Abstract
Human organic anion transporter 3 (hOAT3) is localized at the basolateral membrane of renal proximal tubule cells and facilitates renal secretion of numerous clinical drugs, including anti-HIV therapeutics, anti-tumor drugs, antibiotics, antihypertension drugs, and anti-inflammatories. In the present study, we explored the role of serum and glucocorticoid-inducible kinase 1 (sgk1) in the regulation of hOAT3. We showed that over-expression of sgk1 in hOAT3-expressing cells stimulated hOAT3 transport activity by enhancing the transporter expression at the plasma membrane, kinetically reflected as an increased maximal transport velocity Vmax without substantial change in substrate-binding affinity Km. In contrast, treatment of cells with sgk-specific inhibitor GSK650394 resulted in a dose-dependent inhibition of hOAT3 transport activity. We further provided evidence that sgk1 regulation of hOAT3 activity was mediated by ubiquitin ligase Nedd4-2, an enzyme previously shown to have inhibitory effect on hOAT3. We showed that sgk1 phosphorylated Nedd4-2, weakened the association between Nedd4-2 and hOAT3, and decreased hOAT3 ubiquitination. Functionally, sgk1-stimulated hOAT3 transport activity was attenuated in the presence of a ligase-dead mutant of Nedd4-2. In summary, our investigation established for the first time that sgk1 stimulates hOAT3 transport activity by interfering with the inhibitory effect of Nedd4-2 on the transporter.
Keywords: Drug Transporter, Organic Anion Transporter, Regulation, Serum and Glucocorticoid-Inducible Kinase, Ubiquitin Ligase
Introduction
The organic anion transporter (OAT) family consists of over 10 membrane proteins. Organic anion transporter 3 (OAT3) is localized at the basolateral membrane of the renal proximal tubule cells and facilitates the renal secretion of numerous clinical drugs, including anti-HIV therapeutics, anti-tumor drugs, antibiotics, antihypertension drugs, and anti-inflammatories [1–6]. We previously established that members of OAT family are dynamic membrane transporters, constitutively internalizing from and recycling back to the plasma membrane. Alteration of the trafficking kinetics leads to a change in the amount of OAT at the plasma membrane and therefore leads to a change in OAT transport activity. For instance, activation of protein kinase C (PKC) accelerates the rate of OAT endocytosis/internalization without changing the rate of OAT recycling [7]. Consequently, OAT expression at the plasma membrane is reduced and OAT transport function is decreased.
Further studies from our lab demonstrated that PKC-stimulated OAT internalization requires the attachment of ubiquitin, a polypeptide of 76 amino acids, to OAT at the plasma membrane [8]. The ubiquitin attached onto the transporter can be recognized by the internalization machinery and therefore triggers the internalization step. Ubiquitination of OAT is a reaction, catalyzed by an E3 ubiquitin ligase Nedd4-2 [9, 10]. We showed that Nedd4-2 inhibits OAT transport activity by enhancing OAT ubiquitination, and internalization, and therefore decreasing OAT expression at the plasma membrane. Thus, Nedd4-2 is a significant regulator for OAT ubiquitination, expression, and transport activity.
The serum- and glucocorticoid-inducible kinases (sgk) are a family of three serine/threonine kinase isoforms sgk1, sgk2 and sgk3. These kinases regulate various cellular processes such as sodium Na+ balance, osmoregulation, cell survival and proliferation [11–17]. Their physiological roles in oncology, diabetes, and obesity have also been discovered [18]. Interestingly, there are distinct properties among sgk1, sgk2 and sgk3. Sgk1 and sgk3 are expressed ubiquitously, whereas sgk2 is limited to tissues such as liver, kidney, pancreas, and brain. The expression of sgk1 and sgk3 in the kidney was regulated by aldosterone. In contrast, sgk2 is insensitive to such regulation. Our lab recently demonstrated that sgk2 plays a significant role in the regulation of OATs [19, 20]. However, the role of sgk1 in OAT function has not been explored. In the current study, we established the critical role of the sgk1/Nedd4-2 signaling pathway in the transport function of hOAT3.
Materials and Methods
Materials
COS-7 cells were purchased from ATCC (Manassas, VA). [3H]-labeled estrone sulfate was purchased from PerkinElmer (Waltham, MA). cDNA for human Nedd4-2 was obtained as a generous gift from Dr. Peter M. Snyder of the College of Medicine, University of Iowa (Iowa City, IA). Nedd4-2 ligase-dead mutant Nedd4-2/C821A was generated in our lab by site-directed mutagenesis as described previously (9, 10). cDNAs for mouse Flag-tagged sgk1s (wild-type sgk1, constitutively-active sgk1/S422D and kinase-dead sgk/K127M) were generously provided by Dr. Alan C. Pao from Department of Medicine, Stanford University, (Stanford, CA). Brefeldin A and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and Transient transfection
Parental COS-7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Corning, Corning, NY) supplemented with 10% fetal bovine serum (GIBCO, Grand Island, NY) at 37 °C in 5% CO2. Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used for transfection of cDNAs following the manufacturer’s instructions. 48 hours after transfection, cells were used for further experiments. For some experiments, cells were starved with serum-free medium for 3–6 hours before experiments. Cells stably expressing hOAT3 were maintained in DMEM medium supplemented with 0.2mg/ml G418 (Invitrogen, Carlsbad, CA), 10% fetal bovine serum.
Transport Measurements
Transport activity of hOAT3 was determined by measuring [3H]-labeled estrone sulfate (ES) uptake into hOAT3-expressing cells. The uptake solution consists of phosphate-buffered saline PBS with 1mM CaCl2 and 1mM MgCl2 (PBS/CM) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, 0.1 mM CaCl2, and 1 mM MgCl2, pH 7.3) and [3H] estrone sulfate (300 nM). At the time points indicated, uptake was terminated by removing the uptake solution followed by washing with ice-cold PBS twice. The cells were then lysed in 0.2 N NaOH, neutralized with 0.2 N HCl, and transferred into scintillation vials for liquid scintillation counting.
Cell Surface Biotinylation
Expression level of hOAT3 at the cell surface was examined using biotinylation strategy. Cells in monolayer culture were washed with ice-cold PBS and then incubated with 1 ml of NHS-SS-biotin (0.5 mg/ml in PBS/CM) on ice for two consecutive 20 min under gentle shaking. Biotinylation was stopped by rinsing with 100 mM glycine in PBS/CM. Afterwards, cell extracts were prepared in lysis buffer (10 mM Tris/HCl, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100 with 1/100 protease inhibitor cocktail and 20 mM N-ethylmaleimide) for 30min at 4 °C and cleared by centrifugation at 16,000g at 4 °C. The supernatant was mixed with streptavidin-agarose beads (Pierce, Rockford, IL) to isolate cell surface proteins. Membrane-expressed hOAT3 was detected by SDS-PAGE and immunoblotting with an anti-myc antibody (epitope myc was tagged to hOAT3).
Immunoprecipitation
Detailed procedure has been introduced previously [19, 20]. Cells were lysed with lysis buffer and then precleared with protein G-agarose beads (Pierce, Rockford, IL) at 4 °C for 2 hrs. to reduce nonspecific binding. At the meantime, anti-myc antibody (1:100) was incubated with protein G-agarose beads at 4 °C for 2 hrs. The precleared protein sample was then mixed with antibody-bound protein G-agarose beads at 4 °C overnight. On day 2, after washing with lysis buffer, immunoprecipitated proteins were eluted and immunoblotted with indicated antibodies.
Electrophoresis and Immunoblotting
Protein samples were separated on 7.5% SDS-PAGE minigels (Bio-Rad, Hercules, CA) and electroblotted on to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). The blots were blocked with 5% nonfat dry milk for 1–2 hrs. in PBS-Tween 20 (PBST; 0.05% Tween-20 in PBS) at room temperature, washed, and incubated overnight at 4 °C with appropriate primary antibodies. Primary antibodies included rabbit anti-Nedd4-2, mouse anti-E cadherin (Abcam, Cambridge, MA), rabbit anti-P-Nedd4-2 (Cell Signaling, Danvers, MA), mouse anti-myc (Roche, Indianapolis, IN), mouse anti-β-actin, mouse anti-ubiquitin (Santa Cruz, Santa Cruz, CA). The blots were then incubated with horseradish peroxidase-conjugated secondary antibodies, followed by detection with SuperSignal West Dura Extended Duration Substrate kit (Pierce, Rockford, IL). FluorChem 8000 imaging system (Alpha Innotech Corp., San Leandro, CA) was applied to quantify nonsaturating, immunoreactive protein bands.
Data Analysis
Each experiment was repeated for at least three times. Student’s paired t tests were used to perform statistical analysis. A p value of <0.05 was considered significant.
Results
Sgk1 stimulates hOAT3 transport activity
The role of sgk1 in hOAT3 function was explored. COS-7 cells-expressing hOAT3 were transfected with wild type sgk1 (WT-sgk1), constitutive active form of sgk1 (CA-sgk1), or inactive sgk1 (IN-sgk1), followed by the measurement of hOAT3-mediated uptake of [3H] estrone sulfate. As shown in Fig. 1, WT-sgk1 enhanced the uptake by ~ 40% as compared to that in control cells (hOAT3-expressing cells without WT-sgk1), and CA-sgk1 further amplified the effect of WT-sgk1, whereas the inactive sgk1 (IN-sgk1) resulted in a slight decrease in the uptake, possibly through suppressing the activity of endogenous sgk1.
Fig. 1.
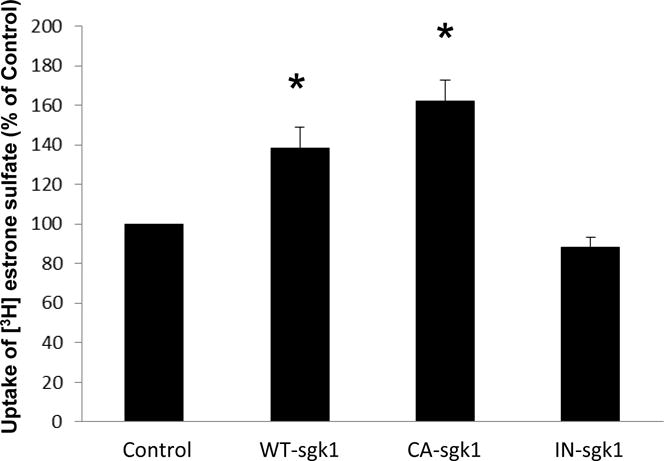
Sgk1 stimulates hOAT3 transport activity. COS-7 cells were co-transfected with hOAT3 and control vector, with hOAT3 and wild type sgk1 (WT-sgk1), with hOAT3 and constitutive active form of sgk1 (CA-sgk1), or with hOAT3 and inactive sgk1 (IN-sgk1). 3-min uptake of [3H]-estrone sulfate (0.3 μM) was then determined. The data represented uptake into hOAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Uptake activity was expressed as a percentage of the uptake measured in control cells. Values are mean ± S.E. (n = 3).
In a separate experiment, we treated hOAT3-expressing cells with a sgk-selective inhibitor GSK650394, followed by the measurement of hOAT3-mediated uptake of [3H] estrone sulfate. GSK650394 induced a concentration-dependent inhibition with 40% and 85% inhibition at concentrations of 100 nM and 1 μM separately (Fig. 2).
Fig. 2.
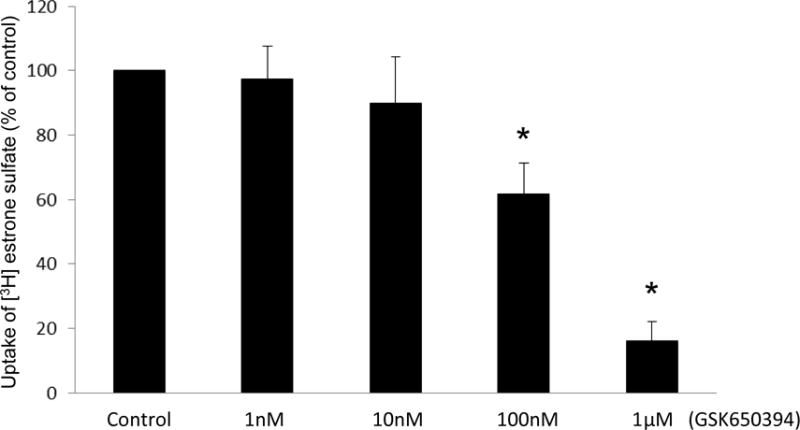
Sgk-specific inhibitor decreases hOAT3 transport activity. COS-7 cells were co-transfected with hOAT3 and CA-sgk1. Transfected cells were incubated with sgk-specific inhibitor GSK650394 for 30 min at indicated concentrations. 3-min uptake of [3H]-estrone sulfate (0.3 μM) was then determined. The data represented uptake into hOAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Uptake activity was expressed as a percentage of the uptake measured in control cells. Values are mean ± S.E. (n = 3).
The influence of sgk1 on the kinetic characteristics of hOAT3-mediated [3H] estrone sulfate uptake was then determined at different substrate concentrations. An Eadie-Hofstee analysis (Fig. 3) showed that there was an increase in the maximal transport velocity Vmax of hOAT3 in CA-sgk1-transfected cells as compared to that in control cells (154.0 ± 13.0 pmol·mg−1·3min−1 with control cells and 219.6 ± 9.7 pmol·mg−1·3min−1 with cells transfected with CA-sgk1), whereas there was no significant difference in the substrate-binding affinity Km of the transporter in both control and CA-sgk1-transfected cells (5.46 ± 0.32 μM with control cells and 5.94 ± 0.19 μM with cells transfected with CA-sgk1).
Fig. 3.
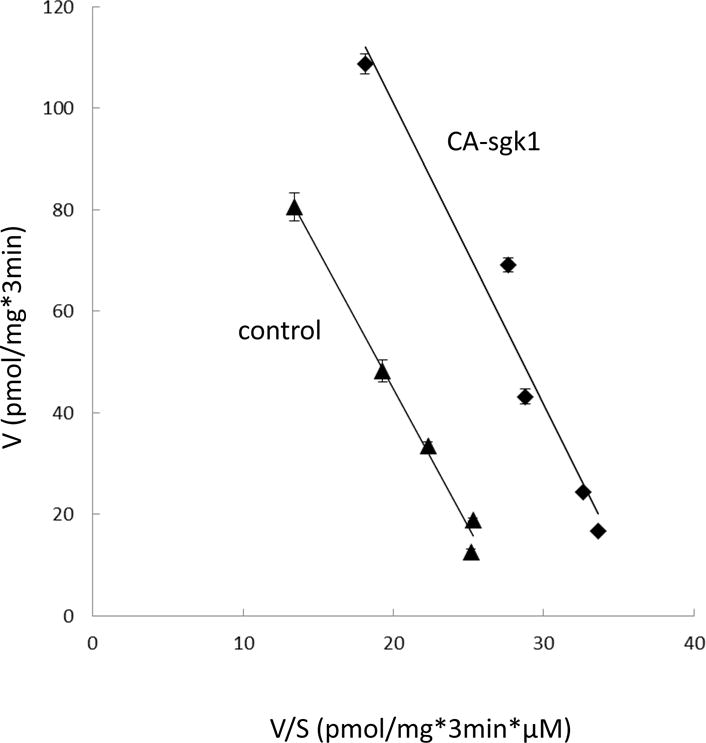
Effect of sgk1 on the kinetics of hOAT3-mediated estrone sulfate transport. COS-7 cells were co-transfected with hOAT3 and control vector or with hOAT3 and the constitutive active form of sgk1 (CA-sgk1). 3-min uptake of [3H] estrone sulfate was then determined at the concentrations of 0.1–10 μM. The data represented uptake into hOAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are means ± S.E. (n = 3). V, velocity; S, substrate concentration.
Sgk1 enhances hOAT3 Expression
Sgk1-induced increase in the maximal transport velocity Vmax of hOAT3 shown above (Fig. 3) could either reflect an increased number of the transporter at the plasma membrane or an increased transporter turnover rate. To differentiate between these possibilities, we examined the transporter expression both at the plasma membrane and in total cell lysates. Our results revealed that hOAT3 expressions at the cell surface (Fig. 4a, top panel) and in total cell lysate (Fig. 4c, top panel) were both increased in cells transfected with CA-sgk1 as compared to that in control cells. The increase in hOAT3 expression was specific as there was no significant changes in the expression of cell surface protein marker E-cadherin (Fig. 4a, bottom panel) and cellular protein marker β-actin (Fig. 4c, bottom panel).
Fig. 4.
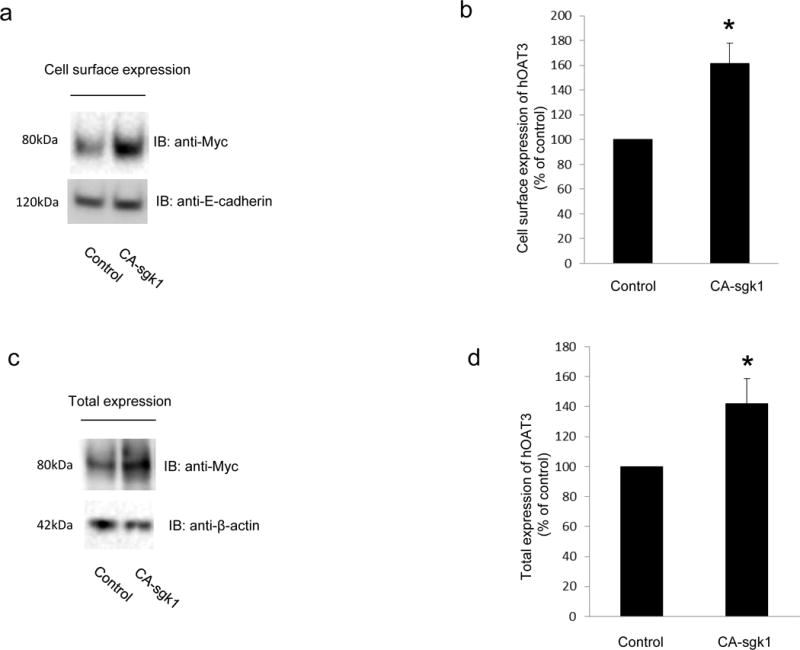
Sgk1 enhances hOAT3 expression. a. Top panel: hOAT3 expression at the cell surface. COS-7 cells were co-transfected with hOAT3 and control vector or with hOAT3 and the constitutive active form of sgk1 (CA-sgk1). Cell surface proteins were labeled with biotin, separated with streptavidin beads, followed by immunoblotting (IB) with an anti-myc antibody (epitope myc was tagged to hOAT3 for immune-detection). Bottom panel: The expression of cell surface protein marker E-cadherin. The same blot from the Top panel was re-probed with anti- E-cadherin antibody. b. Densitometry plot of results from Fig. 4a, Top panel as well as from other experiments. c. Top Panel: Total cell expression of hOAT3. COS-7 cells were co-transfected with hOAT3 and control vector or with hOAT3 and CA-sgk1. Transfected cells were lysed, followed by immunoblotting (IB) with an anti-myc antibody. Bottom panel: Total cell expression of cellular protein marker β-actin. The same blot from Top panel was re-probed with anti-β-actin antibody. (d). Densitometry plot of results from Fig. 4c, Top panel as well as from other experiments. The values are mean ± S.E. (n = 3).
Sgk1 stabilizes hOAT3
An increased expression of hOAT3 at the cell surface observed above (Fig. 4a, top panel) may result from an enhanced insertion of the transporter into the plasma membrane or a decreased removal of the transporter from the plasma membrane. We used a reagent brefeldin A (BFA) to distinguish between these two mechanisms. BFA inhibits secretory mechanisms by blocking the transition of the transporters from endoplasmic reticulum (ER) to Golgi, and preventing formation of vesicles at the Golgi apparatus. As a result, insertion of the transporters into the plasma membrane is inhibited [21]. As shown in Fig. 5a, top panel, after a 6-hr treatment with BFA, the decrease of hOAT3 expression at the plasma membrane was less dramatic in cells transfected with CA-sgk1 (65%) as compared to that in control cells (80%). We also examined the effect of BFA on the total expression of hOAT3 (Fig. 5c, top panel). Under BFA treatment, hOAT3 displayed two bands at molecular sizes of 60 kDa and 80 kDa. Our lab previously demonstrated [22, 23] that OAT undergoes a maturation process when transiting from ER to Golgi. The ER form is a non-glycosylated form of 60 kDa, which matures in Golgi to a glycosylated form of 80 kDa. Only the glycosylated form (80 kDa) can target to plasma membrane. BFA blocked the transition of hOAT3 from ER to Golgi, which led to the appearance of a 60 kDa band after BFA treatment. Therefore, the total expression of hOAT3 is the combination of the band at 60 kDa and the band at 80 kDa. It is important to note that even when we combined the two bands (60 kDa + 80 kDa) together, the intensity was still slightly lower than that without BFA treatment. The possible explanation is that BFA blocked the insertion of hOAT3 into the plasma membrane but did not blocked hOAT3 degradation. After 6-hour period, part of hOAT3 was removed from the plasma membrane and was degraded. The degradation in the presence of sgk1 is slower than that without sgk1. Similarly, the decrease of hOAT3 transport activity was ~61% in cells transfected with CA-sgk1 as compared to a more significant decline of ~74% in control cells (Fig. 5d). These data suggest that sgk1 plays a significant role in stabilizing hOAT3 both at the plasma membrane and in total expression.
Fig. 5.
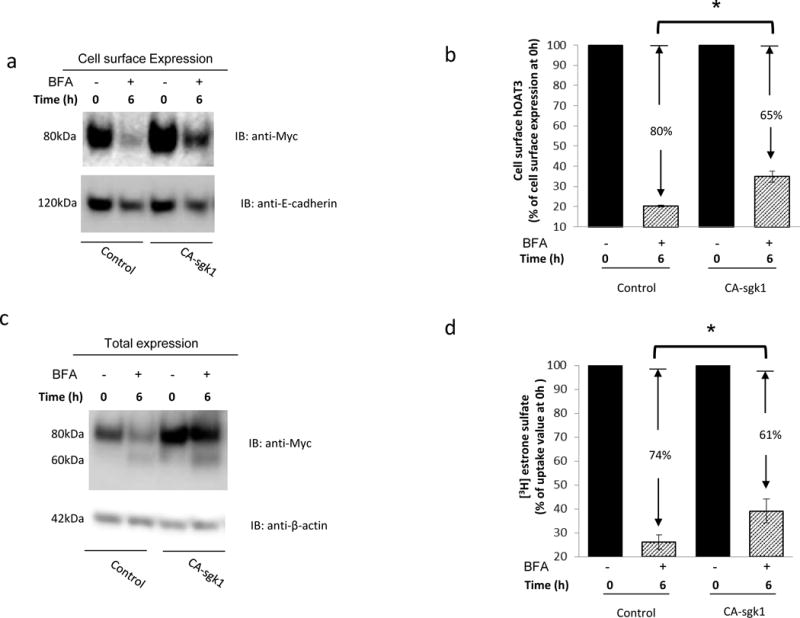
Sgk1 stabilizes hOAT3. a. Top panel: hOAT3 expression at the cell surface. COS-7 cells were co-transfected with hOAT3 and control vector or with hOAT3 and constitutive active form of sgk1 (CA-sgk1). The transfected cells were treated with brefeldin A (BFA, 0.1 μg/ml) for 6 hrs., followed by the determination of hOAT3 expression at the cell surface through a biotinylation approach and immunoblotting with anti-myc antibody (epitope myc was tagged to hOAT3 for immune-detection). Bottom panel: The expression of cell surface marker protein E-cadherin. The same blot from Fig. 5a, Top panel was re-probed with anti- E-cadherin antibody. b. Densitometry plot of results from Fig. 5a, Top panel as well as from other experiments. The amount of cell surface hOAT3 was expressed as % of total initial cell surface hOAT3 pool (at 0 hr. of BFA treatment). Values are mean ± S.E. (n = 3). c. Top Panel: Total cell expression of hOAT3. COS-7 cells were co-transfected with hOAT3 and control vector or with hOAT3 and constitutive active form of sgk1 (CA-sgk1). The transfected cells were treated with brefeldin A (BFA, 0.1 μg/ml) for 6 hrs. Treated cells were lysed, followed by immunoblotting (IB) with an anti-myc antibody. Bottom panel: Total cell expression of β-actin, a cellular protein marker. The same blot from Fig. 5c, Top panel was re-probed with anti-β-actin antibody. d. COS-7 cells were co-transfected with hOAT3 and control vector, or with hOAT3 and CA-sgk1. The transfected cells were treated with brefeldin A (BFA, 0.1 μg/ml) for 6 hrs. [3H] estrone sulfate uptake (3min, 0.3 μM) was then measured. Uptake activity was expressed as % of the uptake value measured at 0 hr. of BFA treatment.
Sgk1 phosphorylates Nedd4-2 in COS-7 cells
Previous investigations [24] in other systems reported that sgk1 regulates its substrates, at least in part, by phosphorylating Nedd4-2 at serine residue 327 (Ser 327). In the current experiment, we examined whether sgk1 also phosphorylated Nedd4-2 in our system. COS-7 cells were co-transfected with Nedd4-2 and control vector or with Nedd4-2 and CA-sgk1. Phospho-Nedd4-2 was examined by immunoblotting with phosphor-Nedd4-2 (Ser327)-specific antibody. As shown in Fig. 6a, the phospho-Nedd4-2-specific antibody recognized a band around the size of Nedd4-2 in control cells, indicating that Nedd4-2 was indeed phosphorylated at Ser327. The phosphorylation signal was increased by 37% in cells transfected with CA-sgk1. The total expression of Nedd4-2 was not affected by CA-sgk1 (Fig. 6c). Therefore, sgk1 enhanced Nedd4-2 phosphorylation in COS-7 cells.
Fig. 6.
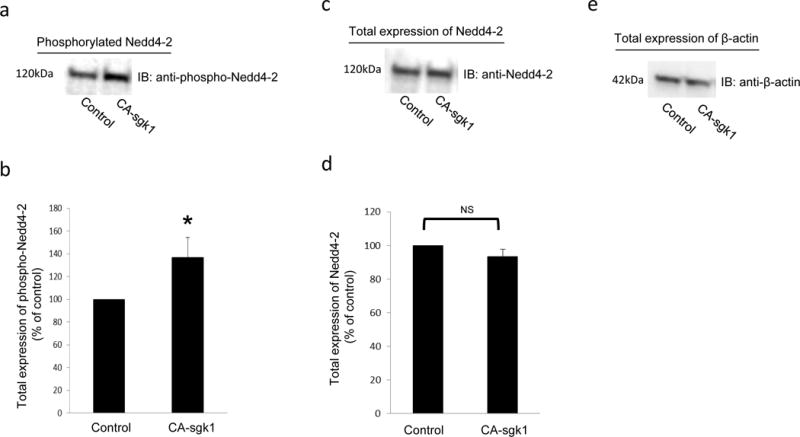
Sgk1 phosphorylates Nedd4-2. a. COS-7 cells were transfected with Nedd4-2 and control vector or with Nedd4-2 and CA-sgk1. Transfected cells were lysed and immunoblotted with anti-phospho-Nedd4-2(Ser327)-specific antibody. b. Densitometry plot of results from Fig. 6a, as well as from other experiments. The values are mean ± S.E. (n = 3). c. The same blot from Fig. 6a was re-probed with anti-Nedd4-2. d. Densitometry plot of results from Fig. 6c as well as from other experiments. The values are mean ± S.E. (n = 3). e. The same blot from Fig. 6a, was re-probed with anti-β actin antibody. β actin is a cellular protein marker.
Sgk1 interferes with the interaction between Nedd4-2 and hOAT3
Our lab recently demonstrated [9, 10] that Nedd4-2, a ubiquitin ligase, binds to OAT and inhibits OAT transport activity by promoting the conjugation of ubiquitin to OAT, which leads to OAT internalization from the plasma membrane and subsequent degradation ([9, 10] and our unpublished results). In this experiment, we examined the relationship among sgk1, Nedd4-2 and hOAT3 using a co-immunoprecipitation strategy. As shown in Fig. 7a, top panel, significant amount of Nedd4-2 was pulled down in hOAT3 immunoprecipitates (lane 1) in cells transfected with Nedd4-2, indicating a direct association between these two proteins. However, in cells transfected with Nedd4-2 and sgk1, much less amount of Nedd4-2 was detected in hOAT3 immunoprecipitates (lane 2). The difference in the amount of Nedd4-2 detected in hOAT3 immunoprecipitates was not due to the difference in the amount of hOAT3 immunoprecipitated as similar amount of hOAT3 was pulled down in all samples (Fig. 7a, bottom panel). These results suggest that sgk1 interfered with the association between hOAT3 and Nedd4-2.
Fig. 7.
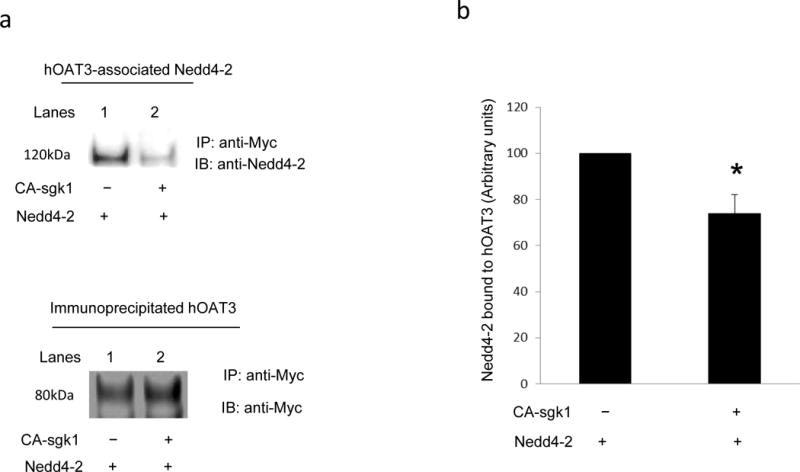
Sgk1 weakens the interaction between Nedd4-2 and hOAT3. a. Top panel: hOAT3-expressing COS-7 cells were transfected with Nedd4-2 and control vector or with Nedd4-2 and CA-sgk1. Transfected cells were lysed and hOAT3 was immunoprecipitated by anti-myc antibody (epitope myc was tagged to hOAT3), followed by immunoblotting with anti-Nedd4-2 antibody. Bottom panel: The same immunoblot from Fig. 7a, Top panel was reprobed by anti-myc antibody. b. Densitometry plot of results from Fig. 7a, Top panel as well as from other experiments. The values are mean ± S.E. (n = 3).
Sgk1 inhibits hOAT3 ubiquitination
The effect of sgk1 on hOAT3 ubiquitination was then examined (Fig 8). In cells transfected with Nedd4-2 alone, hOAT3 was heavily ubiquitinated. However, the ubiquitination of hOAT3 was significantly decreased in cells transfected with both Nedd4-2 and sgk1 (Fig. 8a, top panel). The difference in the amount of ubiquitinated OAT3 was not due to the difference in the amount of OAT3 immunoprecipitated as similar amount of OAT3 was pulled down in all samples (Fig. 8a, bottom panel). These results suggest that sgk1 inhibits Nedd4-2-mediated OAT3 ubiquitination.
Fig. 8.
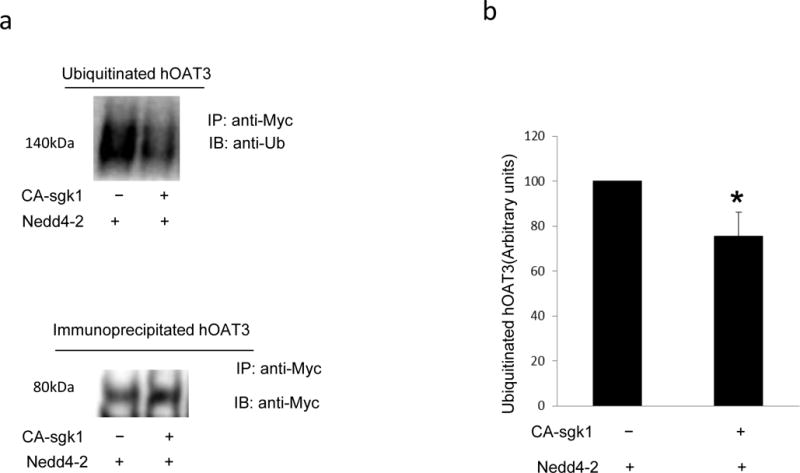
The effect of Sgk1 on OAT3 ubiquitination. a. Top panel: COS-7 cells were transfected with hOAT3, Nedd4-2 and control vector or with hOAT3, Nedd4-2 and CA-sgk1. Transfected cells were treated with the PKC activator PMA (1 μM) for 30 min to enhance hOAT3 ubiquitination. Treated cells were then lysed and hOAT3 was immunoprecipitated by anti-myc antibody (epitope myc was tagged to hOAT3), followed by immunoblotting with anti-ubiquitin antibody. Bottom panel: The same immunoblot from Fig. 8a, Top panel was reprobed by anti-myc antibody. b. Densitometry plot of results from Fig. 8a, top panel as well as from other experiments. The values are mean ± S.E. (n = 3).
Nedd4-2 ligase-dead mutant attenuates sgk1-induced stimulation of hOAT3 transport activity
Our result obtained above demonstrated that sgk1 phosphorylated hOAT3 (Fig. 6), interfered with the binding of Nedd4-2 to hOAT3 (Fig. 7), and inhibited hOAT3 ubiquitination (Fig. 8). The functional consequence due to such action was then assessed. hOAT3-expressing COS-7 cells were transfected with CA-sgk1 and control vector or with CA-sgk1 and a ligase-dead mutant of Nedd4-2 (Nedd4-2/C821A). This mutant is incapable of ubiquitinating OATs. As shown in Fig. 9, sgk1 stimulated hOAT3 transport activity by 38% in control cells, presumably through interfering with the inhibitory effect of the endogenous Nedd4-2 on hOAT3. However, in cells transfected with a ligase-dead mutant of Nedd4-2 (Nedd4-2/C821A), the stimulatory effect of sgk1 on hOAT3 was attenuated.
Fig. 9.
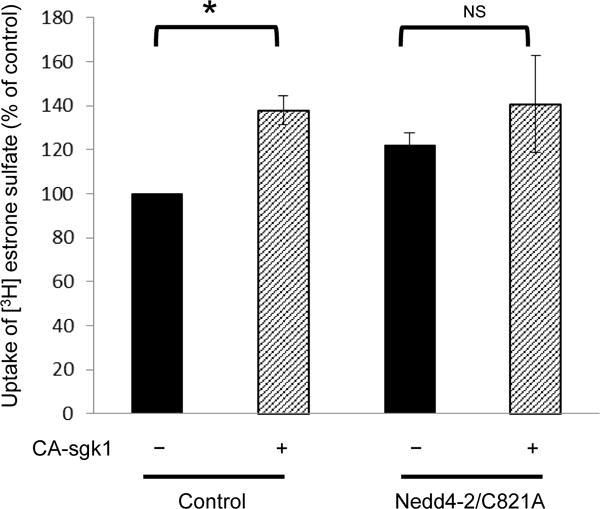
Nedd4-2 ligase-dead mutant attenuates the stimulatory effect of CA-sgk1 on hOAT3 transport activity. hOAT3-expressing COS-7 cells were transfected with sgk1 and control vector or with sgk1 and a ligase-dead mutant of Nedd4-2 (Nedd4-2/C821A). 3-min uptake of [3H]-estrone sulfate (0.3 μM) was then determined. Uptake activity was expressed as a percentage of the uptake measured in cells without the transfection of sgk1. Values are mean ± S.E. (n = 3).
Discussion
Organic anion transporters (OATs) are major players in the efficacy and toxicity of therapeutic agents. Therefore, uncovering the molecular and cellular mechanisms for OAT regulation is clinically and pharmacologically significant. The current investigation revealed that sgk1/Nedd4-2 signaling pathway plays a significant role in the regulation of hOAT3 function.
To carry out our investigation, we chose monkey kidney COS-7 cells, a model cell system that has been widely used for mechanistic studies of many kidney transport processes. Importantly, the characteristic of OAT trafficking in COS-7 cells [25] is similar to that in rat kidney slices [26], where OATs are endogenously expressed. Therefore, our studies in these cells will pave the path for the future exploration in assessing whether similar mechanisms are working in vivo.
From our investigation, we obtained several pieces of important information. First, sgk1 has a significant regulatory role in hOAT3 function. Overexpression of sgk1 resulted in a stimulation of hOAT3-mediated estrone sulfate uptake (Fig. 1). Treatment of the cells with a sgk-specific inhibitor has an opposite effect (Fig. 2), although we cannot exclude the possibility that the effect of GSK650394 may result from the inhibition of sgk1 as well as other Sgk1-related kinases. The stimulation of hOAT3 activity by sgk1 was due to an enhanced maximum transport velocity (Vmax) without changing the binding affinity (Km) of the transporter for its substrates, as revealed by our kinetic analysis (Fig. 3). The enhanced Vmax correlated with an increased amount of the transporter at the plasma membrane (Fig. 4), which resulted, at least in part, from the role of sgk1 in stabilizing hOAT3 at the cell surface (Fig. 5).
Secondly, sgk1 regulates hOAT3 function via ubiquitin ligase Nedd4-2. Sgk kinases were previously shown to modulate their targets by interacting with cytosolic components, one of which is the ubiquitin ligase Nedd4-2. Nedd4-2 is a key regulator of many membrane proteins such as sodium channel ENaC, potassium channel hERG, and the amino acid transporter EAAT2 [27–29]. Our lab recently demonstrated [9, 10] that Nedd4-2 directly binds to OATs and catalyzes the attachment of ubiquitin molecules to these transporters, which leads to the endocytosis/internalization of OATs from plasma membrane to intracellular endosomes and subsequent degradation. Consequently, the amount of OATs at the plasma membrane is reduced and their transport activity is decreased ([9, 10] and our unpublished results). In exploring the relationship among sgk1, Nedd4-2 and hOAT3, we found that sgk1 phosphorylated Nedd4-2 (Fig. 6), weakened the interaction between Nedd4-2 and hOAT3 (Fig. 7), and decreased hOAT3 ubiquitination (Fig. 8). The sgk1-induced phosphorylation of Nedd4-2 may cause a conformational change of the ubiquitin ligase, hinder its binding to hOAT3 and attenuate its inhibition of hOAT3 transport activity. Therefore, the stimulatory effect of sgk1 on hOAT3 transport activity is due, in part, to the ability of sgk1 to interfere with the inhibitory effect of Nedd4-2 on hOAT3.
OATs are under the control of several protein kinases including protein kinase C [30] and sgk1 as demonstrated in our current study. These protein kinases exert their roles through phosphorylating their substrates. Our lab previously demonstrated that PKC regulates OAT activity by altering the trafficking kinetics of OATs without direct phosphorylation of the transporter itself [7, 30]. It is possible that PKC regulates OAT by phosphorylating an OAT-interacting partner. In our current study, we showed that sgk1 phosphorylated Nedd-4-2 (Fig. 6). Whether sgk1 directly phosphorylates hOAT3 is an interesting topic for future investigation.
In summary, our current study demonstrated that hOAT3 activity is regulated through sgk1/Nedd4-2 signaling pathway. Sgk1 stimulated hOAT3 transport activity by interfering with the inhibitory effect of Nedd4-2 on the transporter. Sgk is the downstream signaling molecule for many physiological hormones such as glucocorticoids and mineralocorticoids [31, 32]. Therefore, our current study provides important insights into how hOAT3-mediated drug elimination is regulated in vivo.
Acknowledgments
This work was supported by grant (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123).
Footnotes
Contributor Information
Haoxun Wang, Rutgers The State University of New Jersey, Piscataway, New Jersey, United States.
Guofeng You, Rutgers The State University of New Jersey, 160 Frelinghuysen Road, Piscataway, New Jersey, United States, 08854.
References
- 1.You G. Structure, function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22(6):602–16. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 2.Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38(7–8):889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618(2):185–93. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 4.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31(1):1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76(3):481–90. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terada T, Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol. 2007;73(3):440–9. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, et al. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem. 2008;283(47):32570–9. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, et al. Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis. Mol Pharmacol. 2013;83(1):217–24. doi: 10.1124/mol.112.082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu D, Wang H, You G. An Essential Role of Nedd4-2 in the Ubiquitination, Expression, and Function of Organic Anion Transporter-3. Mol Pharm. 2016;13(2):621–30. doi: 10.1021/acs.molpharmaceut.5b00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D, et al. Nedd4-2 but not Nedd4-1 is critical for protein kinase C-regulated ubiquitination, expression, and transport activity of human organic anion transporter 1. Am J Physiol Renal Physiol. 2016;310(9):F821–31. doi: 10.1152/ajprenal.00522.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SY, et al. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A. 1999;96(5):2514–9. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naray-Fejes-Toth A, et al. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial na+ channels. J Biol Chem. 1999;274(24):16973–8. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 13.Rozansky DJ, et al. Hypotonic induction of SGK1 and Na+ transport in A6 cells. Am J Physiol Renal Physiol. 2002;283(1):F105–13. doi: 10.1152/ajprenal.00176.2001. [DOI] [PubMed] [Google Scholar]
- 14.Waldegger S, et al. Cloning of sgk serine-threonine protein kinase from shark rectal gland - a gene induced by hypertonicity and secretagogues. Pflugers Arch. 1998;436(4):575–80. doi: 10.1007/s004240050674. [DOI] [PubMed] [Google Scholar]
- 15.Leong ML, et al. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278(8):5871–82. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 16.Buse P, et al. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. J Biol Chem. 1999;274(11):7253–63. doi: 10.1074/jbc.274.11.7253. [DOI] [PubMed] [Google Scholar]
- 17.Pao AC, et al. Expression and role of serum and glucocorticoid-regulated kinase 2 in the regulation of Na+/H+ exchanger 3 in the mammalian kidney. Am J Physiol Renal Physiol. 2010;299(6):F1496–506. doi: 10.1152/ajprenal.00075.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessier M, Woodgett JR. Serum and glucocorticoid-regulated protein kinases: variations on a theme. J Cell Biochem. 2006;98(6):1391–407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, et al. Serum- and glucocorticoid-inducible kinase SGK2 regulates human organic anion transporters 4 via ubiquitin ligase Nedd4-2. Biochem Pharmacol. 2016;102:120–9. doi: 10.1016/j.bcp.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, et al. Serum- and glucocorticoid-inducible kinase sgk2 stimulates the transport activity of human organic anion transporters 1 by enhancing the stability of the transporter. Int J Biochem Mol Biol. 2016;7(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Klaus F, et al. Up-regulation of hypertonicity-activated myo-inositol transporter SMIT1 by the cell volume-sensitive protein kinase SGK1. J Physiol. 2008;586(6):1539–47. doi: 10.1113/jphysiol.2007.146191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, et al. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. 2005;67(3):868–76. doi: 10.1124/mol.104.007583. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, et al. Role of glycosylation in the organic anion transporter OAT1. J Biol Chem. 2004;279(15):14961–6. doi: 10.1074/jbc.M400197200. [DOI] [PubMed] [Google Scholar]
- 24.Snyder PM, et al. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279(44):45753–8. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, et al. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. Int J Biochem Mol Biol. 2012;3(2):242–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Phatchawan A, et al. Decreased renal organic anion transporter 3 expression in type 1 diabetic rats. Am J Med Sci. 2014;347(3):221–7. doi: 10.1097/MAJ.0b013e3182831740. [DOI] [PubMed] [Google Scholar]
- 27.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277(1):5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 28.Lamothe SM, Zhang S. The serum- and glucocorticoid-inducible kinases SGK1 and SGK3 regulate hERG channel expression via ubiquitin ligase Nedd4-2 and GTPase Rab11. J Biol Chem. 2013;288(21):15075–84. doi: 10.1074/jbc.M113.453670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehmer C, et al. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J Neurochem. 2006;97(4):911–21. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- 30.You G, et al. Regulation of mOAT-mediated organic anion transport by okadaic acid and protein kinase C in LLC-PK(1) cells. J Biol Chem. 2000;275(14):10278–84. doi: 10.1074/jbc.275.14.10278. [DOI] [PubMed] [Google Scholar]
- 31.Hall BA, et al. Serum and glucocorticoid-regulated kinase 1 (SGK1) activation in breast cancer: requirement for mTORC1 activity associates with ER-alpha expression. Breast Cancer Res Treat. 2012;135(2):469–79. doi: 10.1007/s10549-012-2161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce D. The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol Metab. 2001;12(8):341–7. doi: 10.1016/s1043-2760(01)00439-8. [DOI] [PubMed] [Google Scholar]


