Abstract
Objectives
Specifics of the biochemical pathways that modulate collagen cross-links in the periodontal ligament (PDL) are not fully defined. Better knowledge of the collagen post-translational modifications that give PDL its distinct tissue properties is needed to understand the pathogenic mechanisms of human PDL destruction in periodontal disease. In this study the post-translational phenotypes of human and mouse PDL type I collagen were surveyed using mass spectrometry.
Background
PDL is a highly specialized connective tissue that joins tooth cementum to alveolar bone. The main function of the PDL is to support the tooth within the alveolar bone while under occlusal load after tooth eruption. Almost half of the adult population in the US suffers from periodontal disease resulting from inflammatory destruction of the PDL, leading to tooth loss. Interestingly, PDL is unique from other ligamentous connective tissues as it has a high rate of turnover. Rapid turnover is believed to be an important characteristic in order for this specialized ligament to function within the oral-microbial environment. Like other ligaments, PDL is composed predominantly of type I collagen. Collagen synthesis is a complex process with multiple steps and numerous post-translational modifications including hydroxylation, glycosylation, and cross-linking. The chemistry, placement and quantity of intermolecular cross-links are believed to be important regulators of tissue-specific structural and mechanical properties of collagens.
Methods
Type I collagen was isolated from several mouse and human tissues, including PDL, and analyzed by mass spectrometry for post-translational variances.
Results
The collagen telopeptide cross-linking lysines of PDL were found to be partially hydroxylated in human and mouse, as well as in other types of ligament. However, the degree of hydroxylation and glycosylation at the helical Lys87 cross-linking residue varied across species and between ligaments. These data suggest that different types of ligament collagen, notably PDL, appear to have evolved distinctive lysine/hydroxylysine cross-linking variations. Another distinguishing feature of PDL collagen is that, unlike other ligaments, it lacks any of the known P3H2-catalyzed 3-hydroxyproline site modifications that characterize tendon and ligament collagens. This gives PDL a novel modification profile, with hybrid features of both ligament and skin collagens.
Conclusion
This distinctive post-translational phenotype may be relevant for understanding why some individuals are at risk of rapid PDL destruction in periodontal disease and warrants further investigation. In addition, developing a murine model for studying PDL collagen may be useful for exploring potential clinical strategies for promoting PDL regeneration.
Keywords: Collagen, Post-translational modifications, Periodontal Ligament, Extracellular matrix, Connective tissues
Introduction
Periodontal ligament (PDL) is a highly specialized connective tissue that suspends individual teeth within the alveolar bone. PDL has several biological functions including tooth support, regulating tooth eruption, dissipating masticatory forces and facilitating the movement of teeth through the alveolar bone when under orthodontic force (1). Periodontal disease is a chronic inflammatory disorder of the periodontium that affects 46% of the U.S population and may progress to tooth loss (2). It is speculated that the enzymatic activity of oral pathogens and the ensuing host response erode PDL collagen and impair tooth support (3). The extracellular matrix of PDL is comprised primarily of collagen types I (75%), III (20%) and V (5%) (4). Healthy PDL maintains the structural and biomechanical integrity of type I collagen and transmits occlusal stresses while undergoing rapid turnover (5). Indeed, two defining features of PDL collagen are its notably high rate of turnover and its small fibril diameter in relation to other connective tissues (6).
Post-translational modifications determine the tissue-specific organization of collagen molecules and intermolecular cross-link formation, thereby defining the biomechanical properties of collagen fibers and connective tissue (7). Indeed, it is becoming increasingly clear that unique tissue specific modifications such as prolyl 3-hydroxylation (tendon and sclera) (8), lysyl 5-hydroxylation (bone, tendon, skin) (9) and glycosylation (bone, skin) (10,11) can play essential roles in defining the structural and mechanical properties of specialized connective tissue. Tissue-specific regulation of these enzymatic modifications is essential and fundamental to tissue functionality.
The prolyl 3-hydroxylases (P3Hs) are members of a class of enzymes known as 2-oxoglutarate dependent dioxygenases. The P3H family is composed of 3 isozymes (P3H1, P3H2 and P3H3) and two non-enzymatic proteins (CRTAP and SC65). The physiological significance of these proteins is underscored by the growing spectrum of disorders caused by mutations in their encoding human genes and genetically engineered mouse models. For example, LEPRE1 (P3H1) and LEPREL3 (CRTAP) mutations have been associated with osteogenesis imperfecta (12,13); LEPREL1 (P3H2) gene mutations have been shown to cause high myopia with various other eye defects (14,15), and LEPREL2 (P3H3) and LEPREL4 (SC65) gene mutations have recently been connected with a collagen pathobiology seen in Ehlers Danlos Syndrome type VIA (11). Potential effects of mutations in this family of proteins on periodontal matrix have not been explored. However, it is becoming clear that P3H1 is ubiquitously expressed in all fibrillar collagen-containing tissues, whereas P3H2 and P3H3 appear to display a highly regulated and specific tissue expression profile (16,17). The gene expression patterns across tissues coincides closely with the level of collagen substrate modification (i.e. amount of 3-hydroxyproline), as measured with mass spectrometry (18).
Several features in the primary sequence of collagen can be used to predict the downstream cross-linking pattern of the mature tissue. These include the type of telopeptide aldehyde (Lys or Hyl), extent of hydroxylation of Lys at interaction sites for these aldehydes on neighboring molecules and whether cross-linking Hyl (Lys87) is glycosylated. Hydroxylysine glycosylation has been suggested to function in collagen fibrillogenesis, collagen cross-linking, and collagen-cell interactions (10). In PDL, lysyl hydroxylase-2 (LH2), the enzyme responsible for cross-linking telopeptide lysine hydroxylation, and lysyl oxidase (LOX), the enzyme that catalyzes the conversion of cross-linking telopeptide (hydroxyl-) lysine to (hydroxyl-) lysine aldehyde are overexpressed in teeth under occlusal function (1,19). We speculate that the unique combination of type I collagen modifications or “molecular phenotype” customizes collagen matrices to function in tissue-specific environments.
PDL health depends on the capacity for type I collagen to sustain both rapid turnover and transmission of occlusal load (5). Although a few animal studies have characterized PDL collagen biochemistry (20,21), the post-translational processes defining human PDL type I collagen structure are still not well defined. The current study characterizes the basic biochemistry of PDL type I collagen modifications in mouse and human tissues using a cross-tissue comparison to investigate the functional significance of the post- translational pathway in PDL type I collagen. We propose that modifications unique to PDL type I collagen have evolved to enable the matrix to adapt and function in an environment subjected to compression/tension forces and bacterial exposure. The purpose of this study was to identify evolutionarily conserved, distinguishing post-translational features shared by murine and human type I collagen α-chains that distinguish PDL from other ligaments, tendon and skin.
Experimental procedures
Statement of Ethics
The Institutional Review Board at the University of Washington reviewed the protocol for collection of de-identified extracted human teeth, and determined that this research did not meet the federal regulatory definition of “human subjects research.” The breeding and harvesting of mouse tissues from C57Bl/6 mice was performed under protocols approved by the IACUC at the University of Washington. Mice were euthanized in carbon dioxide chambers. This method is consistent with the recommendations of the American Veterinary Medical Association Guidelines on Euthanasia.
Periodontal ligament isolation
C57Bl/6 mice were sacrificed 35–41 days postnatal and the fully erupted maxillary and mandibular molars were extracted. Human teeth with healthy periodontium were collected from five de-identified donors during routine dental procedures at the University of Washington School of Dentistry Graduate Periodontics clinic. All collected tissue samples were obtained from fully erupted adult teeth in functional occlusion. Health was defined as sulci with probing depths ranging from 1–3mm without any bleeding on probing or suppuration. Teeth were extracted were extracted either for prosthetic reasons or due to non-restorability. Donors were identified as healthy, with no known genetic collagen disorders or immunosuppressive/chemotherapeutic medicines. Mouse and human teeth were frozen at −20C and subsequently, PDL tissue samples were dissected under surgical magnification (2.5–4x) utilizing multiple 15C surgical blades. Human PDL samples were analyzed individually (n=5) and mouse samples were pooled (n=3 groups/8 mice each).
Transmission electron microscopy (TEM)
Whole mandible was harvested from a C57Bl/6 mouse (n=1) sacrificed at 41 days postnatal. An extracted human tooth (n=1) with attached alveolar bone was obtained from a de-identified patient during a routine dental procedure at the University of Washington Graduate Periodontics Clinic. Tissues were fixed with 2% paraformaldehyde, 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4 for 1 hour. Tissues were rinsed with 0.1M cacodylate buffer and decalcified in 0.5M EDTA, pH 7.4 at 4°C for 2 weeks (human sample) and in 0.11M EDTA, pH 7.4 at 4°C for 2 weeks (mouse sample). The mouse specimen included the mesial root of the first mandibular molar and surrounding bone, and measured 1mm in height apico-coronally along the root axis and 1mm mesio-distally and bucco-lingually. The human specimen was subdivided into 0.5 mm by 1 mm tissue blocks in the coronal plane. Tissues were rinsed with several changes of 0.1M cacodylate buffer at 4°C for 2 days. Specimens were post-fixed in ice cold 1% osmium tetroxide in 0.1M cacodylate buffer for 1 hour and rinsed with 0.1M cacodylate buffer. Specimens were stained in 1% uranyl acetate for 1 hour and dehydrated in graded acetonitrile series. Infiltration on a rotary mixer and embedding was done with 1:1 acetonitrile: resin, then 1:2 acetonitrile: resin, then 2× pure resin (Embed812 resin, Hatfield, PA). Polymerization was done for 48 hours at 60°C in a BEEM® embedding capsule (Ted Pella, Inc., Redding, CA). Sections were cut perpendicular to PDL using a diamond ultra-knife at 65 nm to obtain cross-sections of PDL fibers. Contrasting was done with tannic acid 0.01% for 3 minutes at 60°C, uranyl acetate 2% for 30 minutes, and lead citrated for 10 seconds. Sections were examined with a Philips CM100 TEM (Eindhoven, Netherlands) and images were captured using an Olympus Morada camera (Münster, Germany). Survey images at 4600× were obtained from the middle of the PDL apico-coronally and mesio-distally (10 images per mouse sample and 16 images per human sample). Regions where collagen fibers were sectioned transversely were imaged at 64000× and ten regions were chosen for analysis using Image J Software (National Institutes of Health, Baltimore, USA) (22).
Collagen extraction
Type I collagen was solubilized from human and mouse PDL by heat denaturation for 5 min at 95°C in Laemmli buffer (SDS extraction). SDS extraction was also used to extract collagens from mouse medial collateral ligament (MCL), skin and tail tendon as previously described (23). Collagen α-chains were resolved by SDS-PAGE and stained with Coomassie Blue R-250.
Mass spectrometry
Mass spectrometric analysis of the hydroxylation and glycosylation content within collagen α-chains was performed as previously described (24). Collagen α-chains were cut from SDS-PAGE gels and subjected to in-gel trypsin digestion. Electrospray mass spectrometry was carried out on the tryptic peptides using an LTQ XL linear quadrapole ion-trap mass spectrometer equipped with in-line Accela 1250 liquid chromatography and automated sample injection. Thermo Xcalibur software and Proteome Discoverer software (ThermoFisher Scientific) were used for peptide identification. Tryptic peptides were also identified manually by calculating the possible MS/MS ions and matching these to the actual MS/MS spectrum. Hydroxyl differences were determined manually by averaging the full scan MS over several minutes to include all the post-translational variations of a given peptide. Protein sequences used for MS analysis were obtained from the Ensembl genome database.
Results
TEM analysis of PDL collagens
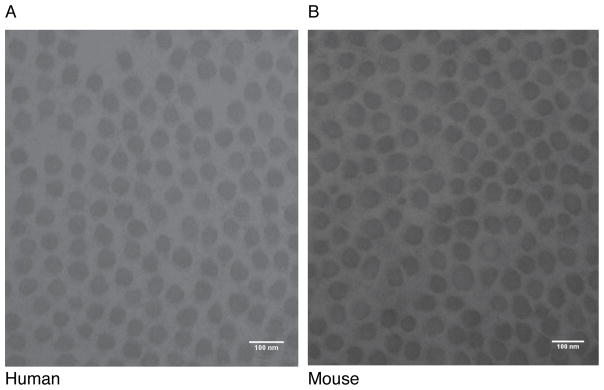
Ultrastructural features of human and mouse PDL collagen were investigated by electron microscopy. The small sample size (n=1) was in part due to the difficulty of obtaining extracted teeth with intact bone attached to the root. Collagen fibril diameters from human and mouse PDL were almost identical as determined by TEM (Figure 1). The collagen fibrils from human and mouse PDL had a similar range of diameters (44–79 nm). The mean fibril diameter was also similar between species, averaging 57.0 ± 6.2 nm (n=2830) in human and 56.6 ± 6.4 nm (n=2589) in mouse. The small fibril diameters observed in both species is consistent with previous reports (6). These similarities in fibril diameter, interfibrillar spacing and parallel organization are common morphological features of PDL in human and mouse. However, due to the small sample size, these TEM data were interpreted qualitatively without emphasis on the statistical significance.
Figure 1. Transmission electron microscopy of collagen fibrils from human and mouse PDL.
Collagen fibril diameter was similar between human and mouse PDL. Representative TEM images showing similarities between the PDL collagen fibrils of human (A) and mouse (B), 64000x. Scale bar is 100 nm.
PDL collagen has reduced prolyl 3-hydroxylation
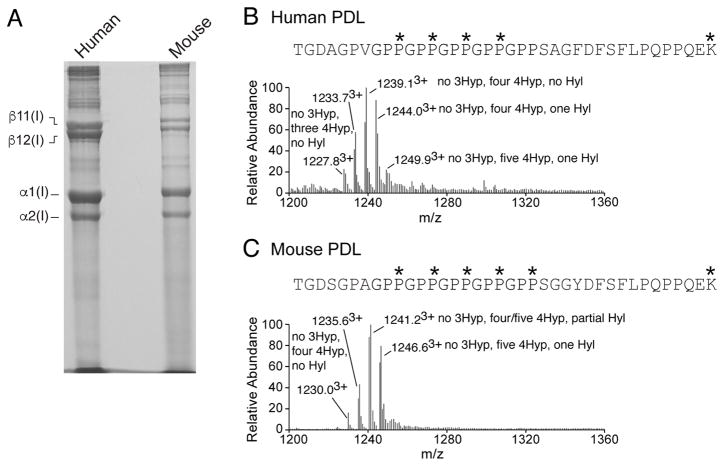
SDS-extraction of human and mouse PDL tissues produced a similar banding pattern of collagen α-chains on SDS-PAGE (Figure 2A). The predominant collagen type extracted from both species was type I collagen. Mass spectrometric analysis of the collagen α-chains revealed that PDL has a distinctive post-translational phenotype from other ligaments. Our group and others have previously identified several post-translational biomarkers of tendon and ligament type I collagen (23,25,26). These sites include unique P3H2-catalyzed 3-hydroxyproline modifications, such as the (GPP)n motif, located at the C-terminus of the triple helix and the Pro707 site of the α1(I) chain. Interestingly, PDL type I collagen from both human and mouse lacked prolyl 3-hydroxylation at the (GPP)n (Figure 2B&C) and α1(I)Pro707 (Table 1). In contrast, the medial collateral ligament (MCL) phenocopied tendon post-translationally with a high occupancy of prolyl 3-hydroxylation at both these sites (Table 1). PDL shares this site-specific lack of 3-hydroxyproline with skin and bone type I collagen (18). Table 1 summarizes the prolyl-3-hydroxylation patterns found in type I collagen from PDL.
Figure 2. Collagen from PDL gives a distinct post-translational fingerprint from other ligaments.
Representative SDS-PAGE of collagens extracted from PDL tissue. Human (n=1) and mouse (n=6) PDL were subjected to SDS-extraction and resolved on 6% SDS-PAGE (A). Mass spectra of (GPP)n containing tryptic peptides from human and mouse PDL. Full scan spectra from LC-MS profiles of in-gel trypsin digests of α1(I) from human PDL (B) and mouse PDL (C). P*, 4Hyp; P#, 3Hyp; K*, Hyl.
Table 1. Comparison of post-translational variances in type I collagens from mouse and human.
Percent hydroxylation at the major 3Hyp sites and the C-telopeptide cross-linking lysine in type I collagen from PDL, MCL, tendon and skin. The percentages (mean±SD) were determined based on the ratio of the m/z peaks of each post-translational variant.
| 3Hyp | Hyl | ||||
|---|---|---|---|---|---|
| α1(I)986 | α1(I)707 | α2(I)707 | α1(I)(GPP)n | C-telo | |
| Human | |||||
| PDL (n=5) | 90±0.4% | 0% | 85±2% | 0% | 46±4% |
| Mouse | |||||
| PDL (n=3) | 96±1% | 0% | 15±2% | 0% | 86±4% |
| MCL (n=2) | 95±4% | 59±3% | 85±7% | 75±5% | 58±10% |
| Tendon (n=3) | 91±3% | 50±5% | 87±4% | 65±5% | - |
| Skin (n=2) | 89±1% | 0% | 10±1% | 0% | - |
Post-translational modifications of linear cross-linking lysine residues
The post-translational nature of the type I collagen linear cross-linking lysine residues was also compared between PDL, MCL, tendon and skin tissues. Using the same tryptic peptides that were used for the (GPP)n motif (Figure 2), the degree of hydroxylation on the α1(I) telopeptide cross-linking lysine was estimated. Table 1 summarizes the percentages of hydroxylation at the C-telopeptide cross-linking Lys across tissues. The high percentage of hydroxylysine at the telopeptide cross-linking site (~85%), suggests that PDL type I collagen is largely cross-linked using the hydroxylysine aldehyde cross-linking pathway. A similar cross-linking pathway is likely used for human PDL collagen (~50% Hyl) and mouse MCL collagen (~65% Hyl). Skin collagen is distinct from PDL in that it is solely cross-linked via the lysine aldehyde pathway (27).
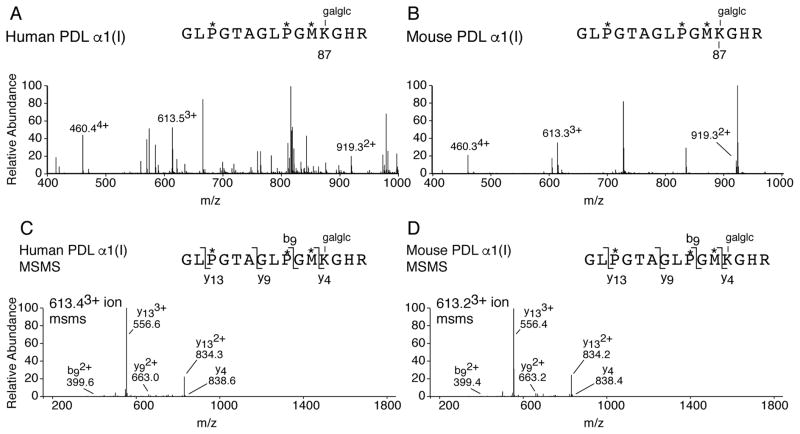
The type I collagen helical cross-linking lysines (Lys87) were also investigated in PDL. In human, the post-translational profile at Lys87 from both α-chains of type I collagen was fully glycosylated in PDL (Figure 3). This finding is in agreement with what has previously been published for bovine PDL (21). In mouse, the post-translational profile of Lys87 was similar in PDL and skin. For example, in mouse PDL and skin, the α1(I) Lys87 residue was fully glycosylated with glucosylgalactosyl, and the α2(I) Lys87 residue had an identical proportion of lysine hydroxylation with little to no glycosylation (Table 2). Mouse type I collagen from MCL and PDL differed post-translationally at residue Lys87; with mouse MCL more resembling tendon at this important cross-linking residue. Indeed, MCL and tendon were unique among soft tissues with Lys87 being 100% hydroxylated and non-glycosylated on both α-chains of type I collagen. In higher order mammals, Lys87 is fully hydroxylated and glycosylated in postcranial ligaments such as MCL (Hudson and Eyre unpublished). Table 2 summarizes the percentages of hydroxylation at the helical cross-linking Lys across tissues.
Figure 3. Post-translational variances on linear cross-linking lysines in PDL type I collagen.
LC-MS profiles of in-gel trypsin digests of the α1(I) collagen chains from human and mouse PDL. MS profile of α1(I) from human PDL (A) and mouse PDL (B) reveals complete glycosylation (glucosyl-galactosyl-Hyl) at cross-linking residue α1(I) Hyl87 (~ 4604+, 6133+ and 9192+). (C) MS/MS fragmentation spectrum of the parent ion (613.43+) from human PDL α1(I). (D) MS/MS fragmentation spectrum of the parent ion (613.23+) from mouse PDL α1(I). The trypsin digested peptide is shown with P* indicating 4Hyp, M* indicating methionine sulfoxide and galglc indicating glucosyl-galactosyl.
Table 2. Post-translational variances in linear cross-linking lysine 87 from type I collagens.
Modifications on Lys87 from both α-chains of type I collagen in human PDL and mouse PDL, MCL, tendon, and skin were measured using mass spectrometry. Lysine modifications include unmodified lysine (Lys), hydroxylysine (Hyl), galactosyl-hydroxylysine (G-Hyl) and glucosylgalactosyl-hydroxylysine (GG-Hyl). The percentages (mean±SD) were determined based on the ratio the m/z peaks of each post-translational variant.
| Modification | |||||
|---|---|---|---|---|---|
| Human | Lys | Hyl | G-Hyl | GG-Hyl | |
| PDL (n=5) | α1(I) | - | - | - | 100±0% |
| α2(I) | - | - | - | 100±0% | |
| Mouse | Lys | Hyl | G-Hyl | GG-Hyl | |
| PDL (n=3) | α1(I) | - | - | - | 100±0% |
| β2(I) | 32±2% | 60±1% | - | 8±3% | |
| MCL (n=2) | α1(I) | - | 100±0% | - | - |
| α2(I) | - | 100±0% | - | - | |
| Tendon (n=3) | α1(I) | - | 100±0% | - | - |
| α2(I) | - | 100±0% | - | - | |
| Skin (n=2) | α1(I) | - | - | - | 100±0% |
| α2(I) | 32±4% | 68±4% | - | - | |
Discussion
Our results indicate that, compared to other connective tissue sources, type I collagen from PDL has a unique post-translational phenotype. For example, specific prolyl 3-hydroxylation substrate sites from the α1-chain of type I collagen, Pro707 and (GPP)n, which are highly modified in most ligaments and tendons from both mouse and human, are completely lacking in PDL tissue from both species. Another noteworthy difference between PDL and other ligaments was found in the highly conserved helical cross-linking Lys87 residue, which was significantly more modified in mouse PDL collagen than other mouse ligaments. The post-translational quality of Lys87 is vital in controlling the cross-link chemistry of the collagen fibril. For example, the degree and combination of Lys hydroxylation and glycosylation (galactosyl- or glucosylgalactosyl-) will have a significant influence on defining the intermolecular cross-links that form and determine the structural and mechanical properties of the tissue. In higher mammals, the helical collagen cross-linking lysines are similarly modified in PDL and other ligaments (1). Higher levels of modified Lys were identified in the C-telopeptide cross-linking site of mouse (85%) than human (50%). This is likely the result of a general trend in higher levels of collagen modifications in mice compared to higher mammals (8, 18); however, potential differences in cross-linking cannot be ruled out. Overall however, mouse and human PDL type I collagen displayed several cross-species collagen post-translational similarities.
Collagen post-translational modifications are fundamental to defining the structural properties of a tissue. The collagen superfamily is composed of at least 45 genes and gene translational products each with variably regulated post-translational modifications. Although this post-translational variability is still not fully understood, it is becoming increasingly evident for fibril-forming collagens that these modifications can influence covalent intermolecular cross-links that are fundamental to tissue function (7). For example, the high degree of hydroxylation on the cross-linking telopeptide lysine of PDL collagen is comparable to the post-translational phenotype of load-bearing ligaments. In contrast, the lack of specific prolyl 3-hydroxylated substrate sites is identifiable with skin and bone collagen, which have relatively high rates of turnover (5). It is therefore tempting to speculate that the unique post-translational phenotype of PDL type I collagen has evolved to generate a structure suited to function in an environment of load-bearing and rapid turnover. Cross-linking of PDL collagen fibrils enables the transmission of tensile stresses through PDL collagen fibrils during crown loading, and the high rate of PDL collagen turnover rebuilds these load-bearing elements. However, when disease processes promote collagen degradation and/or impair turnover, periodontal stress transmission and tooth attachment are expected to be affected.
The function of the evolutionarily conserved residue, 3-hydroxyproline, in collagen biology is still unclear. However, tissues such as tendon, sclera and ligament have been shown to exhibit unusually high levels of P3H2-catalyzed prolyl 3-hydroxylations in their fibrillar collagens. Furthermore, P3H2 appears to be developmentally regulated, as the level of substrate modification has been shown to increase in older animals and tissues (23,28). Indeed, the P3H2-catalyzed prolyl 3-hydroxylation of the (GPP)n motif has an apparent association with tendon-like tissue, which needs to grow linearly with growing skeleton, yet turns over slowly during adulthood (23,25). This developmental regulation (and requirement for prolyl 3-hydroxylation), which appears to be tissue-specific to tendons and ligaments, may be functionally distinct from PDL. Type I collagen production is epigenetically controlled in PDL, with collagen expression decreasing with age (29). Furthermore, PDL-derived cells have been shown to up- and down-regulate various genes under mechanical stress (30). It is unknown whether the lack of prolyl 3-hydroxyaltion in PDL is the result of low P3H2 gene expression or if the rapid turnover of collagen substrate potentially encumbers enzyme activity.
Early studies have quantified some of the unique structural and functional features of PDL collagen (5,31). A comparison of collagen from multiple connective tissues reported that PDL collagen had the fastest rate of synthesis and turnover. For example, PDL was reported to have a half-life of 1 day, significantly faster than both skin dermis (15 days) and alveolar bone (6 days). These rates of synthesis and turnover support the high rate of collagen remodeling in PDL. The high level of di-glycosylation on PDL cross-linking residue Lys87 may also negatively regulate cross-link maturation (32). Indeed, PDL has mostly immature/reducible cross-links with only minute amounts of mature/stable cross-links (9,21,33). It has been reported that skin and soft oral tissues share a similar collagen divalent cross-linking profile (34); however, PDL has a significantly higher level of immature cross-links compared to skin (9,27). This distinct pattern of cross-linking potentially imparts the high tensile strength and capacity for rapid turnover to PDL.
The combination of rapid turnover and immature collagen cross-links could help to explain the smaller diameter of PDL collagen fibrils. Collagen fibril diameter varies between species, tissues, tissue sites and age (35). Previous studies have shown that PDL fibril diameter in both rodent and human teeth is relatively small, ranging from ~45–55 nm (36,37). Our TEM data support that collagen fibrils have comparable diameters in mouse and human PDL (~57 nm). Tissues that are under high tension or compressive stress, such as PDL, typically have smaller diameter collagen fibrils with more interfibrillar cross-links, which facilitates the return to the tissues original form. Conversely, tissues that experience high tensile strength, such as tendon, have larger diameter collagen fibrils and more intrafibrillar cross-links (35). Indeed, tendon and ligament collagen fibrils have been shown to attain diameters as large as 300 nm (38).
Previously only limited data were available to compare PDL collagen post-translational modification in different species. The evolutionary conserved similarities between human and murine PDL collagen post-translational modifications and fibril diameter presented here support the use of murine models for future PDL collagen studies. The development of this model for human PDL collagen enables further focus on how specific components of post-translational pathways contribute to cross-link formation, and development of the PDL collagen’s capacity for load-bearing and high turnover. Thus, future study may define key steps in PDL collagen formation that must be replicated in regenerative treatments in order to re-establish a healthy PDL. Furthermore, we propose that the unique tissue-specific characteristics revealed in the current study may predispose the PDL to increased susceptibility to microbial and inflammatory proteases. Mouse models could be useful for investigating how periodontal disease affects collagen fibrillogenesis, and may lead to a better understanding of susceptibility of the PDL collagen structure to specific microbial and host proteases.
Acknowledgments
We thank Wai Pang Chan for his help with the TEM imaging. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers AR037318 and AR036794 (DE), as well as the Hack Memorial Fund of the Department of Periodontics, University of Washington. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest.
Abbreviations
- PDL
Periodontal ligament
- P3H
prolyl 3-hydroxylase
- MCL
medial collateral ligament
- TEM
transmission electron microscopy
Footnotes
Author Contributions
DMH, TP, DRD and DRE contributed to the conception and design of the study; DMH and MG performed experiments. DMH wrote the initial draft of the manuscript and all authors contributed to the revisions. All authors gave final approval and agree to be accountable for all aspects of the work. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Kaku M, Yamauchi M. Mechano-regulation of collagen biosynthesis in periodontal ligament. J Prosthodont Res. 2014;58(4):193–207. doi: 10.1016/j.jpor.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eke PI, Dye BA, Wei L, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JD, Lee J, Ozcoban H, Schneider GA, Ho SP. Biomechanical adaptation of the bone-periodontal ligament (PDL)-tooth fibrous joint as a consequence of disease. J Biomech. 2014;47(9):2102–2114. doi: 10.1016/j.jbiomech.2013.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler WT, Birkedal-Hansen H, Beegle WF, Taylor RE, Chung E. Proteins of the periodontium. Identification of collagens with the [alpha1(I)]2alpha2 and [alpha1(III)]3 structures in bovine periodontal ligament. J Biol Chem. 1975;250(23):8907–8912. [PubMed] [Google Scholar]
- 5.Sodek J. A comparison of the rates of synthesis and turnover of collagen and non-collagen proteins in adult rat periodontal tissues and skin using a microassay. Arch Oral Biol. 1977;22(12):655–665. doi: 10.1016/0003-9969(77)90095-4. [DOI] [PubMed] [Google Scholar]
- 6.Luder HS, Zimmerli I, Schroeder HE. Do collagen fibrils of the periodontal ligament shrink with age? J Periodont Res. 1988;23(1):46–52. doi: 10.1111/j.1600-0765.1988.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 7.Eyre DR, Weis MA. Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif Tissue Int. 2013;93(4):338–347. doi: 10.1007/s00223-013-9723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson DM, Joeng KS, Werther R, et al. Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations. J Biol Chem. 2015;290(13):8613–8622. doi: 10.1074/jbc.M114.634915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays In Biochemistry. 2012;52:113–133. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terajima M, Perdivara I, Sricholpech M, et al. Glycosylation and cross-linking in bone type I collagen. J Biol Chem. 2014;289(33):22636–22647. doi: 10.1074/jbc.M113.528513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heard ME, Besio R, Weis M, et al. Sc65-Null Mice Provide Evidence for a Novel Endoplasmic Reticulum Complex Regulating Collagen Lysyl Hydroxylation. In: Bateman JF, editor. PLoS genetics. 4. Vol. 12. 2016. p. e1006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morello R, Bertin TK, Chen Y, et al. CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006;127(2):291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39(3):359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mordechai S, Gradstein L, Pasanen A, et al. High myopia caused by a mutation in LEPREL1, encoding prolyl 3-hydroxylase 2. American journal of human genetics. 2011;89(3):438–445. doi: 10.1016/j.ajhg.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H, Tong P, Peng Y, et al. Homozygous loss-of-function mutation of the LEPREL1 gene causes severe non-syndromic high myopia with early-onset cataract. Clin Genet. 2013 Oct; doi: 10.1111/cge.12309. [DOI] [PubMed] [Google Scholar]
- 16.Tiainen P, Pasanen A, Sormunen R, Myllyharju J. Characterization of recombinant human prolyl 3-hydroxylase isoenzyme 2, an enzyme modifying the basement membrane collagen IV. J Biol Chem. 2008;283(28):19432–19439. doi: 10.1074/jbc.M802973200. [DOI] [PubMed] [Google Scholar]
- 17.Vranka J, Stadler HS, Bächinger HP. Expression of prolyl 3-hydroxylase genes in embryonic and adult mouse tissues. Cell Struct Funct. 2009;34(2):97–104. doi: 10.1247/csf.09002. [DOI] [PubMed] [Google Scholar]
- 18.Hudson DM, Eyre DR. Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification? Connect Tissue Res. 2013;54(4–5):245–251. doi: 10.3109/03008207.2013.800867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y-J, Jeng J-H, Chang H-H, Huang M-Y, Tsai F-F, Yao C-CJ. Differential regulation of collagen, lysyl oxidase and MMP-2 in human periodontal ligament cells by low- and high-level mechanical stretching. J Periodont Res. 2013;48(4):466–474. doi: 10.1111/jre.12028. [DOI] [PubMed] [Google Scholar]
- 20.Plecash JM, Bentley JP. Crosslink analysis as an indicator of collagen turnover in periodontal ligament from functioning and non-functioning teeth in the dog. Arch Oral Biol. 1982;27(6):463–468. doi: 10.1016/0003-9969(82)90085-1. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi M, Katz EP, Mechanic GL. Intermolecular cross-linking and stereospecific molecular packing in type I collagen fibrils of the periodontal ligament. Biochemistry (Mosc) 1986;25(17):4907–4913. doi: 10.1021/bi00365a027. [DOI] [PubMed] [Google Scholar]
- 22.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson DM, Werther R, Weis M, Wu J-J, Eyre DR. Evolutionary origins of C-terminal (GPP)n 3-hydroxyproline formation in vertebrate tendon collagen. In: Ruggiero F, editor. PloS one. 4. Vol. 9. 2014. p. e93467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weis MA, Hudson DM, Kim L, Scott M, Wu JJ, Eyre DR. Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J Biol Chem. 2010;285(4):2580–2590. doi: 10.1074/jbc.M109.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyre DR, Weis M, Hudson DM, Wu JJ, Kim L. A novel 3-hydroxyproline (3Hyp)-rich motif marks the triple-helical C terminus of tendon type I collagen. J Biol Chem. 2011;286(10):7732–7736. doi: 10.1074/jbc.C110.195768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa Y, Vranka JA, Boudko SP, et al. Mutation in cyclophilin B that causes hyperelastosis cutis in American Quarter Horse does not affect peptidylprolyl cis-trans isomerase activity but shows altered cyclophilin B-protein interactions and affects collagen folding. J Biol Chem. 2012;287(26):22253–22265. doi: 10.1074/jbc.M111.333336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 28.Taga Y, Kusubata M, Ogawa-Goto K, Hattori S. Developmental Stage-dependent Regulation of Prolyl 3-Hydroxylation in Tendon Type I Collagen. J Biol Chem. 2016;291(2):837–847. doi: 10.1074/jbc.M115.686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takatsu M, Uyeno S, Komura J, Watanabe M, Ono T. Age-dependent alterations in mRNA level and promoter methylation of collagen alpha1(I) gene in human periodontal ligament. Mech Ageing Dev. 1999;110(1–2):37–48. doi: 10.1016/s0047-6374(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 30.Kaku M, Rosales Rocabado JM, Kitami M, et al. Mechanical Loading Stimulates Expression of Collagen Cross-Linking Associated Enzymes in Periodontal Ligament. J Cell Physiol. 2015;231(4):926–933. doi: 10.1002/jcp.25184. [DOI] [PubMed] [Google Scholar]
- 31.Sodek J, Ferrier JM. Collagen remodelling in rat periodontal tissues: compensation for precursor reutilization confirms rapid turnover of collagen. Coll Relat Res. 1988;8(1):11–21. doi: 10.1016/s0174-173x(88)80032-3. [DOI] [PubMed] [Google Scholar]
- 32.Sricholpech M, Perdivara I, Yokoyama M, et al. Lysyl hydroxylase 3-mediated glucosylation in type I collagen: molecular loci and biological significance. J Biol Chem. 2012;287(27):22998–23009. doi: 10.1074/jbc.M112.343954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi M, Shiiba M. Post-Translational Modifi Cations of Proteins. Vol. 446. Totowa, NJ: Humana Press; 2008. Lysine Hydroxylation and Cross-linking of Collagen; pp. 95–108. Methods in Molecular Biology™. [DOI] [PubMed] [Google Scholar]
- 34.Kuboki Y, Takagi T, Sasaki S, Saito S, Mechanic GL. Comparative collagen biochemistry of bovine periodontium, gingiva, and dental pulp. J Dent Res. 1981;60(2):159–163. doi: 10.1177/00220345810600021201. [DOI] [PubMed] [Google Scholar]
- 35.Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978;203(1152):305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- 36.Berkovitz BK, Weaver ME, Shore RC, Moxham BJ. Fibril diameters in the extracellular matrix of the periodontal connective tissues of the rat. Connect Tissue Res. 1981;8(2):127–132. doi: 10.3109/03008208109152132. [DOI] [PubMed] [Google Scholar]
- 37.Shore RC, Moxham BJ, Berkovitz BK. A quantitative comparison of the ultrastructure of the periodontal ligaments of impeded and unimpeded rat incisors. Arch Oral Biol. 1982;27(5):423–430. doi: 10.1016/0003-9969(82)90153-4. [DOI] [PubMed] [Google Scholar]
- 38.Ruggeri A, Motta PM, editors. Ultrastructure of the Connective Tissue Matrix. Boston, MA: Springer US; 1984. [DOI] [Google Scholar]