Abstract
The mammalian nuclear envelope (NE) forms a stable physical barrier between the nucleus and the cytoplasm, normally breaking down only during the cell cycle phase of mitosis. However, spontaneous transient NE rupture in interphase can occur when NE integrity is compromised such as when the nucleus experiences mechanical stress. For instance, deficiencies in the nuclear lamins and their associated proteins can cause NE rupture that is promoted by forces exerted by actin filaments. NE rupture can allow cytoplasmic nucleases to access chromatin, potentially compromising genome integrity. Importantly, spontaneous NE rupture was noted in several human cancer cell lines but the cause of this defect is not known. Here, we investigated the mechanistic contributions of two major tumor suppressors, p53 (TP53) and Rb (RB1), to the repression of NE rupture. NE rupture was induced in normal human epithelial RPE-1 cells upon impairment of either Rb or p53 achieved by shRNA knockdown and CRISPR/Cas9 gene editing. NE rupture did not involve diminished expression of NE components or greater cell motility. However, cells that underwent NE rupture displayed a larger nuclear projection area. In conclusion, the data indicate that NE rupture in cancer cells is likely due to loss of either the Rb or the p53 pathway.
Implications
These findings imply that tumor suppression by Rb and p53 includes the ability to prevent NE rupture, thereby protecting against genome alterations.
Keywords: Cancer, Nuclear Envelope Rupture, Tumor Suppressor, p53, Rb
Introduction
The nuclear envelope (NE) is composed of the inner nuclear membrane (INM), to which the nuclear lamina is attached, and the outer nuclear membrane (ONM), which is contiguous with the endoplasmic reticulum (ER). The NE prevents macromolecules from diffusing into and out of the nucleus, thus maintaining the separation between cytoplasm and nucleoplasm and allowing the nuclear pore complexes (NPCs) to regulate the trafficking of macromolecules (1). In prophase, NE fragmentation and dissolution allow mitotic chromosomes to attach to the microtubule spindle. In telophase, the NE reforms, surrounding the decondensing chromatin and re-establishing the physical barrier between the nucleus and the cytoplasm (2).
Although NE breakdown was thought to occur only during mitosis, it has recently become clear that NE rupture can occur transiently during interphase under specific conditions (3). Cells derived from patients with laminopathy, a genetic disorder caused by mutations in lamin or lamin-associated proteins, show transient NE rupture in interphase (4). In addition, during parvovirus infection, the NE and lamina break down, allowing viral particles to enter the nucleus (5). Although the NE is usually resealed rapidly, NE rupture can have detrimental effects due to mis-localization of cytoplasmic proteins and organelles (4,6).
Recent studies have pointed to mechanical stress as a major source of NE rupture in cells with lamin deficiencies. Contractile actin bundles that increase nuclear pressure contribute to NE rupture and chromatin herniation (7). This finding is consistent with the role of the Linker of Nucleoskeleton and Cytoskeleton complex (LINC complex, composed of SUN and Nesprin proteins) in exacerbating NE rupture in cells with deficiencies in the nuclear lamina (8) and with the finding that increased stiffness of the substratum promotes NE rupture in cells with Lamin A mutations (9). Mechanical stress is also implicated in NE rupture in primary cells that migrate through tight spaces (10,11) and NE rupture can be induced by simply flattening HeLa nuclei mechanically (12). Furthermore, mechanical stress exerted by pulling forces on nuclei could explain NE rupture resulting from the chromatin bridges formed by dicentric chromosomes (13). In this setting, NE rupture allows a cytoplasmic nuclease, TREX1, to fragment the chromatin in the bridges, giving rise to chromothripsis (13). This DNA fragmentation exemplifies the risk of genome alterations associated with NE rupture.
Interestingly, a permanent form of NE rupture has been noted in micronuclei (14). This lack of NE integrity is thought to promote fragmentation of the resident chromatin and has been implicated in the chromothripsis of micronuclear chromosomes (15,16).
Cells are protected against deleterious consequences of NE rupture by the ESCRT-III complex, which can rapidly re-establish NE integrity (10,11). In addition, perinuclear actin nucleated by Formin-2 (FMN2) protects nuclei from NE rupture and ensuing DNA damage during cell migration (17). Interestingly, FMN2 is upregulated in melanoma, potentially allowing cells to survive extravasation during the development of metastatic disease.
Importantly, several human cancer cell lines (HeLa, U2OS and SJSA), which have no known lamin deficiency, show spontaneous NE rupture in the absence of extraneous mechanical stress (6). The reason for this propensity of cancer cell lines for NE rupture is not clear. We found that human RPE-1 cells show increased spontaneous NE rupture when both p21 and Rb are knocked down with shRNAs (13), potentially explaining NE rupture in cancer cell lines deficient in Rb or p53. Here we test the individual contribution of p53 and Rb to this phenotype and report that loss of either p53 or Rb in RPE-1 cells enhances the frequency of NE rupture. Thus, loss of p53 or Rb function may be one of the causes of NE rupture in cancer cell lines.
Materials and Methods
Cell culture procedures and plasmids
hTERT RPE-1 cells were obtained from the American Tissue Culture Collection (ATCC) and cultured in a 1:1 mixture of Dulbecco’s Modified Eagle Medium (DMEM) and Ham’s F-12 medium (DMEM/F12) (Gibco). The cells have been tested to be free of Mycoplasma contamination by DAPI staining. Phoenix virus packaging cells were grown in DMEM. Media were supplemented with 10% fetal bovine serum (Gibco), 100 U/ml penicillin/streptomycin (Life Technologies), and 2.5 mM L-glutamine (Life Technologies), 1× Minimum NEAA (Gibco).
NLS-3xmTurquoise2 (13) was cloned into the retroviral pQCXIP vector. Retroviral constructs were transfected into Phoenix amphotropic packaging cells using calcium phosphate precipitation. Retroviral supernatants were filtered, mixed 1:1 with target cell media, and supplemented with 4 μg/ml polybrene. Target cells were infected with retroviral supernatants four times at 12 h intervals. Transduced cells were isolated by FACS.
pMKO.1-EV, pMKO.1-Rb shRNA, and pMKO.1-p53 shRNA#2 plasmids were purchased from Addgene (#8452, #10670, #10672). Constructs were transfected into Phoenix amphotropic packaging cells, and target cells were infected as above. Infected cells were selected using Puromycin (10 μg/ml).
Target sequences for CRISPR/Cas9-mediated gene knockouts were identified by ZiFit (http://zifit.partners.org): sgTP53: 5′-GGCAGCTACGGTTTCCGTC-(PAM)-3′, sgRb: 5′-GGCCGCTGTCCTGCTCT-(PAM)-3′. Target sequences were incorporated into a BbsI-linearized sgRNA-cloning vector that also contains mCherry-tagged Cas9 (Addgene # 64324). Plasmids were then transfected into target cells by nucleofection (Lonza apparatus). 700,000 cells in electroporation buffer (freshly mixed 125 mM Na2HPO4, 12.5 mM KCl, 55 mM MgCl2 pH 7.75) were mixed with 10 μg gRNA plasmid, transferred to an electroporation cuvette (BTX), and electroporated with program T23 for RPE-1 cells. Cells were then allowed to recover for 48 h before FACS sorting for mCherry positive cells. FACS-sorted cells were then collected in bulk and tested by western blot for Rb or p53 knockout.
Immunoblotting
For immunoblotting, cells were harvested by trypsinization and lysed in 1× Laemmli buffer (50 mM Tris, 10% glycerol, 2% SDS, 0.01% bromophenol blue, 2.5% β-mercaptoethanol) at 107 cells/ml. Lysates were denatured at 100°C and DNA was sheared with a 281/2 gauge insulin needle. Lysate equivalent to 105 cells was resolved on 8% or 10% SDS/PAGE (Life Technologies) and transferred to nitrocellulose membranes. Membranes were blocked in 5% milk in TBS with 0.1% Tween-20 (TBS-T) and incubated with primary antibody overnight at 4°C, washed 3 times in TBS-T, and incubated for 1 h at room temperature with horseradish-peroxidase-conjugated sheep anti-mouse or donkey anti-rabbit secondary antibodies. After three washes in TBS-T, membranes were rinsed in TBS and proteins were developed using enhanced chemiluminescence (Amersham). Band intensity was quantified in Fiji.
The following primary antibodies were used: anti-Rb (mouse monoclonal, BD Pharmingen, #554136); anti-p53 (mouse monoclonal, Santa Cruz, sc-126); anti-γ tubulin (mouse monoclonal, Abcam, ab11316); anti-Lamin A/C (mouse monoclonal, Santa Cruz, sc-7293); anti-Lamin B1 (rabbit polyclonal, Abcam, ab16048); anti-SUN1 (rabbit polyclonal, Abcam, ab74758); anti-SUN2 (rabbit polyclonal, Abcam, ab87036); anti-LAP2 (rabbit polyclonal, Bethyl, A304-838A-T); anti-FMN2 (rabbit polyclonal, Abcam, ab72052); anti-CHMP2A (rabbit polyclonal, Proteintech, 10477-1-AP); anti-CHMP4B (rabbit polyclonal, Proteintech, 13681-1-AP).
FACS
For cell cycle analysis, cells were labeled with 10 μM BrdU for 30 min, fixed with cold 70% ethanol and stored overnight. BrdU-incorporated DNA was denatured with 2N HCl and 0.5% Triton X-100 for 30 min at room temperature. After neutralized with 0.1 M Na2B4O7·10H2O (pH 8.5), cells were incubated with fluorescein-isothiocyanate-conjugated anti-BrdU antibody (BD Biosciences) in PBS with 0.5% Tween 20 and 0.5% BSA for 30 min at room temperature. Cells were washed and stained with Propidium iodide (2 mM EDTA, 0.2 mg/ml RNASEA, 10 μg/ml Propidium iodide in PBS). FACS was performed with an AccuriC6 (BD Biosciences) and data were analyzed by FlowJo software.
Live-cell Imaging
200,000 cells were plated onto 35 mm glass bottom dishes (MatTek) 24 h before imaging. Live-cell imaging was performed using a CellVoyager CV1000 spinning disk confocal system (Yokogawa, Olympus) equipped with 405, 488, and 561 nm lasers, and a Hamamatsu 512 × 512 EMCCD camera. Pinhole size was 50 μm. Images were acquired at the indicated intervals using a UPlanSApo 60x/1.3 silicone oil objective with the correction collar set to 0.17. The pixel size in the image was 0.27 μm. 480/40 emission filter was used for image acquisition for NLS-3xmTurquoise2. 16 z-stacks were collected at 1.33 μm steps. Temperature was maintained at 37°C in a temperature-controlled enclosure with CO2 support. Maximum intensity projection of z-stacks and adjustment of brightness and contrast were performed using Fiji software. Image stitching was done with the Fiji plugin Grid/Collection stitching (18) with 20% tile overlap, linear blending, a 0.30 regression threshold, a 2.50 max/avg. displacement threshold, and a 3.50 absolute displacement threshold. Images were cropped and assembled into figures using Photoshop CS5.1 (Adobe). Cell tracking was done with Fiji plugin Manual Tracking (Fiji version 2.00-rc-54/1.51h). Nuclear surface area was measured by manual tracing of nuclear borders in Fiji.
Results
Loss of either Rb or p53 enhances NE rupture
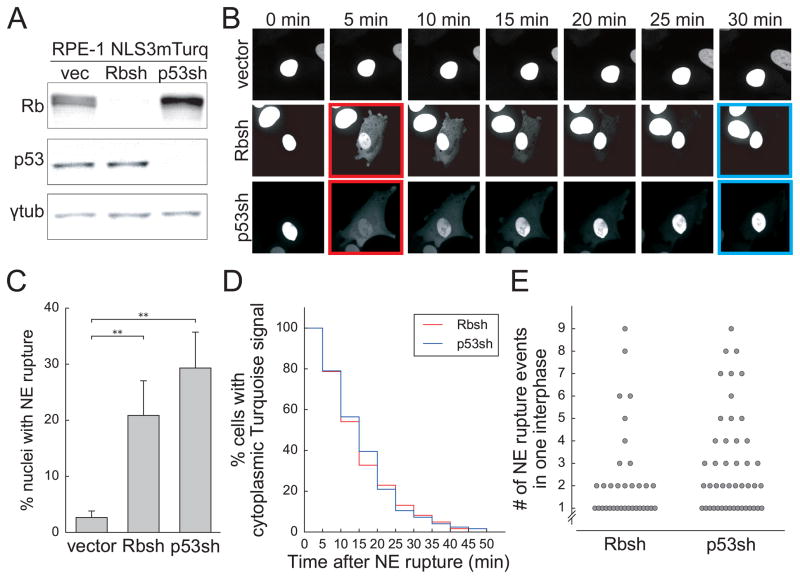
In order to visualize NE rupture, we used NLS-3xmTurquoise2 (NLS3mTurq, three copies of mTurquoise2 fused to the nuclear localization signal of SV40 large T antigen) as the marker for NE integrity (13). After retroviral transduction of the marker into RPE-1 cells, cells were sorted for Turquoise fluorescence using FACS. The NLS3mTurq marker was stably expressed in the FACS-sorted RPE-1 cells and showed nuclear localization. To determine the effect of Rb or p53 deficiency, RPE-1 NLS3mTurq cells were infected with empty vector (vector), Rb shRNA (Rbsh) or p53 shRNA (p53sh) (19,20), resulting in a significant depletion of Rb or p53 protein (Figure 1A). Rb or p53 depletion did not significantly change the ploidy of the cell population (Supplementary Figure 1A).
Figure 1. Depletion of Rb or p53 with shRNAs in RPE-1 cells leads to NE rupture.
(A) Immunoblotting of Rb and p53 in RPE-1 cells infected with shRNAs with γ-tubulin as the loading control.
(B) Examples of transient NE rupture after Rb or p53 depletion in RPE-1 NLS3mTurq cells. The red squares indicate cells undergoing NE rupture, and the blue squares indicate cells with NLS3mTurq marker localization fully restored.
(C) Quantification of the percentage of nuclei with NE rupture. Error bars indicate SD and are derived from three separate experiments with at least 50 cells tracked in each experiment. **p<0.01 (Student’s t-test). Fisher’s exact test, p<0.0001.
(D) Timing of the full restoration of nuclear localization of NLS3mTurq in RPE-1 cells that had undergone NE rupture. Full restoration is marked when the cytoplasmic Turquoise signal was reduced to the original level before NE rupture. 60 NE rupture events were analyzed in each cell line. p=0.98 (Kolmogorov–Smirnov test).
(E) Quantification of the number of NE rupture events per cell per cell cycle. Only cells that underwent at least one NE rupture event are plotted.
To evaluate NE rupture, populations of cells were imaged for 24 h at 5 min intervals using spinning disk confocal microscopy. Ninety adjacent fields were then computationally stitched together to allow hundreds of cells to be tracked. The two daughter cells from individual cells that divided in early time frames were followed until they had reached the second mitosis. NE rupture was scored based on the reduction of the nuclear Turquoise fluorescence intensity coincident with transient appearance of Turquoise in the cytoplasm (6) (Figure 1B).
Upon depletion of either Rb or p53, cells showed increased frequency of NE rupture, manifested by the rapid efflux of NLS3mTurq marker from the nucleus into the cytoplasm (Figure 1B). Fifty daughter cells from 25 dividing cells in each cell line were examined. The percentage of cells undergoing at least one NE rupture event was 21±3% and 29±3% for the Rb- and p53-depleted cells, respectively (Figure 1C). This frequency of NE rupture was significantly increased compared to cells infected with the empty vector (Figure 1C), which showed NE rupture at 3% consistent with a previous analysis of NE rupture of RPE-1 cells (13).
In all cases of NE rupture, the nuclear localization of NLS3mTurq was gradually re-established, usually within half an hour (Figure 1D), indicating that the ruptured NE had been re-sealed and nuclear import was functional. The rate at which the nuclear localization of NLS3mTurq was restored was consistent with a previous report (6), and there was no difference between the cells with or without Rb or p53 (p-value = 0.98, Kolmogorov–Smirnov test). Strikingly, some Rb or p53 deficient cells underwent 8 or 9 NE rupture events in one cell cycle (Figure 1E). All cells with a NE rupture event appeared to survive and progressed through the next mitosis, indicating that a prior NE rupture event does not inhibit cell division.
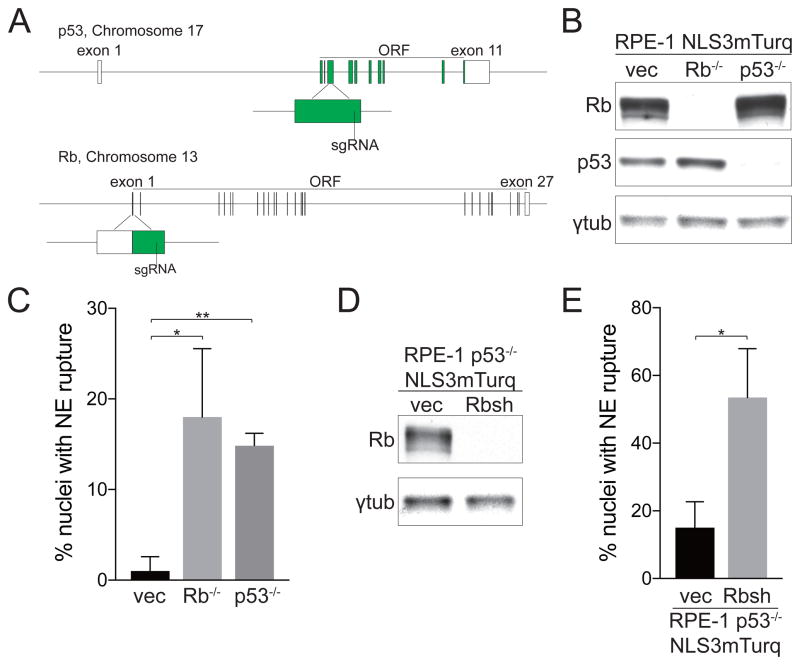
To confirm the role of Rb and p53 in preventing NE rupture we used CRISPR/Cas9 to generate Rb and p53 knockout RPE-1 cells. RPE-1 NLS3mTurq cells were nucleofected with plasmids containing mCherry-Cas9 and sgRNA targeting the Rb or p53 locus (Figure 2A) and mCherry positive cells were collected in bulk. Western blot showed efficient knockout of Rb and p53 (Figure 2B), and FACS analysis showed no significant difference in ploidy after Rb or p53 knockout (Supplementary figure 1B). Similar to cells with Rb and p53 depletion by shRNA, Rb and p53 knockout cell populations also showed significantly increased frequency of NE rupture compared to the empty vector control cells, reinforcing the idea that both Rb and p53 are important in protecting cells against NE rupture (Figure 2C). To determine whether the combined loss of p53 and Rb further exacerbates NE rupture, we knocked down Rb with shRNA in p53 knockout cells (Figure 2D). After Rb depletion, p53 knockout cells have a significantly increased frequency of NE rupture, indicating that Rb and p53 function in different pathways that prevent NE rupture (Figure 2E).
Figure 2. Rb or p53 knockout by CRISPR/Cas9 in RPE-1 cells leads to NE rupture.
(A) Schematic of Rb and p53 gene editing with CRISPR/Cas9. The rectangles represent exons with green indicating coding sequences. The position of each sgRNA is depicted.
(B) Verification of Rb and p53 knockout by western blot.
(C) Quantification of the percentage of nuclei with NE rupture in Rb and p53 knockout cells. Error bars indicate SD and are derived from three separate experiments. *p<0.05, **p<0.01 (Student’s t-test). Fisher’s exact test, p<0.0001.
(D) Verification of Rb shRNA knockdown by western blot in the p53 knockout population shown in (B).
(E) Quantification of the percentage of nuclei with NE rupture in p53 knockout cells with or without Rb knockdown. Error bars indicate SD and are derived from three separate experiments. *p<0.05 (Student’s t-test). Fisher’s exact test, p<0.0001.
NE rupture does not require progression through S phase
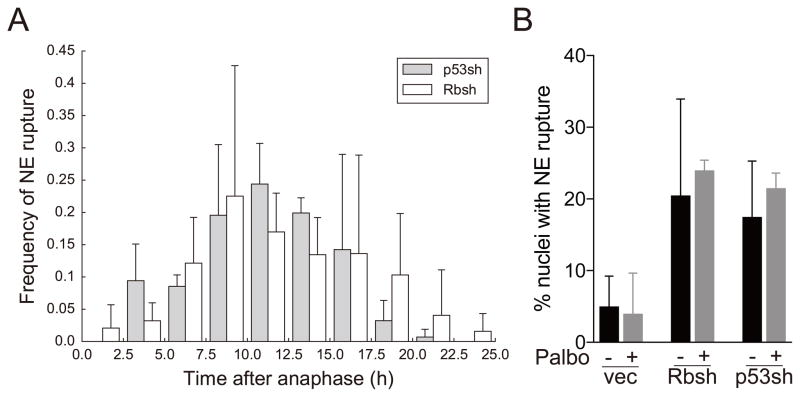
We analyzed the timing of NE rupture after mitosis of the Rb- and p53-depleted cells. In both cases, NE rupture frequency gradually increased after mitosis and peaked at around 10 hours after mitosis. The data indicate that NE rupture is less likely to occur immediately after mitosis and becomes more frequent as cells go through G1 and enter S phase.
To understand whether G1-S transition has a role in promoting NE rupture, we treated the cells with the CDK4/6 inhibitor Palbociclib at the beginning of the imaging sessions. As expected, 1 μM Palbociclib treatment completely blocked the cells to G1 in the case of empty vector and p53-depleted cells (Supplementary Figure 1C). In Rb-depleted cells, Palbociclib treatment only halved the number of cells in S phase (Supplementary Figure 1C), probably because the main pathway by which CDK4/6 promote G1-S transition is by phosphorylating and inactivating Rb. However, treatment with Palbociclib did not reduce the occurrences of NE rupture in Rb- and p53-depleted cells (Figure 3B). These data indicate that NE rupture can take place in G1 and that the effect of Rb and p53 knockdown is unlikely to be directly related to a change in G1/S transition in these cells.
Figure 3. NE rupture can take place in G1.
(A) Frequency of NE rupture at the indicated time intervals after anaphase in Rb- or p53-depleted cells. Error bars indicate SD of three separate experiments. All NE rupture events in all analyzed cells are included.
(B) Quantification of the percentage of nuclei with NE rupture in cells treated with or without 1 μM Palbociclib (Palbo). Error bars indicate SD and are derived from two separate experiments.
Effects of Rb and p53 loss on expression of NE components and regulatory proteins
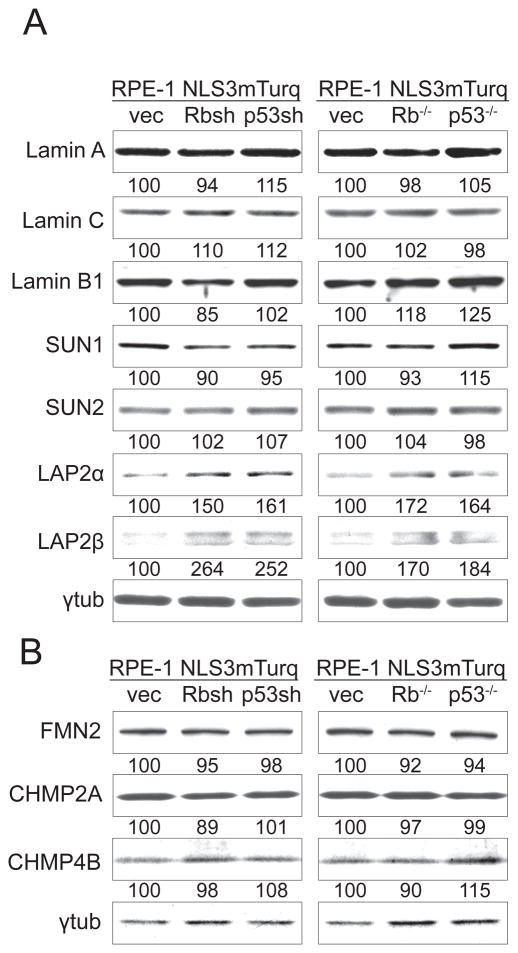
In order to understand how loss of Rb and p53 affected NE integrity, we performed immunoblots to examine lamins and lamin-associated proteins that were previously implicated in NE rupture (4,6,8). To avoid any off-target effects of shRNA and CRISPR/Cas9 editing, we only considered those proteins that showed similarly significant difference both in cells with shRNA-mediated knockdown and CRISPR/Cas9 knockout. There was no significant difference in lamin A/C (LMNA/C) or lamin B1 (LMNB1) expression upon loss of Rb or p53 (Figure 4A). We also did not observe any significant changes in SUN1 or SUN2 expression level upon Rb or p53 depletion (Figure 4A).
Figure 4. Examination of NE protein expression after loss of Rb or p53.
(A) Immunoblotting of lamins or lamin associated proteins with γ-tubulin as the loading control. The numbers below each band represent the band intensity normalized to the empty vector controls.
(B) Immunoblotting of FMN2, and the ESCRT-III proteins CHMP2A and CHMP4B with γ-tubulin as the loading control.
Since increased levels of LAP2 (TMPO) can suppress NE rupture in cells with chromatin bridges (13), we also monitored the expression of LAP2 in Rb- and p53-depleted cells. There was no indication of LAP2 deficiency in the cells with elevated NE rupture. On the contrary, both splice variant of LAP2, LAP2α and LAP2β, were slightly elevated in Rb- and p53- depleted cells (Figure 4A).
We also examined the level of FMN2, because of its role in maintaining NE integrity during migration through confined spaces (17) and because FMN2 is downregulated by the Rb-controlled factor E2F1 (21). However, immunoblotting did not indicate significant changes in FMN2 protein level upon Rb or p53 depletion (Figure 4B).
Finally, we looked at the expression of two ESCRT-III complex proteins, CHMP2A and CHMP4B, because of their role in repairing NE rupture after cells migrate through restrictions (10,11). Also in this case, there was no significant decrease in the expression level of these two proteins upon Rb or p53 depletion (Figure 4B).
Effects of Rb and p53 loss on cell motility and nuclear projection area
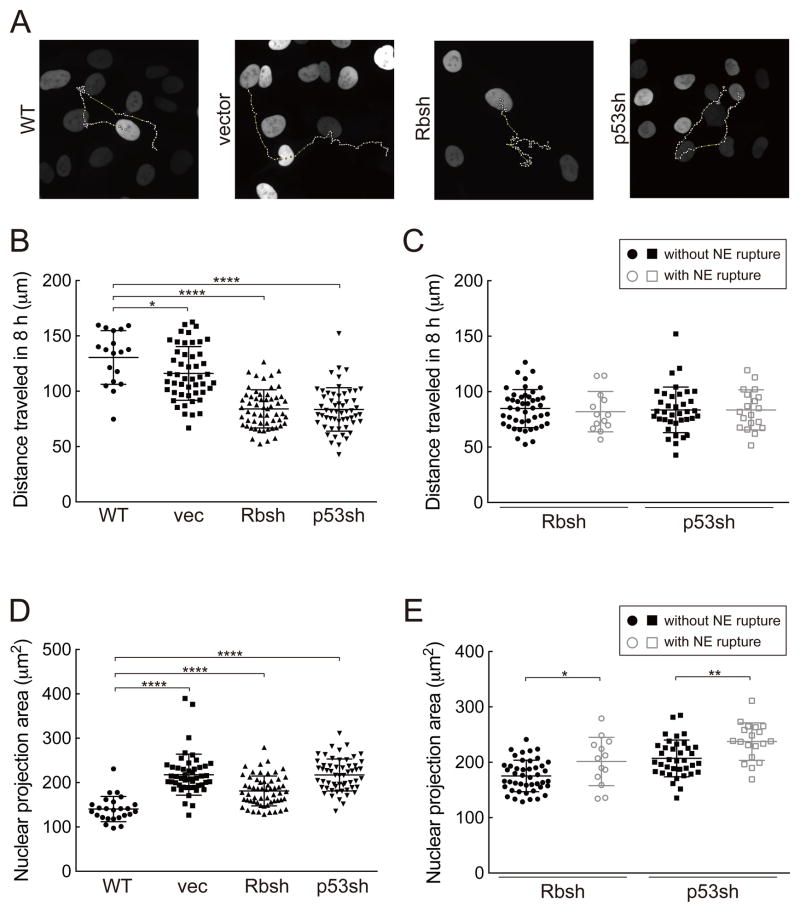
It has been shown that Rb or p53 depletion can increase cell motility (22–24). Given that both the actin cytoskeleton and the stiffness of the cell substratum can affect NE rupture (7,9), we tested whether the enhanced NE rupture in p53- and Rb-depleted cells could be ascribed to a change in cell motility. We manually tracked the movement of cells (Figure 5A) and found a significant decrease, rather than an increase, in cell motility upon loss of Rb or p53 (Figure 5B). We also compared the cells with and without NE rupture and found that there is no difference in their migration behavior (Figure 5C).
Figure 5. Migration rate and projected nuclear area in cells with NE rupture.
(A) Examples of cell tracking over an 8-hour time period.
(B and C) Quantification of the distance that individual cells traveled in 8 hours. The error bars indicate SDs. Statistical significance analysis by Student’s t-test (*p<0.05; ****p<0.0001). One-way ANOVA in (B), p<0.0001.
(D and E) Quantification of the nuclear projection area of individual cells. The error bars indicate SDs. Statistical significance analysis by Student’s t-test (*p<0.05; **p<0.01; ****p<0.0001). Where no stars are given, there was no significant difference. One-way ANOVA in (D), p<0.0001.
Finally, we sought to determine the effect of Rb and p53 loss on the nuclear projection area. An increase in nuclear projection area could be an indication of increased nuclear size or a flattened nucleus, which is correlated with an increase in the mechanical stress. Unexpectedly, we found that there was a significant increase in nuclear projection area upon infection of the cells with the shRNAs vectors (Figure 5D). However, this effect was also observed with the vector not expressing shRNAs, indicating that it not relevant to the increased NE rupture upon p53 or Rb knockdown.
We also compared the nuclear projection area of cells that did or did not undergo NE rupture, using the Rb- or p53-depleted cells. Interestingly, those cells undergoing NE rupture displayed slightly larger nuclear projection area (Figure 5E).
Discussion
Given that the majority of human cancers are deficient in either the Rb or p53 pathway, our finding that both Rb and p53 prevent NE rupture has significant implications. Indeed, in each of the three cancer cell lines in which NE rupture has so far been documented, HeLa, U2OS and SJSA (6), either Rb or p53 pathway has been shown to be defective. In HeLa cells, the tumor suppressor p53 is undetectable owing to the expression of HPV E6 (25,26). In U2OS cells, the CDKN2A locus is silenced by DNA methylation (27), and as a result, Rb is constitutively hyper-phosphorylated and inactivated (28). In the case of SJSA, the p53 pathway is inactivated because of the amplification of the MDM2 gene (29). Therefore, our findings could potentially explain the frequent occurrence of NE rupture in these cancer cell lines.
NE rupture could have an impact on gene regulation, not only because of mislocalized transcription factors, but also due to disruption of the lamin network, which can affect gene regulation (30). Furthermore, NE rupture has been reported to lead to DNA damage (10,11), potentially resulting from the introduction of cytoplasmic nucleases into the nucleoplasm. In micronuclei as well as in cells experiencing telomere damage, NE rupture can lead to chromothripsis (13,16,31,32). Thus, NE rupture may change the genomic landscape in cancer cells, thereby contributing to carcinogenesis. Since we show that p53 or Rb deficient cells can undergo several rounds of NE rupture without losing the ability to progress into mitosis, this process could substantially enhance their carcinogenic potential.
We have not been able to identify the mechanism by which Rb or p53 deficiency leads to NE rupture. Expression levels of a number of relevant proteins, including NE components, the ESCRT-III complex, and FMN2, were not overtly changed by the loss of Rb and p53. Further work on the (ultra) structure of the NE in these cells will be required to define the pathway by which Rb and p53 ensure NE integrity.
Interestingly, we found that the level of LAP2α and LAP2β, the two splicing variants of LAP2, were slightly increased after Rb depletion. Mouse LAP2α has been implicated in tethering Rb to A-type lamins, thereby preventing the proteosomal degradation of the protein (33,34). In addition, in mouse, LAP2β can recruit Germ-cell-less (mGCL) protein to the lamina and repress the activity of E2F-DP3 complex, the main target of Rb (35). Furthermore, overexpression of LAP2 is able to suppress the NE rupture phenotype (13). Upon Rb depletion, cells with increased LAP2 may experience a selective advantage and/or there may be a feedback loop that gives rise to higher LAP2 levels when Rb is repressed.
Our data provide a critical link between tumor suppressors and NE integrity. It will therefore be of interest to uncover the detailed mechanism by which tumor suppressors Rb and p53 prevent NE rupture. In addition, it is important to determine whether NE rupture could occur in primary tumors, and if so, whether targeting pathways that maintain NE could induce synthetic lethality in Rb or p53 deficient cancers.
Supplementary Material
Acknowledgments
Financial Support: J. Maciejowski, NIH, K99CA212290, T. de Lange, NCI, R35CA210036, T. de Lange, BCRF, T. de Lange, the Starr Foundation Cancer Consortium, I9-A9-047
We thank members of the de Lange lab for comments on this manuscript. JM is supported by a grant from the NIH (K99CA212290). Research reported in this publication was supported by an OIA to TdL from the NCI (R35CA210036) and grants to TdL from the BCRF and the Starr Foundation Cancer Consortium (I9-A9-047). TdL is an American Cancer Society Rose Zarucki Trust Research Professor.
We thank K. Thomas, T. Tao, and A. North of the RU Bio-imaging Core Facility for their expert assistance with microscopy. Bio-Imaging Center at RU is supported by a grant (S10RR031855) from the National Center For Research Resources to the Bio-Imaging Center at RU. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Abbreviations
- NE
Nuclear Envelope
- INM
Inner Nuclear Membrane
- ONM
Outer Nuclear Membrane
- NPC
Nuclear Pore Complexes
- LINC
Linker of Nucleoskeleton and Cytoskeleton Complex
References
- 1.Hetzer MW. The nuclear envelope. Cold Spring Harbor Perspectives in Biology. 2010;2:a000539. doi: 10.1101/cshperspect.a000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Güttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol. 2009;10:178–91. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 3.Hatch E, Hetzer M. Breaching the nuclear envelope in development and disease. J Cell Biol. 2014;205:133–41. doi: 10.1083/jcb.201402003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, et al. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Human Molecular Genetics. 2011;20:4175–86. doi: 10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- 5.Porwal M, Cohen S, Snoussi K, Popa-Wagner R, Anderson F, Dugot-Senant N, et al. Parvoviruses Cause Nuclear Envelope Breakdown by Activating Key Enzymes of Mitosis. In: Linden RM, editor. PLoS Pathog. Vol. 9. 2013. p. e1003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus. 2014;3:88–100. doi: 10.4161/nucl.18954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatch EM, Hetzer MW. Nuclear envelope rupture is induced by actin-based nucleus confinement. J Cell Biol. 2016;215:27–36. doi: 10.1083/jcb.201603053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C-Y, Chi Y-H, Mutalif RA, Starost MF, Myers TG, Anderson SA, et al. Accumulation of the Inner Nuclear Envelope Protein Sun1 Is Pathogenic in Progeric and Dystrophic Laminopathies. Cell. 2012;149:565–77. doi: 10.1016/j.cell.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamiello C, Kamps MAF, van den Wijngaard A, Verstraeten VLRM, Baaijens FPT, Broers JLV, et al. Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations. Nucleus. 2014;4:61–73. doi: 10.4161/nucl.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–62. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- 11.Denais CM, Gilbert RM, Isermann P, McGregor AL, Lindert te M, Weigelin B, et al. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–8. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Berre M, Aubertin J, Piel M. Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integr Biol. 2012;4:1406. doi: 10.1039/c2ib20056b. [DOI] [PubMed] [Google Scholar]
- 13.Maciejowski J, Li Y, Bosco N, Campbell PJ, de Lange T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell. 2015;163:1641–54. doi: 10.1016/j.cell.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic Nuclear Envelope Collapse in Cancer Cell Micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53–8. doi: 10.1038/nature10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C-Z, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, et al. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–84. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skau CT, Fischer RS, Gurel P, Thiam HR, Tubbs A, Baird MA, et al. FMN2 Makes Perinuclear Actin to Protect Nuclei during Confined Migration and Promote Metastasis. Cell. 2016;167:1571–1585. e18. doi: 10.1016/j.cell.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–5. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, et al. Telomerase Maintains Telomere Structure in Normal Human Cells. Cell. 2003;114:241–53. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 20.Boehm JS, Hession MT, Bulmer SE, Hahn WC. Transformation of human and murine fibroblasts without viral oncoproteins. Molecular and Cellular Biology. 2005;25:6464–74. doi: 10.1128/MCB.25.15.6464-6474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada K, Ono M, Perkins ND, Rocha S, Lamond AI. Identification and Functional Characterization of FMN2, a Regulator of the Cyclin-Dependent Kinase Inhibitor p21. Molecular Cell. 2013;49:922–33. doi: 10.1016/j.molcel.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadea G, de Toledo M, Anguille C, Roux P. Loss of p53 promotes RhoA–ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178:23–30. doi: 10.1083/jcb.200701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K-J, Godarova A, Seedle K, Kim M-H, Ince TA, Wells SI, et al. Rb Suppresses Collective Invasion, Circulation and Metastasis of Breast Cancer Cells in CD44-Dependent Manner. In: Mehta K, editor. PLoS ONE. Vol. 8. 2013. p. e80590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang C-I, Matoso A, Corney DC, Flesken-Nikitin A, Körner S, Wang W, et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc Natl Acad Sci USA. 2011;108:14240–5. doi: 10.1073/pnas.1017536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matlashewski G, Banks L, Pim D, Crawford L. Analysis of human p53 proteins and mRNA levels in normal and transformed cells. European Journal of Biochemistry. 1986;154:665–72. doi: 10.1111/j.1432-1033.1986.tb09449.x. [DOI] [PubMed] [Google Scholar]
- 26.Scheffner M, Münger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proceedings of the National Academy of Sciences. 1991;88:5523–7. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park Y-B, Park MJ, Kimura K, Shimizu K, Lee SH, Yokota J. Alterations in the INK4a/ARF locus and their effects on the growth of human osteosarcoma cell lines. Cancer Genetics and Cytogenetics. 2002;133:105–11. doi: 10.1016/s0165-4608(01)00575-1. [DOI] [PubMed] [Google Scholar]
- 28.Broceño C, Wilkie S, Mittnacht S. RB activation defect in tumor cell lines. Proceedings of the National Academy of Sciences. 2002;99:14200–5. doi: 10.1073/pnas.212519499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–3. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 30.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, et al. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes & Development. 2008;22:832–53. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, et al. Massive Genomic Rearrangement Acquired in a Single Catastrophic Event during Cancer Development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, et al. Mutational Processes Molding the Genomes of 21 Breast Cancers. Cell. 2012;149:979–93. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, et al. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proceedings of the National Academy of Sciences. 2004;101:9677–82. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nitta RT, Jameson SA, Kudlow BA, Conlan LA, Kennedy BK. Stabilization of the retinoblastoma protein by A-type nuclear lamins is required for INK4A-mediated cell cycle arrest. Molecular and Cellular Biology. 2006;26:5360–72. doi: 10.1128/MCB.02464-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nili E, Cojocaru GS, Kalma Y, Ginsberg D, Copeland NG, Gilbert DJ, et al. Nuclear membrane protein LAP2β mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less) Journal of Cell Science. 2001;114:3297–307. doi: 10.1242/jcs.114.18.3297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.