Abstract
Objective
Women with a history of preeclampsia are at an increased risk of hypertension and structural brain changes. However, the combined effect of both preeclampsia and late-life hypertension on brain structural changes is not known and was investigated in this study.
Methods
Participants were identified from the population-based Rochester Epidemiology Project cohort. Four groups of women, were recruited and investigated in this study: (1) women with a history of normotensive pregnancy who have late-life hypertension (n=8, median age=62), (2) women with a history of normotensive pregnancy who do not have late-life hypertension (n=32, median age=59), (3) women with a history of preeclampsia who have late-life hypertension (n=24, median age=60), and (4) women with a history of preeclampsia who do not have late-life hypertension (n=16, median age=57). Cerebrovascular disease lesions on MRI, and total gray matter volumes were assessed.
Results
Total gray matter volumes were smaller in women with a history of preeclampsia and late-life hypertension compared to the other groups. Voxel based morphometry demonstrated that the volume changes were localized to the posterior brain regions, particularly the occipital lobe gray matter in women with a history of preeclampsia and late-life hypertension.
Conclusion
Having late-life hypertension superimposed on a history of preeclampsia affects the brain structure differently than having either a history of preeclampsia alone or a history of normotensive pregnancy either with or without late-life hypertension.
Keywords: hypertensive pregnancy, preeclampsia, hypertension, MRI, neuroimaging, atrophy, cerebrovascular disease
Introduction
Preeclampsia is a hypertensive pregnancy disorder that affects around 3–8% of all pregnancies worldwide [1]. Women who experienced preeclampsia that resolved after delivery have a fourfold increased risk of developing hypertension, on average 8–10 years earlier, compared to women who had normotensive pregnancies [2]. Additionally, women with histories of hypertensive pregnancy disorders have smaller brain volumes measured with magnetic resonance imaging (MRI) decades after the pregnancy compared to women with histories of normotensive pregnancies[3]. However, studies have not determined the combined effect of both a history of preeclampsia and late-life hypertension on the risk of abnormal findings on brain MRI later in life.
MRI provides a sensitive measure of the presence of cerebrovascular disease lesions defined by white matter hyperintensities (WMH), cortical and subcortical infarctions, and microbleeds. WMH are areas of increased signal in subcortical and periventricular white matter on T2-weighted images that are associated with cerebral small vessel disease in older adults[4]. Infarctions and microbleeds are cerebrovascular disease–related lesions that are visible on MRI. Gray matter atrophy, a marker of neuronal loss, is also associated with increased severity of cerebrovascular disease and, therefore, can reflect a global burden of cerebrovascular disease on the brain [4].
The purpose of this study was to determine the impact of a history of preeclampsia and late-life hypertension on MRI findings associated with cerebrovascular disease and structural MRI measures of gray matter atrophy.
Methods
Study design and participants
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards and all participants provided written informed consent. The Rochester Epidemiology Project (REP) medical records-linkage system was used to identify women (n=40) with a history of preeclampsia and age- and parity-matched women (n=40) with a history of normotensive pregnancy, as described previously [5]. Briefly, to be eligible, the women had to be residents of Olmsted County, MN when delivering a baby from a pregnancy lasting >20 weeks between 1976 through 1982 (n=990). Medical records of women identified by Hospital International Classification of Diseases Adapted (HICDA) codes were reviewed for indication of a possible hypertensive pregnancy disorder. A possible hypertensive pregnancy disorder was then confirmed as preeclampsia if a woman had at least 1 preeclamptic pregnancy from 1976 through 1982 and met the following criteria: (1) ≥2 BP readings of a systolic BP >140 mm Hg or a diastolic BP >90 mm Hg that occur at least 4 hours apart after >20 weeks’ gestation; and (2) proteinuria. Preeclampsia could have been experienced at any pregnancy and there were no twin pregnancies. After confirmation of history of preeclampsia, 77 eligible women from the REP were invited to participate in the study. Of those, 25 (32%) refused, 7 (9%) did not respond, and 5 (6%) were found to be ineligible due to temporal lobe epilepsy (n=1), lung cancer (n=1), contraindication for MRI (n=1), possible TIA (n=1), medication that interferes with blood tests (n=1). after screening for additional medical conditions not identified in the medical record. Finally, we identified 40 women with histories of preeclampsia that met the inclusion and exclusion criteria of this study. Additionally, women were identified as having “severe disease” if they had 1.) SBP>160 or DBP > 110 during pregnancy, 2.) recurrence in another pregnancy, 3.) intrauterine fetal demise or 4.) delivery < 34 weeks gestation due to preeclampsia[6].
Women with histories of normotensive pregnancies, who were identified as those with none of the possible hypertensive pregnancy codes were age- and parity-matched to the 40 woman with a history of preeclampsia. Of the 104 women with histories of normotensive pregnancies who were contacted, 18 (17%) refused, 41 (39%) did not respond, and 5 (5%) were interested in participating, but they were not included in the study because other matched controls (n=40) were already in the study.
The medical records were then fully abstracted for demographic and clinical information at the time of each pregnancy: date of birth, ethnicity, education, marital status, family history, prenatal blood pressures, weight, laboratory values (such as creatinine, proteinuria, liver enzymes), medications, tobacco use, alcohol use, pregnancy complications, seizures, persistent headache, epigastric pain, coma, chronic hypertension, diabetes, stroke, cardiac disease, renal disease, hepatic disease, autoimmune disorder, admission into the intensive care unit, postpartum depression, and hyperreflexia[5]. Women with a clinical diagnosis of cardiac disease (e.g. congestive heart failure), stroke, any cancer (with the exception of non-melanoma skin cancer), autoimmune disease (e.g., multiple sclerosis, lupus) were excluded because these entities can cause structural changes on MRI and confound investigations of preeclampsia-related vascular damage.
The medical records of the women were abstracted to identify whether they had chronic hypertension at the time of enrollment in the current study. Presence of hypertension was defined by either the current use of antihypertensive drugs as abstracted from the medical record or by an average of three measurements with a mean systolic blood pressure (SBP) > 140 mm Hg and/or mean diastolic blood pressure (DBP) > 90 mm Hg. Four groups of women were investigated in this study: (1) women with a history of normotensive pregnancy who have current hypertension, (2) women with a history of normotensive pregnancy who do not have current hypertension, (3) women with a history of preeclampsia who have current hypertension, and (4) women with a history of preeclampsia who do not have current hypertension.
MRI Methods
Image Acquisition
All women agreed to have MRI acquired for this study. All participants underwent MRI at 3 Tesla on a GE Signa scanner (GE Healthcare, Milwaukee, WI). 3D Magnetization Prepared Rapid Acquisition Gradient Echo (T1-weighted MRI; TR/TE/T1 = 2300/3/900 ms; flip angle = 8°; FOV = 26 cm; in-plane matrix = 256 × 256; phase FOV = 0.94; slice thickness = 1.2 mm); Fluid Attenuated Inversion Recovery (FLAIR; TR/TI/TE = 11,000/2,250/147 ms, matrix = 256 × 192, FOV = 24 cm, slice thickness = 3 mm interleaved) MRI; and T2* Gradient Recalled Echo (GRE; TR/TE = 200/20 ms; flip angle = 12°; FOV = 20 cm; in-plane matrix = 256 × 224; phase FOV = 1.00; slice thickness = 3.3 mm) images were acquired for all patients.
Quantification of White Matter Hyperintensities
The total volume of white matter hyperintensities (WMH), a marker of cerebral small vessel disease, was quantified using a semi-automated algorithm [7]. T1-weighted images were segmented using SPM5[8] and aligned to FLAIR images, and corresponding brain masks were used to remove non-brain tissue and voxels that had a high probability of being cortical gray matter. Voxels that were deemed candidate WMH locations were clustered via connected-components. Clusters were removed if they consisted of a single isolated voxel, were mostly located outside regions classified as white matter, or contained no supra-threshold FLAIR voxels after blurring. Remaining clusters were then manually edited by a trained image analyst (S.M.Z.) who was blinded to the diagnostic group assignment to correct for WMH misclassifications. Following assessment of infarcts (described in the next section), the hyperintense voxels associated with infarcts were marked and excluded from the total WMH volume measurement as they are attributed to distinct pathophysiologic processes. The total WMH volume was adjusted for total intracranial volume. This accounts for differences in the relative head size/stature of the participants.
Assessment of Infarcts and Microbleeds
Cerebral infarcts were identified on FLAIR MRI and microbleeds on T2* GRE images by a trained image analyst (S.M.Z.) and confirmed by a radiologist (K.K.) who were both blinded to the diagnostic group category. We have previously shown excellent intra-rater reliability in assessment of cortical and subcortical infarctions using this method [9, 10]. Cortical infarcts were defined as areas of increased signal intensity on FLAIR MRI in the cortical gray matter. Subcortical infarcts were defined as lesions of ≥3 mm surrounded by a hyperintense rim in the subcortical gray matter or white matter. Subcortical infarcts were differentiated from dilated perivascular spaces based on the shape of the hypointense center and the surrounding hyperintesity. Microbleeds were defined as hypointense lesions ≤10 mm in diameter on T2* GRE.
Gray Matter Volumes
Total gray matter volume was calculated using FreeSurfer version 5.3 (www.freesurfer.net; access year 2013) and adjusted for total intracranial volume using a linear regression.
Voxel-Based Morphometry
Voxel-based analysis was performed to determine regional structural differences in cortical gray matter using our previously described voxel based morphometry technique using SPM5 (http://www.fil.ion.ucl.ac.uk/spm; access year 2005)[8, 11, 12].
Statistical analysis
Characteristics of participants and differences among the four groups were assessed using Fisher’s Exact Tests for categorical variables and Kruskal-Wallis One-way Analysis of Variance (ANOVA) by Ranks Tests for continuous variables (Table 1). Differences in MRI measures across the four groups were first assessed using two-way factorial ANCOVA, with preeclampsia and current hypertension as our categorical predictors of interest, and adjustment for age and total intracranial volume on logarithmic scale due to skewness [log(TIV)]. Results from voxel based morphometry were assessed at a statistical threshold of p<0.001 with a cluster size correction of ≥500 voxels.
Table 1.
Participant characteristics. Table shows demographics and cardiovascular risk factor information of the participants, comparing the 4 groups of women. P-values were generated using Fisher’s Exact and Kruskal-Wallis ANOVA by Ranks Tests.
| History of Normotensive Pregnancy (N = 40) | History of Preeclampsia (N = 40) | P-value | |||
|---|---|---|---|---|---|
|
| |||||
| Current Hypertension (N = 8) | No Current Hypertension (N = 32) | Current Hypertension (N = 24) | No Current Hypertension (N = 16) | ||
|
| |||||
| median (IQR) | median (IQR) | median (IQR) | median (IQR) | ||
|
| |||||
| Age at MRI (years) | 62 (59, 66) | 59 (56, 62) | 60 (58, 63) | 57 (54, 60) | 0.05 |
| Age at menopause (years) | 50 (46, 50) | 50 (48, 53) | 50 (48, 54) | 50 (49, 51) | 0.78 |
| Education (years) | 14 (13, 16) | 14 (13, 16) | 14 (12, 15) | 14 (13, 16) | 0.70 |
| Mean systolic blood pressure (mm Hg) | 160 (131, 164) | 123 (114, 138) | 137 (130, 143) | 123 (119, 134) | 0.004 |
| Mean diastolic blood pressure (mm Hg) | 82 (76, 86) | 74 (69, 80) | 80 (72, 87) | 78 (69, 82) | 0.17 |
| Hypertension duration (years) | 7 (5, 11) | 13 (8, 14) | |||
| Body mass index (kg/m2) | 24.2 (22.9, 27.6) | 25.6 (23.2, 32.6) | 32.0 (28.5, 34.5) | 26.1 (23.8, 31.4) | 0.02 |
|
| |||||
| n (%) | n (%) | n (%) | n (%) | ||
|
| |||||
| Menopausal hormone therapy use | 4 (50) | 13 (41) | 10 (42) | 7 (44) | 0.97 |
| Migraine | 3 (38) | 5 (16) | 6 (25) | 3 (19) | 0.54 |
| Dementia | 0 (0) | 0 (0) | 1 (4) | 0 (0) | |
| Hyperlipidemia | 6 (75) | 23 (72) | 19 (79) | 13 (81) | 0.92 |
| Diabetes | 0 (0) | 2 (6) | 4 (17) | 0 (0) | 0.29 |
| Severe Preeclampsia | -- | -- | 3 (12) | 4 (25) | 0.41* |
Results from Fisher’s Exact test between two groups with history of Preeclampsia only
Significant p-value shows that within each pregnancy group, mean SBP was greater in women with current hypertension than without hypertension (P<0.02). Non-significant p-values indicate that none of the four groups differed on those characteristics.
Abbreviations: MRI: magnetic resonance imaging; WMH: white matter hyperintensity.
Results
The characteristics of the 4 groups of women in this study are listed in Table 1. At the time of MRI, women were similar in age, years past menopause, years of education, menopausal hormone therapy use, migraine, and diagnoses of dementia, hyperlipidemia, and diabetes. Of the 40 women with a history of preeclampsia, 24 had current hypertension (60%). Of the 40 women with a history of normotensive pregnancy, 8 (20%) had current hypertension. There were 7 women with a history of preeclampsia who had a “severe disease” as defined earlier. Three of these women had current hypertension and four did not have current hypertension.
Within each pregnancy group, mean SBP was greater in women with current hypertension than without hypertension (P<0.02; Table 1). However, mean SBP did not differ between women with current hypertension, regardless of a history of normotensive pregnancy or preeclampsia (P>0.1) nor did it differ between women with no current hypertension regardless of pregnancy group (P>0.99). There were 15 women who were being treated with diuretics for the treatment of current hypertension. Of the 8 women with a history of normotensive pregnancy and current hypertension, 3 (38%) were being treated with a diuretic. Of the 24 women with a history of hypertensive pregnancy with current hypertension, 12 (50%) were being treated with a diuretic.
Although all women had some WMH present on FLAIR MRI, the volume of WMH, adjusted for total intracranial volume and age, did not differ among the groups (p=0.51). Furthermore, cerebrovascular disease-associated lesions such as infarcts (p=0.66) and microbleeds (p>0.11) did not differ among groups.
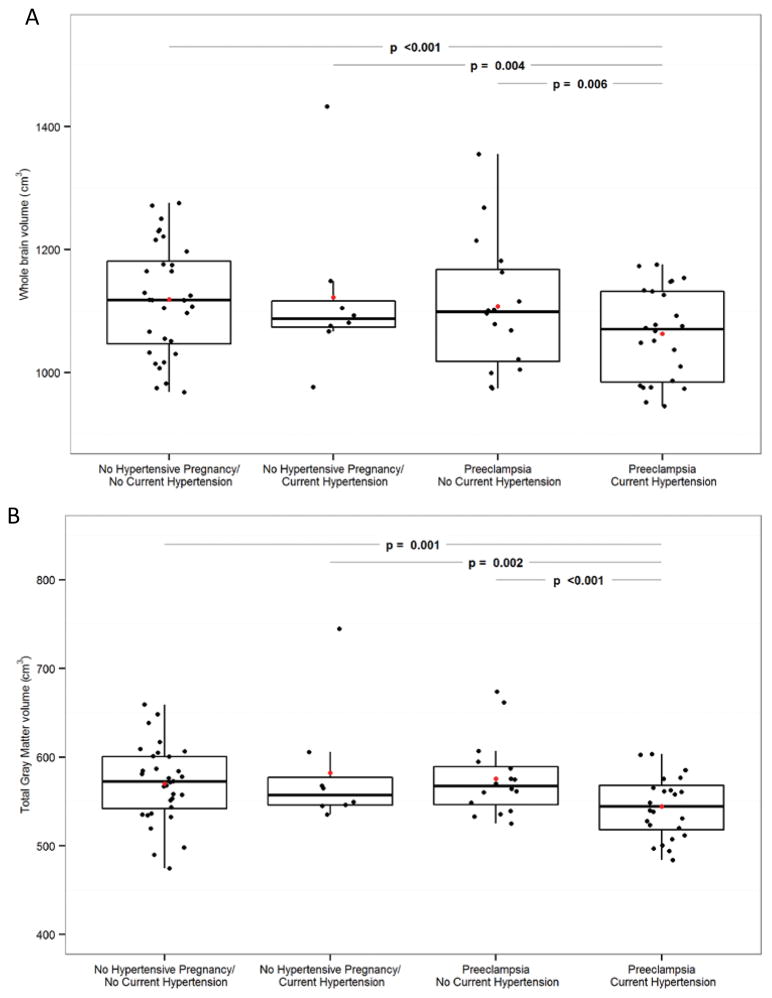
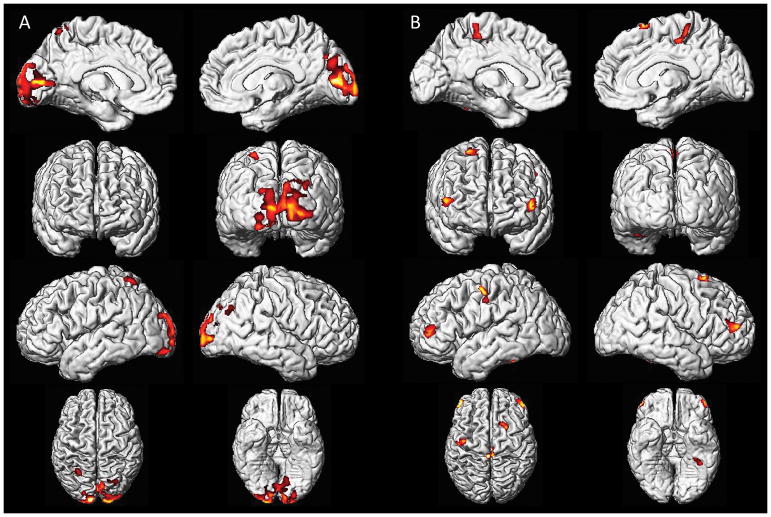
Total gray matter volumes were smaller in women with a history of preeclampsia and current hypertension compared to all other groups (Figure 1). After adjusting for age and log(TIV) the interaction between the a history of preeclampsia and current hypertension were associated with smaller gray matter volumes (p=0.04). Voxel-based morphometry identified smaller gray matter volumes in the occipital cortex, spanning much of Brodmann areas 17 and 18, and medial aspects of 19 in both hemispheres in women with a history of preeclampsia and current hypertension (Figure 2a) compared to women with a history of preeclampsia and no current hypertension. These Brodmann areas include the primary visual and visual association cortices. Furthermore, women with a history of preeclampsia and current hypertension had smaller gray matter volumes in the prefrontal and sensorimotor cortices in both hemispheres compared to women with a history of normotensive pregnancy and no current hypertension (Figure 2b).
Figure 1. Group differences in Gray Matter Volumes.
Boxplots of total gray matter volumes by groups (B). Red dots indicate mean for each group. Plots show age adjusted p-values.
Figure 2. Voxel-based analysis.
Voxel-based analysis comparing mean data from different study groups, assessed at a statistical threshold of p<0.001, shows (A) women with a history of preeclampsia and current hypertension had smaller gray matter volumes in the occipital cortex than women with a history of preeclampsia and no current hypertension and (B) women with a history of preeclampsia and current hypertension have smaller gray matter volumes than women with a history of normotensive pregnancy and no current hypertension in the prefrontal and sensorimotor cortices.
Discussion
This study investigated the impact of pregnancy history and late-life hypertension on structural changes in the brain decades after the pregnancy. Women who had a history of preeclampsia and later developed chronic hypertension had smaller gray matter volumes than women with a history of preeclampsia but did not later develop hypertension and women with a history of normotensive pregnancy with or without later-life hypertension after adjusting for age. This result is in agreement with previous studies reporting both smaller brain volumes in women with histories of hypertensive pregnancy disorders compared to women with histories of normotensive pregnancies[3] as well as data from the Women’s Health Initiative Memory Study reporting an association between late-life hypertension and smaller brain volumes in older women[13]. Therefore, the present study brings clarity to the risk of future brain changes following a hypertensive pregnancy by indicating contribution of late life hypertension in these women as they age. However, in both the present study and the previous study in women [3] with a history of hypertensive pregnancy, women did not differ in their MRI markers of cerebrovascular disease. This result could be due to the women’s relatively young age (i.e. mid-life) since the strongest risk factor for cerebrovascular disease is older age [10, 14–19], the current use of antihypertensive medication, or to exclusion of women with cardiovascular disease in this study. Alternatively, the relatively small sample sizes of the four groups might have limited our power to detect group differences in the presence of cerebrovascular disease lesions on MRI.
Voxel-based analysis showed that women with a history of preeclampsia and current hypertension had smaller gray matter volumes in the occipital lobe involving the primary visual and visual association cortices than women with a history of preeclampsia and no current hypertension (Figure 2a). The localization of these changes is similar to those identified from neuroimaging studies of women who experienced eclampsia and a clinical syndrome known as Posterior Reversible Encephalopathy Syndrome (PRES). PRES is characterized by neurologic symptoms, such as headache, altered consciousness, visual abnormalities, and seizures, together with vasogenic edema in posterior brain regions on neuroimaging [20]. However, none of the preeclemptic patients in our study had a diagnosis of PRES. In the present study, the increased atrophy was seen in the occipital lobe only in women with a history of preeclampia who went on to develop hypertension and was not seen in areas typically affected in patients with cardiovascular disease (prefrontal, sensorimotor cortices). This observation could indicate that all of the women who experienced preeclampsia have a similar burden of cardiovascular disease-related neuronal loss, but those who went on to develop hypertension later in life show increased neuronal loss in the posterior circulation. A previous study suggests that age combined with hypertension most severely affects the occipital and temporal lobes [21]. As women with a history of preeclampsia are at a fourfold increased risk for developing hypertension later in life [2], it might be expected that women with a history of preeclampsia who go on to develop hypertension would develop atrophy in the occipital gray matter as they age. Alternatively, the atrophy of the occipital gray matter might have occurred at an earlier age in the women with a history of preeclampsia and current hypertension. That is, the combined effect of preeclamptic pregnancy and hypertension may shift the aging processes. The small numbers of women with a history of normotensive pregnancy and hypertension later in life (n=8) limited our ability to investigate the segregated impact of pregnancy history alone on the late-life hypertension and gray matter loss. However, the higher percentage of women with hypertension later in life in the women with a history of preeclampsia (60%; n=24) compared to the women with a history of normotensive pregnancy (20%; n=8) is consistent with the literature.
Another limitation is that it was not possible to ascertain the age of onset of hypertension, and therefore length of hypertension duration in these women. Thus it was not possible to determine whether the length of hypertension had an impact on the gray matter volumes. A longitudinal study beginning from the time of the pregnancy to the onset of hypertension is needed to clarify the temporal relationships among disease processes. Also, it is possible that chronic use of diuretics could impact brain volumes, however the effects would are expected to be global. Additionally, evidence from prior literature indicates that diuretic use for treating hypertension does not appear to impact brain structure[22].
Atrophy of gray matter regions in the prefrontal and sensorimotor cortices characterize patients with vascular disease including hypertension [23–27]. Atrophy in these brain areas were found only between women with a history of preeclampsia and current hypertension compared to women with a history of normotensive pregnancy and no current hypertension (Figure 2B). We did not find any differences in these gray matter volumes between women based on their pregnancy history who did not have hypertension. That is, we saw no effect of history of preeclampsia alone, without the added effect of current hypertension in these brain regions. However, the small sample of women with a history of normotensive pregnancy and hypertension later in life may be the reason we were not able to find any regional patterns of atrophy in voxel-based analysis when women with a history of preeclampsia and current hypertension were compared to this group.
In conclusion, our findings indicates suggest that women who have pregnancy-associated hypertension are particularly susceptible to posterior brain injury. Injury in posterior brain regions is commonly associated with cardiovascular disease and is seen in women affected by PRES, thereby similar mechanisms may be contributing to these posterior brain changes in preeclamptic women who developed hypertension later in life. While our study cannot address the temporal sequence of chronic hypertension and brain atrophy after the preeclamptic pregnancy, the observed brain structural abnormalities among these women may indicate a more severe disease process during the preeclamptic pregnancy, which may have led to development of both hypertension and cortical atrophy later in life. In this case, late-life hypertension reflects more severe preeclampsia that led to volume loss in the gray matter. Alternatively, preeclampsia and hypertension synergistically may have contributed to the gray matter volume loss.
Table 2.
Imaging findings comparing the 4 groups of women. P-values were generated using Fisher’s Exact and Kruskal-Wallis ANOVA by Ranks Tests. None of the four groups differed on MRI findings.
| History of Normotensive Pregnancy (N = 40) | History of Preeclampsia (N = 40) | P-value | |||
|---|---|---|---|---|---|
|
| |||||
| Current Hypertension (N = 8) | No Current Hypertension (N = 32) | Current Hypertension (N = 24) | No Current Hypertension (N = 16) | ||
|
| |||||
| WMH (cm3), median (IQR) | 4.91 (2.81, 6.61) | 3.94 (3.24, 6.00) | 4.74 (2.79, 7.01) | 4.10 (2.09, 6.96) | 0.36* |
| TIV (l), median (IQR) | 1.34 (1.32, 1.38) | 1.35 (1.28, 1.45) | 1.33 (1.22, 1.41) | 1.35 (1.27, 1.44) | 0.73 |
| Cerebral Infarct, n (%) | 0 (0) | 4 (12) | 1 (4) | 1 (6) | 0.66 |
| Cerebral microhemorrhages, n (%) | 1 (12) | 0 (0) | 1 (4) | 2 (12) | 0.11 |
WMH analysis used generalize linear regression adjusting for the TIV
Abbreviations: MRI: magnetic resonance imaging; WMH: white matter hyperintensity; TIV: total intracranial volume
Acknowledgments
Study Funding
P50 AG044170, R01 AG034676, R01 AG040042, R01 AG011378.
Footnotes
Disclosures
Dr. Raman, Ms. Tosakulwong, Ms. Zuk, Dr. White, Dr. Fields, Mr. Lesnick, and Dr. Garovic report no disclosures.
Mr. Senjem reports stock/options equity in Inovio Pharmaceuticals Inc, Gilead Sciences, Inc., Celgene Corporation, Medtronic, Inc., and PAREXEL International Corporation, outside the submitted work.
Dr. Mielke served as a consultant for Lysosomal Therapeutics, Inc. She receives funding from the National Institutes of Health (R01 AG 049704 [PI], P30 MH075673 [PI of sub-contract], P50 AG16574/Project 1 [Co-I], U01 AG006786 [Co-I]), Department of Defense (W81XWH-15-1-0573-1 [co-PI]) and the Michael J. Fox Foundation [PI].
Dr. Bailey serves as a consultant for Novartis-- CANTOS study (canakinumab in prevention of CHD) Novartis-- Pioneer-HF (drug trial on heart failure). In both cases, he is the DSMB statistical member.
Dr. Jack serves as a consultant for Eli Lilly. He receives research funding from the National Institutes of Health (R01 AG011378, R01 AG041851, R01 AG037551, U01 AG032438, U01 AG024904), and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation Family.
Dr. Miller is funded by grants from the NIH P50 AG44170 and the Mayo Foundation.
Dr. Kantarci serves on the data safety monitoring board for Pfizer Inc. and Takeda Global Research & Development Center, Inc; and she is funded by the NIH (R01 AG040042 [PI], P50 AG44170/Project 2 [PI], P50 AG16574/Project 1 [Co-I], U19 AG10483[Co-I], U01 AG042791[Co-I]) and Minnesota Partnership for Biotechnology and Medical Genomics (PO03590201[Co-PI]).
References
- 1.Abalos E, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Zoet GA, et al. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas. 2015;82(2):153–61. doi: 10.1016/j.maturitas.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Mielke MM, et al. Impaired Cognition and Brain Atrophy Decades After Hypertensive Pregnancy Disorders. Circulation Cardiovascular quality and outcomes. 2016;9(2 Suppl 1):S70–6. doi: 10.1161/CIRCOUTCOMES.115.002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013;12(8):822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White WM, et al. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. American journal of obstetrics and gynecology. 2016 doi: 10.1016/j.ajog.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White WM, et al. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obstet Gynecol. 2016;214(4):519 e1–8. doi: 10.1016/j.ajog.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raz L, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80(10):911–8. doi: 10.1212/WNL.0b013e3182840c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Kantarci K, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72(17):1519–25. doi: 10.1212/WNL.0b013e3181a2e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarci K, et al. Focal hemosiderin deposits and beta-amyloid load in the ADNI cohort. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9(5 Suppl):S116–23. doi: 10.1016/j.jalz.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 12.Senjem ML, et al. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26(2):600–8. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resnick SM, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72(2):135–42. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernooij MW, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70(14):1208–14. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 15.Jeerakathil T, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke; a journal of cerebral circulation. 2004;35(8):1831–5. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 16.Poels MM, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke; a journal of cerebral circulation. 2010;41(10 Suppl):S103–6. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 17.Sveinbjornsdottir S, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. Journal of neurology, neurosurgery, and psychiatry. 2008;79(9):1002–6. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazekas F. Magnetic resonance signal abnormalities in asymptomatic individuals: their incidence and functional correlates. European neurology. 1989;29(3):164–8. doi: 10.1159/000116401. [DOI] [PubMed] [Google Scholar]
- 19.de Leeuw FE, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. Journal of neurology, neurosurgery, and psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinchey J, et al. A reversible posterior leukoencephalopathy syndrome. The New England journal of medicine. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 21.Strassburger TL, et al. Interactive effects of age and hypertension on volumes of brain structures. Stroke; a journal of cerebral circulation. 1997;28(7):1410–7. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- 22.Salerno JA, et al. Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension. 1992;20(3):340–8. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- 23.Skoog I, et al. A population-based study on blood pressure and brain atrophy in 85-year-olds. Hypertension. 1998;32(3):404–9. doi: 10.1161/01.hyp.32.3.404. [DOI] [PubMed] [Google Scholar]
- 24.Anazodo UC, et al. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. NeuroImage. Clinical. 2013;3:388–95. doi: 10.1016/j.nicl.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, et al. The pattern of brain gray matter impairments in patients with subcortical vascular dementia. Journal of the neurological sciences. 2014;341(1–2):110–8. doi: 10.1016/j.jns.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral neuroscience. 2003;117(6):1169–80. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 27.Raman MR, et al. Antemortem MRI findings associated with microinfarcts at autopsy. Neurology. 2014;82(22):1951–8. doi: 10.1212/WNL.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]