose IQ was either < 85 or ≥ 85
Of the 776 children assessed at age 10 years, 628 had IQ ≥ 85 and 148 had IQ < 85. Sixteen percent of children whose IQ was >85 at age 10 years had SRS total scores ≥ 65 (i.e., social impairment); among the subset of 148 children who had IQ < 85, 27% had SRS-defined social impairment. The prevalence of high SRS component scores ranged from 11%–32% in both groups, about 3–8 times higher than the 4% prevalence expected based on normative population data, and all 95% confidence intervals of the estimated prevalence rates exceeded the general population norm.
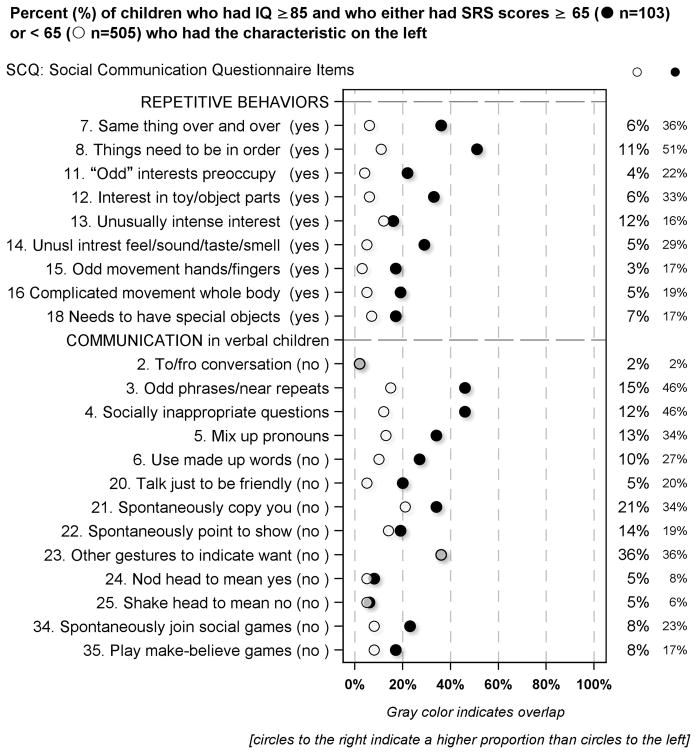
Social Communication Questionnaire (SCQ) item correlates of the SRS in children with IQ ≥ 85 [Figure 2 and Supplement Figure S1]
Figure 2.
Percent of children who had IQ ≥ 85 and who had SRS scores either below or ≥ 65 who had the SCQ defined characteristics described on the left
Based on parent-completed SCQ forms, children who had high SRS scores were far more likely than others to have limitations of verbal communication and reciprocal social interaction, and to have repetitive behaviors. On the other hand, they were no more likely than their peers with lower SRS scores to be classified as not spontaneously copying their parents (item #21) and not playing make-believe games (item #35).
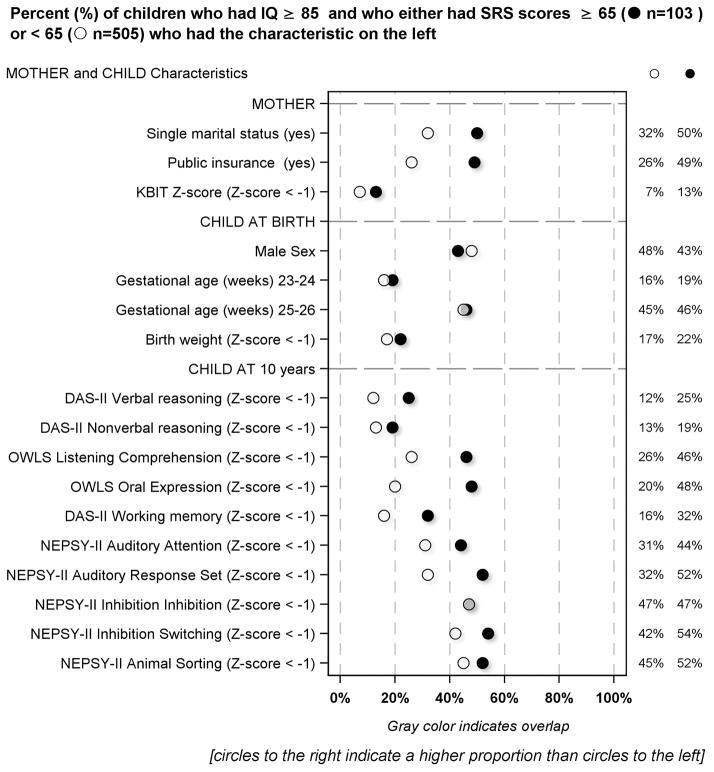
Maternal, newborn, and 10-year neurocognitive correlates in children with IQ ≥ 85 [Figure 3]
Figure 3.
Percent of children who had IQ ≥ 85 and who had SRS scores either below or ≥ 65 who had or were exposed to the characteristics described on the left
Compared to the mothers of children who had lower SRS scores, those whose child had SRS scores ≥ 65 were more likely to report they were not married, and to be eligible for government-provided medical care insurance (Medicaid).
Children born weighing more than one standard deviation below the expected mean for gestational age had SRS-defined social impairment more frequently than their expected birth- weight- for-gestational-age peers. About half (48%) of the children who had SRS scores ≥ 65 were male. By and large, children who had high SRS scores were more likely than their peers to have low scores on the DAS-II, NEPSY-II and OWLS assessments. The two exceptions were DAS-II Nonverbal Reasoning and the NEPSY-II Inhibition subtest.
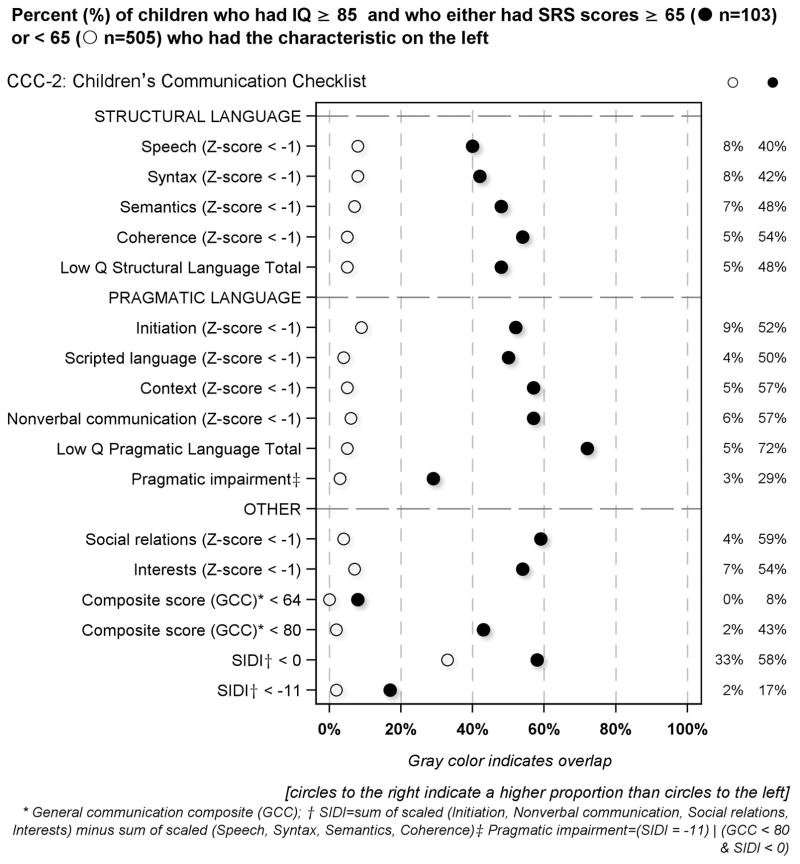
Children’s Communication Checklist (CCC-2) component correlates of the SRS in children with IQ ≥ 85 [Figure 4]
Figure 4.
Percent of children who had IQ ≥ 85 and who had SRS scores either below or ≥ 65 who had the CCC-2 defined characteristics described on the left
Children who had high SRS scores were much more likely than others to have low scores on each of the structural language and pragmatic language subtests of the CCC-2. Consequently, they were also more likely to have low general communication composite scores. Children who had high SRS scores had low quartile pragmatic language scores more frequently than low quartile structural language scores.
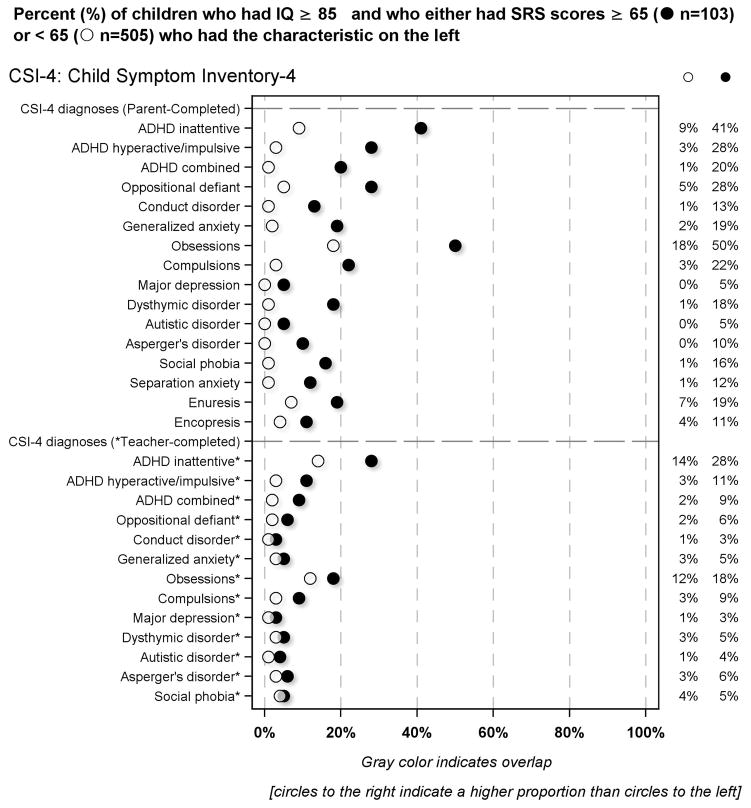
Parent-completed Child Symptom Inventory-4 item correlates of the SRS in children with IQ ≥ 85 [Figure 5]
Figure 5.
Percent of children who had IQ ≥ 85 and who had SRS scores either below or ≥ 65 who had the CSI-4 defined characteristics described on the left
According to parent report, children who had SRS-defined social impairment were more likely than others to be classified as screening positive for ADHD, oppositional defiant or conduct disorder, generalized or separation anxiety, major depression, dysthymic disorder and/or social phobia.
According to teacher report, children who had SRS-defined social impairment were more likely than others to screen positive for ADHD. Teachers preferentially identified the inattentive form of ADHD among children with an SRS-defined social impairment. Children who had high SRS scores were also much more likely than others to be classified by their teachers as having generalized anxiety.
Social impairment in children with IQ < 85 [Figure S2–S5]
Children who had high SRS scores more often had deficits than their peers with lower SRS scores across all cognitive and behavioral domains assessed.
Discussion
We have two main findings. First, the prevalence of SRS-defined social impairment was four times greater than expected based on general population norms among ten year-olds who were born before the 28th week of gestation, even after excluding children who met diagnostic criteria for ASD at age 10 (IQ ≥ 85: 16% and IQ < 85: 27% vs. 4%). Second, children who had SRS-defined social impairment tended to have neurocognitive, language, communication, and emotion and behavior regulation deficits more often than children who had lower SRS scores, irrespective of IQ.
Preterm behavioral phenotype
Our finding of a multiplicity of social deficits in our sample of children born extremely preterm is in keeping with the concept of a “preterm behavioral phenotype”.2 Support for the view that children who are born extremely preterm have deficits of multiple developmental systems, including those that influence the development of social abilities comes from observations that social limitations in children born preterm are frequently accompanied by attention and behavior problems.2,16
Some of the increased risk for social impairment and other deficits in children born extremely preterm might reflect the vulnerability of brain maturation processes, paucity of neuroprotective factors, physiologic instability after birth,17 inflammation-related phenomena that appear to increase the risk of brain damage in children born extremely preterm,18 or more likely a mix of these and perhaps other factors.
One possible explanation for the co-morbidities we found is that social impairment and other indicators of damage to brain function or structure share antecedents (that are correlates of very low gestational age)17 and/or share structural brain characteristics (with or without having shared antecedents). A systematic review of 23 studies that used an array of instruments not including the SRS to assess children born very preterm found that neonatal brain abnormalities are associated with social competence, though only two of the studies assessed children at or beyond age10 years, and none assessed a cohort of children born extremely preterm. 1
Some of the neonatal brain abnormalities identified are diffuse (ventriculomegaly, abnormal signals widely dispersed throughout the white matter) and involve brain structures/pathways that have also been associated with autism.19 More recent work further suggests that the “autistic brain” is characterized by hypo-connectivity rather than focal cortical abnormalities.20
It seems reasonable to assume that non-autistic children who have social limitations also have diffuse structural abnormalities similar to those of children with autism, but less severe. Perhaps some of these abnormalities are similar to the structural abnormalities associated with impaired attention,21 executive dysfunction,22 and/or language and communication impairment,23 all of which are associated with social impairment.
Although the above discussion emphasizes common antecedents and altered neuroconectivity in general, it is also possible that some dysfunctions are a consequence of, or are exacerbated by other dysfunctions. We are unable to identify primary and possibly secondary disturbances.
Implications
While similar phenomena have been observed in the general population,24,25 the recognition of the magnitude of social limitations (and the correlates reported here) among children born very or extremely preterm serves as an impetus to mobilize those who might be capable of preventing or ameliorating such dysfunctions.
Some raise the possibility that, “Just leaving extremely preterm infants alone in an incubator for several weeks may lead to a generation of incubator children” with social limitations.26 Proponents of Neonatal Individualized Developmental Care Assessment Program (NIDCAP) continue to make a strong argument for potential social and developmental benefits at minimal downside risk of their model, which emphasizes developmental support, minimization of stress, and relationship-based family-integrated approach.27
Early intervention to minimize the social limitations of children considered at risk for an autism spectrum disorder appears to hold some promise. Not all children considered at risk for an autism spectrum diagnosis will fulfill diagnostic criteria years later. Thus, it seems reasonable to consider the possibility that some of the children identified as “likely to develop ASD” might have had the preterm behavioral profile. Indeed some of the success attributed to early intervention might reflect the lack of severity of limitations among these children. At a minimum, the success of early interventions should be assessed in children who have (characteristics of) the preterm behavioral profile.
As mentioned above, very little is known about the antecedents of the preterm behavioral profile; we will address this in our future work. The most rewarding directions of investigation will likely be those that emphasize potentially brain-damaging exposures and non-genetic exposures linked to autism spectrum disorders.
With increasing recognition that epigenetic phenomena are likely to be involved,28 a new emphasis on those early exposures that seem to increase the probability of aberrant fetal programming seems promising.29
Limitations and strengths
The strengths of our study are the large number of infants enrolled based on extremely preterm gestational age rather than birth weight,30 and the large number of standardized instruments used to assess social impairment and deficits across other domains of neurocognitive and neurobehavioral development at age 10 years.
We were also able to screen almost all children for ASD, and we excluded children who had rigorously-diagnosed ASD.11 As in all observational studies, inferences of causation from associations are limited.
Conclusion
Among non-autistic, normal IQ children born extremely preterm, the prevalence of SRS-defined social impairment was several times higher than would be expected based on general population norms. Children with these social impairments were more likely than others to have neurocognitive, language and communication deficits, as well as behavior and emotion regulation problems.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069 - 06A2), the National Institute of Child Health and Human Development (5P30HD018655-28), and the Wayne State University Perinatal Initiative. The funders were not involved in study design, data collection, data analysis, manuscript preparation, and/or publication decisions.
The authors express their gratitude to the children and their families who participated in this study. They also gratefully acknowledge the contributions of the ELGAN Study Investigators, listed on the next page.
-
Boston Children’s Hospital, Boston MA
Janice Ware, Taryn Coster, Brandi Henson, Rachel Wilson, Kirsten McGhee, Patricia Lee, Aimee Asgarian, Anjali Sadhwani
-
Tufts Medical Center, Boston MA
Ellen Perrin, Emily Neger, Kathryn Mattern, Jenifer Walkowiak, Susan Barron
-
University of Massachusetts Medical School, Worcester MA
Jean Frazier, Lauren Venuti, Beth Powers, Ann Foley, Brian Dessureau, Molly Wood, Jill Damon-Minow
-
Yale University School of Medicine , New Haven, CT
Richard Ehrenkranz, Jennifer Benjamin, Elaine Romano, Kathy Tsatsanis, Katarzyna Chawarska, Sophy Kim, Susan Dieterich, Karen Bearrs
-
Wake Forest University Baptist Medical Center, Winston-Salem NC
T. Michael O’Shea, Nancy Peters, Patricia Brown, Emily Ansusinha, Ellen Waldrep, Jackie Friedman, Gail Hounshell, Debbie Allred
-
University Health Systems of Eastern Carolina, Greenville, NC
Stephen C. Engelke, Nancy Darden-Saad, Gary Stainback
-
North Carolina Children’s Hospital, Chapel Hill, NC
Diane Warner, Janice Wereszczak, Janice Bernhardt, Joni McKeeman, Echo Meyer
-
Helen DeVos Children’s Hospital, Grand Rapids, MI
Steve Pastyrnak, Wendy Burdo-Hartman, Julie Rathbun, Sarah Nota, Teri Crumb,
-
Sparrow Hospital, Lansing, MI
Madeleine Lenski, Deborah Weiland, Megan Lloyd
-
University of Chicago Medical Center, Chicago, IL
Scott Hunter, Michael Msall, Rugile Ramoskaite, Suzanne Wiggins, Krissy Washington, Ryan Martin, Barbara Prendergast, Megan Scott
-
William Beaumont Hospital, Royal Oak, MI
Judith Klarr, Beth Kring, Jennifer DeRidder, Kelly Vogt
Abbreviations
- CCC-2
Children’s Communication Checklist-2
- CSI-4
Child Symptom Inventory-4
- DAS-II
Differential Ability Scales–II
- NEPSY-II
Developmental NEuroPSYchological Assessment-II
- OWLS
Oral and Written Language Scales
- SCQ
Social Communication Questionnaire
- SRS
Social Responsiveness Scale
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Ritchie K, Bora S. Social development of children born very preterm: a systematic review. 2015;57(10):899–918. doi: 10.1111/dmcn.12783. [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Wolke D. Behavioural outcomes and psychopathology during adolescence. Early Hum Dev. 2013;89(4):199–207. doi: 10.1016/j.earlhumdev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Linsell L, Malouf R, Johnson S, et al. Prognostic Factors for Behavioral Problems and Psychiatric Disorders in Children Born Very Preterm or Very Low Birth Weight: A Systematic Review. J Dev Behav Pediatr. 2016;37(1):88–102. doi: 10.1097/DBP.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund LK, Vik T, Lydersen S, et al. Mental health, quality of life and social relations in young adults born with low birth weight. Health and quality of life outcomes. 2012;10:146. doi: 10.1186/1477-7525-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escovar E, Rosenberg-Lee M, Uddin LQ, et al. The Empathizing-Systemizing Theory, Social Abilities, and Mathematical Achievement in Children. Scientific reports. 2016;6:23011. doi: 10.1038/srep23011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. The lancet Psychiatry. 2016 doi: 10.1016/S2215-0366(16)30376-5. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe KR, Vannatta K, Nelin MA, et al. Executive functions, social information processing, and social adjustment in young children born with very low birth weight. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2015;21(1):41–54. doi: 10.1080/09297049.2013.866217. [DOI] [PubMed] [Google Scholar]
- 8.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 9.Hong DS, Dunkin B, Reiss AL. Psychosocial functioning and social cognitive processing in girls with Turner syndrome. J Dev Behav Pediatr. 2011;32(7):512–520. doi: 10.1097/DBP.0b013e3182255301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph RM, O’Shea TM, Allred EN, et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism research : official journal of the International Society for Autism Research. 2016 doi: 10.1002/aur.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yudkin PL, Aboualfa M, Eyre JA, et al. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 13.Mazen I, Hiort O, Bassiouny R, et al. Differential diagnosis of disorders of sex development in Egypt. Horm Res. 2008;70(2):118–123. doi: 10.1159/000137657. [DOI] [PubMed] [Google Scholar]
- 14.Frazier TW, Ratliff KR, Gruber C, et al. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the social responsiveness scale-2. Autism : the international journal of research and practice. 2014;18(1):31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- 15.Joseph RM, O’Shea TM, Allred EN, et al. Neurocognitive and Academic Outcomes at Age 10 Years of Extremely Preterm Newborns. Pediatrics. 2016;137(4) doi: 10.1542/peds.2015-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott MN, Taylor HG, Fristad MA, et al. Behavior disorders in extremely preterm/extremely low birth weight children in kindergarten. J Dev Behav Pediatr. 2012;33(3):202–213. doi: 10.1097/DBP.0b013e3182475287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leviton A, Blair E, Dammann O, et al. The wealth of information conveyed by gestational age. J Pediatr. 2005;146(1):123–127. doi: 10.1016/j.jpeds.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75(3):376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23(2–3):183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Conti E, Calderoni S, Marchi V, et al. The first 1000 days of the autistic brain: a systematic review of diffusion imaging studies. Front Hum Neurosci. 2015;9:159. doi: 10.3389/fnhum.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg MD, Finn ES, Scheinost D, et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19(1):165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostgard HF, Solsnes AE, Bjuland KJ, et al. Executive function relates to surface area of frontal and temporal cortex in very-low-birth-weight late teenagers. Early Hum Dev. 2016;95:47–53. doi: 10.1016/j.earlhumdev.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Kwon SH, Scheinost D, Vohr B, et al. Functional magnetic resonance connectivity studies in infants born preterm: suggestions of proximate and long-lasting changes in language organization. Dev Med Child Neurol. 2016;58(Suppl 4):28–34. doi: 10.1111/dmcn.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hus V, Bishop S, Gotham K, et al. Factors influencing scores on the social responsiveness scale. Journal of child psychology and psychiatry, and allied disciplines. 2013;54(2):216–224. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havdahl KA, Hus Bal V, Huerta M, et al. Multidimensional Influences on Autism Symptom Measures: Implications for Use in Etiological Research. J Am Acad Child Adolesc Psychiatry. 2016;55(12):1054–1063. e1053. doi: 10.1016/j.jaac.2016.09.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagercrantz H. Are extremely preterm born children with autism the victims of too much isolation in the incubator? Acta Paediatr. 2017 doi: 10.1111/apa.13874. [DOI] [PubMed] [Google Scholar]
- 27.Als H. NIDCAP: testing the effectiveness of a relationship-based comprehensive intervention. Pediatrics. 2009;124(4):1208–1210. doi: 10.1542/peds.2009-1646. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow S, Manning JR, Cartier J, et al. Epigenomic profiling of preterm infants reveals DNA methylation differences at sites associated with neural function. Transl Psychiatry. 2016;6:e716. doi: 10.1038/tp.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell KJ, Meaney MJ. Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis. The American journal of psychiatry. 2017;174(4):319–328. doi: 10.1176/appi.ajp.2016.16020138. [DOI] [PubMed] [Google Scholar]
- 30.Arnold CC, Kramer MS, Hobbs CA, et al. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.