Abstract
Introduction
No previous population-based study has addressed the contribution of activation procedures to the yield of epileptiform abnormalities on serial EEGs. We assessed yield of activation-related epileptiform abnormalities and predictors of finding an activation-related abnormality with multiple EEGs in a population-based study of newly diagnosed epilepsy.
Methods
We used the resources of the Rochester Epidemiology Project to identify 449 residents of Rochester, Minnesota with a diagnosis of newly diagnosed epilepsy at age one year or older, between 1960 and 1994, who had at least one EEG. Information on all activation procedures (i.e. sleep, hyperventilation, photic activation) and seizure/epilepsy characteristics was obtained by comprehensive review of medical records.
Results
At the first EEG, the yield of epileptiform abnormalities was greatest for individuals 1–19 years of age at diagnosis, for each activation procedure. The yield in patients aged 1–19 versus ≥20 years was 21.6% vs. 10.3% for sleep, 6.5% vs. 3.3% for photic stimulation, and 10.3% vs. 5% for hyperventilation. Among young people (aged 1–19 years) sleep was associated with an increased likelihood of finding an activation-related abnormality on any EEG. The likelihood of finding an activation-related abnormality on any EEG was decreased for postnatal symptomatic and for unknown etiology.
Conclusions
Among activation procedures, sleep showed the highest yield of epileptiform abnormalities. There was a low yield for photic stimulation and hyperventilation. Within each activation procedure, younger age at diagnosis had the greatest yield. Sleep is the most effective activation procedure, especially in younger patients, and should be performed when possible.
Keywords: Population-based, epidemiology, epileptiform abnormality, activation procedures, EEG
The first EEG does not always show an epileptiform abnormality in epilepsy and serial EEGs may all be normal (Carpay, de Weerd et al. 1997). After the first EEG fails to show an epileptiform abnormality, the yield of epileptiform abnormalities can be increased if the EEG is repeated during sleep (Carpay, de Weerd et al. 1997, Binnie and Stefan 1999), but previous estimates of the yield due only to sleep vary (Pratt, Mattson et al. 1968, Degen 1980, Degen and Degen 1983, Shinnar, Kang et al. 1994). Hyperventilation and photic stimulation are also widely used as activating procedures on routine EEGs (Miley and Forster 1977), though some authors have questioned their yield (Holmes, Dewaraja et al. 2004). Others have called for reevaluation of the usefulness of routine use of photic stimulation, even for generalized onset epilepsy (Wolf and Goosses 1986, King, Newton et al. 1998, Holmes, Dewaraja et al. 2004, Baykan, Matur et al. 2005).
The aims of this study were to evaluate the contribution of activation procedures to the yield of epileptiform abnormalities and the predictors of finding epileptiform abnormalities in relation to activation procedures. To address these aims, we carried out a population-based study of newly diagnosed epilepsy, and assessed findings on serial EEGs carried out from first diagnosis. No previous study of the yield of activation procedures has been carried out in a population-based setting on serial EEGs performed from onset. Use of this design provides important information for planning cost-effective diagnostic work-ups for patients with newly diagnosed epilepsy because it minimizes selection bias related either to epilepsy prognosis or to factors other than the presence of epilepsy. In contrast, studies restricted to cases from tertiary care centers may be biased by the inclusion of patients with more severe epilepsy, who have high seizure frequency and EEG abnormalities elicited either with or without activation.
Methods
The methods have been described in detail elsewhere (Baldin, Hauser et al. 2014). Briefly, data were obtained from the “Genetic Epidemiology of Seizure Disorders in Rochester” (GESDR) study (Ottman, Barker-Cummings et al. 2010, Ottman, Barker-Cummings et al. 2011, Peljto, Barker-Cummings et al. 2014), a population-based study using the resources of the Rochester Epidemiology Project (REP) (Hauser, Annegers et al. 1993, Hauser, Annegers et al. 1996, Melton 1996, St Sauver, Grossardt et al. 2011, Rocca, Yawn et al. 2012). The original study included all residents of Rochester, Minnesota with either a single unprovoked seizure or newly diagnosed epilepsy between 1935 and 1994. Our current analyses are restricted to patients diagnosed with newly diagnosed epilepsy between 1960 and 1994, and to EEGs recorded in 1960 or later. Only records with prior authorization for medical record review were included (more than 99% of those identified for the study).
Newly diagnosed epilepsy was defined as a history of two or more unprovoked seizures, separated by >24 h and diagnosed by a physician. Subjects were classified as having newly diagnosed epilepsy if they were initially diagnosed with epilepsy while residing in Rochester from 1960 to 1994, or had a first unprovoked seizure from 1960 to 1994 and a recurrence at any time while residing locally during the follow-up period ending in 2008. For each resident with newly diagnosed epilepsy, we examined number of EEGs, age at diagnosis, gender, seizure type, etiology (Berg, Berkovic et al. 2010), presence of status epilepticus, and number of seizures per year of follow up. We excluded prolonged (longer than 2 hours), video- and intra-operative EEGs. The study was approved by the Mayo Clinic and Columbia University Institutional Review Boards.
For each EEG, trained nurses abstracted detailed information on date, activation procedures (sleep, photic stimulation and hyperventilation), and activation-related findings. Two epileptologists (WAH, JRB) reviewed the abstracted information to confirm it was correct. The EEG report did not indicate the duration of sleep, and whether sleep was spontaneous, induced with sleep deprivation, or only drug-induced was not abstracted. Similarly, no technical descriptions of hyperventilation and photic stimulation were provided. However, all EEGs and activation procedures were performed using methods that met or exceeded standard guidelines of the American Clinical Neurophysiology Society (www.acns.org/pdf/guidelines/Guideline-1), with a 21 channels 10–20 system. For almost the whole period of the study, the clinical neurophysiology laboratory of the Mayo Clinic was the only laboratory where EEGs were performed in the region. All EEG tracings were interpreted by board certified clinical neurophysiologists. Information on antiepileptic drug use at the time of the EEG recording was not included in the EEG report.
Epileptiform abnormalities were defined by the presence of generalized epileptiform abnormalities (typical generalized 3Hz spike-wave, atypical spike-wave, slow spike-wave, generalized epileptiform fast, hypsarrhythmia, electrodecremental), focal epileptiform abnormalities (spike, spike-wave, sharp wave, periodic lateralized epileptiform discharges [PLEDS], multifocal, bilateral, independent, or synchronous) or epileptiform abnormality not determined whether generalized or focal (Baldin, Hauser et al. 2014).
Activation-related epileptiform abnormalities were defined as those only due to the specific activation procedure regardless of the concomitant use and effect of other activation procedures on the same EEG. For each activation procedure, the EEG results were categorized as “procedure not done,” “procedure performed but no epileptiform/no additional epileptiform abnormality detected,” or “procedure performed and additional epileptiform abnormality detected.” An “additional epileptiform abnormality” was defined as any epileptiform abnormality not previously present, with or without an activation procedure.
Subjects younger than one year of age were excluded from the activation-related analyses because they are more likely to sleep spontaneously than older subjects and photic stimulation and hyperventilation are less feasible in infants.
Statistical analysis
Descriptive statistical analyses were performed to analyze demographic and clinical characteristics, using frequencies and percentages to summarize categorical variables, and medians and interquartile ranges for continuous variables. Statistical significance was determined using the χ2 statistic (or Fisher's exact test) and Wilcoxon-Rank sum test for continuous variables. The 0.05 level of significance and two-sided tests were used for all analyses. All analyses were conducted using SAS® software, v 9.2., SAS Institute Inc., Cary, NC, USA.
Cumulative yield of any epileptiform abnormality
We estimated the cumulative yield of epileptiform EEG abnormalities for the different activation procedures according to the number of EEGs using a life table approach for clustered observations, with clusters defined by individual subjects and observations defined by EEGs. Subjects were followed until the date an epileptiform abnormality was recorded, the date of last visit to a REP provider, the date of death or the end of the study period (1 January 2008), whichever came first.
We evaluated the yield of any epileptiform abnormality detected only through activation procedure(s) (i.e., sleep, photic stimulation, or hyperventilation) conducted at the time of the routine EEG. An abnormality was considered to be activation-related if it occurred during an EEG at the time of a specific activation procedure and not in periods of the EEG without that procedure. Each activation procedure was considered, regardless of the use or effect of the other two on the same EEG. The analysis was performed by age group at diagnosis (1–19 years vs. ≥20 years) and by seizure type (excluding subjects classified as having both focal and generalized seizure types, since only two subjects were in this group).
The log-rank statistic was used to compare the estimate of cumulative proportion of epileptiform abnormality in newly diagnosed epilepsy among groups.
To determine the additional impact of activation procedures, we also considered the cumulative proportion with any epileptiform abnormality detected in awake EEGs where the epileptiform abnormality was not due to photic stimulation or hyperventilation. The analysis was repeated by seizure type and by syndrome, for subjects aged 1–19 at diagnosis.
Cox proportional hazard regression analysis
A Cox proportional hazard regression model was used to evaluate the hazard of recording an activation-related epileptiform abnormality due only to any of the activation procedures with increasing time since diagnosis. In the model, adjusted for the number of seizures during the first five years of follow-up after diagnosis (categorized as none, one, two or more, or unknown), each activation procedure and age at EEG (dichotomized as 1–19 years of age and 20 years or older) were included as time-dependent covariates. Etiology was also included. A second model included the same variables and age at EEG was categorized as 1–9, 10–19, with 20 years or older as the referent.
To account for the clustering of EEGs within subjects, we used the marginal Cox model approach for clustered data, applying the Wei, Lin and Weissfel method (Wei, Lin et al. 1989). The proportional hazard assumption was tested graphically and by testing for interaction.
Results
We studied 449 Rochester, Minnesota residents aged one year or older with newly diagnosed epilepsy from 1960 through 1994, who had at least one EEG. Among them, only 10 subjects (2.2%) had no activation procedure performed.
Cumulative percentage of subjects with an epileptiform abnormality due to an activation procedure on routine EEGs
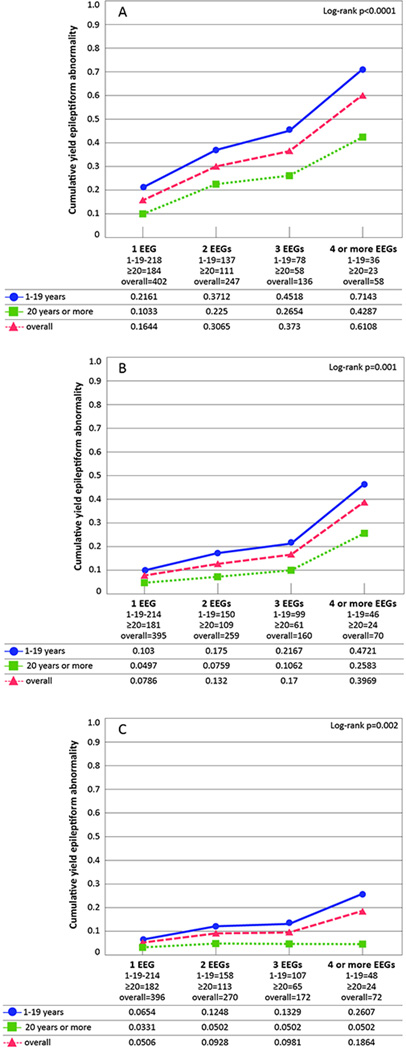
Overall, activation by sleep yielded the greatest proportion of activation-related epileptiform abnormalities (Fig. 1A) followed by hyperventilation (Fig. 1B) and then photic stimulation (Fig. 1C). Within each activation procedure, the yield was greatest for individuals 1–19 years old.
Figure 1. Cumulative proportion of activation-related epileptiform abnormalities by age group.
A. Epileptiform abnormality due to sleep activation among all subjects with incident epilepsy that slept, by age group at diagnosis (1 year or more).
B. Epileptiform abnormalities due to hyperventilation among all subjects with incident epilepsy who had a hyperventilation procedure, by age group at diagnosis (1 year or more).
C. Epileptiform abnormalities due to photic stimulation among all subjects with incident epilepsy who had a photic stimulation procedure, by age group at diagnosis (1 year or more).
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
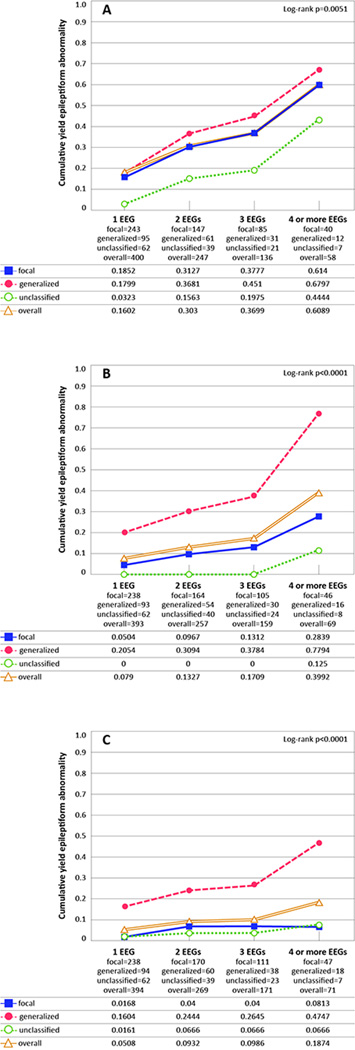
The cumulative yield of epileptiform abnormalities due to sleep activation was similar in those with focal and generalized seizure types (18.5% and 18.0% respectively at the first EEG) while it was lower for those with unclassified seizures (3.2%) (Fig.2A). For both hyperventilation and photic stimulation, the cumulative yield of epileptiform abnormalities was greater in those with generalized seizures (hyperventilation 20.5%, photic stimulation 16.0%) than in focal (hyperventilation 5.0%, photic stimulation 1.7%) or unclassified seizures (hyperventilation 0%, photic stimulation 1.6%, p< 0.0001; Figs. 2B, 2C).
Figure 2. Cumulative proportion of activation-related epileptiform abnormalities by seizure type.
A. Epileptiform abnormalities due to sleep activation among all subjects with incident epilepsy who slept, by seizure type (1 year or more).
B. Epileptiform abnormalities due to hyperventilation among all subjects with incident epilepsy who had a hyperventilation procedure, by seizure type (1 year or more).
C. Epileptiform abnormalities due to photic stimulation among all subjects with incident epilepsy who had a photic stimulation procedure, by seizure type (1 year or more).
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
Abnormalities not due to activation procedures
On the first EEG, the yield of ictal or interictal epileptiform abnormalities that were not due to activation, in awake-EEGs, was 44% (Fig.1 Suppl.). Among children aged 1–19 years, the cumulative yield of ictal or interictal epileptiform abnormalities that were not due to activation was similar in generalized and focal seizures (49% and 56%; Fig.2 Suppl.), and greater in focal and generalized non-GGE syndromes compared to genetic generalized epilepsies (60% and 63% versus 40%; Fig.3 Suppl.).
Distribution of epileptiform abnormalities according to activation procedures
In subjects aged one year or older, 51.9% (N=233) had no epileptiform abnormality on the first EEG, whereas 23.8% had an activation-related abnormality (due to sleep activation in 53.3%), and 24.3% had an abnormality that was not activation-related (Table 1). With regard to abnormalities that were not activation-related, sleep, photic stimulation and hyperventilation might have been performed, but the epileptiform abnormality reported occurred only or also during wakefulness in the absence of these activating procedures.
Table 1.
Distribution of epileptiform abnormality, for the first, second and third EEG, among subjects aged ≥ 1 year.
| EEG 1 (N=449) |
EEG 2 $ (N=164) |
EEG 3 $ (N= 82) |
|
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| No Epileptiform abnormality | 233 (51.9) | 122 (74.4) | 71 (86.6) |
|
Epileptiform abnormality, excluding activation and sleep* |
109 (24.3) | 14 (8.5) | 6 (7.3) |
| Epileptiform abnormality activation-related | 107 (23.8) | 28 (17.1) | 5 (6.1) |
| Sleep only** | 57 (53.3) | 18 (64.3) | 2 (40) |
| Photic stimulation only** | 15 (14.0) | 5 (17.9) | 0 (0) |
| Hyperventilation only** | 25 (23.4) | 1 (3.6) | 3 (60) |
| Any combination of the above** | 10 (9.3) | 4 (14.3) | 0 (0) |
In some cases sleep, photic stimulation and hyperventilation were performed during these EEGs, but the epileptiform abnormality reported occurred during wakefulness without these activating procedures. This epileptiform abnormality might also have occurred during sleep, photic stimulation or hyperventilation, in those with these procedures.
Percent computed among all EEGs with an epileptiform abnormality due to an activation procedure.
# of EEGs performed with no epileptiform abnormality in the previous EEG.
Among 164 subjects who had a second EEG following a first EEG without any epileptiform abnormality, 17.1% (N=28) had an activation-related epileptiform abnormality, mostly by sleep (64.3%). Only 8.5% had an epileptiform abnormality that was not due to activation (Table 1).
Among 82 subjects who had a third EEG following a first and second EEG without any epileptiform abnormality, 6.1% had an activation-related epileptiform abnormality and 7.3% had an epileptiform abnormality that was not due to activation (Table 1).
A complete distribution of activation procedures for each EEG is shown in Supplemental Table 1.
Factors associated with an activation-related epileptiform abnormality across all EEGs
Adjusting for the number of seizures during the first five years of follow-up, the hazard of an epileptiform abnormality due to an activation procedure was increased for children aged 1–19 years (HR=1.8, Table 2); sleep-EEG also increased the hazard (HR=2.7). The hazard was decreased for activation related epileptiform abnormality in those with postnatal symptomatic etiology and those with unknown etiology compared to those with genetic etiology (Table 2).
Table 2.
Cox proportional hazard regression analysis for any new activation-related epileptiform abnormality in 449 people with epilepsy aged 1 year or older who had at least one EEG
| Factors | N (%) | Crude HR (95% CI) | Adjusted HR* (95% CI) |
|---|---|---|---|
| Age at EEG** | |||
| 1–19 years | 2.3 (1.6– 3.4) | 1.8 (1.1– 2.9) | |
| ≥20 years | 1.0 (referent) | 1.0 (referent) | |
| Gender | |||
| Male | 238 (53%) | 1.0 (referent) | 1 |
| Female | 211 (47%) | 1.1 (0.9–1.5) | |
| Sleep done** | |||
| No | 1.0 (referent) | 1.0 (referent) | |
| Yes | 1.8 (1.3– 2.7) | 2.7 (1.8– 4.2) | |
| Photic stimulation done** | |||
| No | 1.0 (referent) | 1.0 (referent) | |
| Yes | 0.9 (0.6– 1.4) | 0.8 (0.5– 1.6) | |
| Hyperventilation done** | |||
| No | 1.0 (referent) | 1.0 (referent) | |
| Yes | 1.0 (0.7– 1.4) | 1.2 (0.7– 2.1) | |
| Etiology^ | |||
| Genetic | 83 (18.5%) | 1.0 (referent) | 1.0 (referent) |
| Prenatal/developmental | 55 (12.2%) | 0.6 (0.4–0.9) | 0.5 (0.3–1.02) |
| Postnatal symptomatic | 104 (23.2%) | 0.3 (0.2–0.5) | 0.3 (0.1–0.5) |
| Unknown | 207 (46.1%) | 0.5 (0.4–0.7) | 0.4 (0.3–0.7) |
| Status epilepticus | |||
| No | 438 (97.6%) | 1.0 (referent) | 1 |
| Yes | 11 (2.4%) | 0.4 (0.1–1.6) | |
accounting for the number of seizures during each of the first five years of follow up (categorized as none, one, two or more, or unknown).
Time–dependent variable The status of each EEG with regard to use of any activation technique (i.e., sleep, photic stimulation, hyperventilation). It is not possible to give the N for all the variables because the time dependent covariates change over time. Therefore, we provide the remaining variables that are not time dependent.
Genetic etiology includes epilepsy formerly classified as idiopathic (e.g. idiopathic generalized, N=67, 80.7%; idiopathic focal, N=16, 19.3%).
HR= Hazard Ratio; 95% CI= 95% Confidence Interval;
Factor not included in the model;
Compared to the ≥20 year old age group, in the 1–9 year olds, there was an increased hazard (HR=1.8, p<0.05) for epileptiform abnormality, while in 10 to 19 year age group, there was no statistically significant difference.
There was no multiplicative or additive interaction between age at EEG and each specific time-dependent activation procedure, implying that the combination of the activation procedure and young age was not associated with an additional increase in the hazard of epileptiform abnormalities.
Discussion
This is the first population-based study of the yield of activation-related epileptiform abnormalities over multiple EEGs in people with newly diagnosed epilepsy. Previous studies on EEG activation procedures have focused mostly on selected patients with prevalent epilepsy. In these studies the contribution of activation was not evaluated on multiple EEGs.
The literature is inconsistent regarding the ability of activation to trigger an epileptiform abnormality in different epilepsy syndromes and age groups (Miley and Forster 1977, Degen and Degen 1983, Wolf and Goosses 1986, el-Ad, Neufeld et al. 1994, Holmes, Dewaraja et al. 2004, Guaranha, Garzon et al. 2005, Mendez and Brenner 2006). In our analysis of epileptiform abnormalities due solely to an activation procedure, the yield was highest for sleep and lower for hyperventilation and photic stimulation. Additionally, the yield of activation was greater in children than in adults, across all three procedures.
Younger age at EEG recording and presence of sleep on EEG were significantly and independently associated with an increased likelihood of epileptiform abnormality, suggesting that the role of activation in detecting epileptiform abnormalities is greater in young people.
Sleep
Sleep has been reported as an effective procedure to increase the yield of epileptiform activity after a normal EEG (Gibbs and Gibbs 1947). Systematic studies of the yield of epileptiform abnormality in sleep have mostly considered sleep-deprived or unspecified EEGs (Mattson, Pratt et al. 1965), although sleep deprivation, spontaneous sleep, or drug induced sleep is not always specified. Studies on children with incident seizure(s) reported a yield of 4.1% due only to sleep activation on the first EEG (Shinnar, Kang et al. 1994) and 19.8% on a second EEG after sleep deprivation (Carpay, de Weerd et al. 1997). In prevalent samples of patients with epilepsy, the yield on the first EEG varies from 11.4% to 22% (Pratt, Mattson et al. 1968, Degen 1980, Degen and Degen 1983), consistent with our findings (Table 3). A previous study found that older age at epilepsy onset was associated with fewer epileptiform abnormalities in both awake and asleep EEGs (Degen 1980), consistent with our finding of higher yield in children than adults. Previous studies found that epileptiform abnormalities were more likely to be activated by sleep or sleep deprivation in patients with idiopathic generalized epilepsy than focal epilepsy (Pratt, Mattson et al. 1968, Degen 1980, Carpay, de Weerd et al. 1997), whereas we found that the yield did not differ between generalized and focal seizure types.
Table 3.
Studies of yield of activation-related epileptiform abnormalities
| Author | Age range | Source population | Type of population | Yield due to activation |
|---|---|---|---|---|
| Current study | All ages (≥1 year) |
Population based. All subjects with incident epilepsy in Rochester within study period (1960–1994) |
Incident | 1st EEG: Sleep: 16.4% Photic stimulation: 5.1% Hyperventilation: 7.9% |
| Shinnar et al, 1994 (Shinnar et al., 1994) | Children (1 month–19 years) |
Prospective, first unprovoked afebrile seizure examined at centers in the Bronx between 1983–1990 |
Incident | Sleep: 4.1% |
| Carpay et al, 1997 (Carpay et al., 1997) | Children (1 month–16 years) |
Prospective, idiopathic or remote symptomatic single unprovoked seizure or epilepsy seen at a center between 1988–1992 |
Incident | Sleep deprived: 19.8% |
| Pratt et al, 1968 (Pratt et al., 1968) | All ages (mostly young adults) |
Consecutive patients with epilepsy with non- epileptiform routine awake EEG |
Prevalent | Sleep: 11.4% |
| Degen and Degen, 1983 (Degen et al., 1983) | Adults (≥15 years) | Patients with atypical absences whose routine EEG was non- epileptiform |
Prevalent | Sleep deprived: 21.9% |
| Degen, 1980 (Degen, 1980) | All ages | Subjects with epilepsy. Sleep deprived EEGs recorded at an EEG center in 1974 |
Prevalent | Sleep: 19.6% |
| Herrlin, 1954 (Herrlin, 1954) | Children (<15 years) | Series with epilepsy and photic stimulation from an EEG laboratory (Sept 1949–Nov 1953) |
Prevalent | Photic stimulation: 11% |
| Roy et al, 2003 (Roy et al., 2003) | Children (8–20 years) | Series of patients with epilepsy | Prevalent | Photic stimulation: 3% |
| Wolf and Gooses, 1986 (Wolf et al., 1986) | All ages | Series of patients with epilepsy at an EEG laboratory (Jan 1974 – May1982) |
Prevalent | Photic stimulation: 9.9% |
| Holmes et al, 2004 (Holmes et al., 2004) | 10–64 years | Consecutive patients with epilepsy | Prevalent | Hyperventilation: 4.6% |
| Gabor and Marsan, 1969 (Gabor et al., 1969) | All ages (not specified) |
Retrospective series of patients with focal epilepsy. Cases focal and bilaterally epileptiform discharges Controls unilateral discharges |
Prevalent | Sleep: 14.3% cases; about 12% controls Photic stimulation: 14.3 % cases; none in controls Hyperventilation:31.4 cases; 6% controls |
| Miley and Forster, 1977 (Miley et al., 1977) | All ages (not specified) |
Series of patients with complex partial epilepsy between 1971– 1975 |
Prevalent | Hyperventilation: 11% |
Photic stimulation
Only 5% of subjects in our study had an activation-related epileptiform abnormality due to photic stimulation on the first EEG. While this was greatest in children and for generalized compared to focal seizures, the yield was low, especially when compared with sleep. Our findings are consistent with studies reporting a prevalence of photoparoxysmal response in epilepsy of 3%–11%, higher in children than in adults and in generalized than in focal seizure types (Herrlin 1954, Wolf and Goosses 1986, Roy, Pinheiro et al. 2003).
Hyperventilation
The yield of epileptiform abnormalities is also low for hyperventilation compared to sleep. We found that the yield of activation-related epileptiform abnormalities accounted for 7.9% of epileptiform abnormalities on the first EEG, higher in children and in generalized seizure types. This result is similar to the existing literature, reporting an overall yield of less than 5% (Holmes, Dewaraja et al. 2004) and of 6%–11% in focal epilepsies (Gabor and Marsan 1969, Miley and Forster 1977) (Table 3).
Detection of activation in serial EEGs
Our study evaluated whether the increased yield of any epileptiform abnormality after a first non-epileptiform EEG is due to activation procedures. We found that in subjects aged ≥1 year with epilepsy, who had a first EEG without an epileptiform abnormality, most activation-related epileptiform abnormalities on the second EEG were due to sleep.
Strengths and limitations
Strengths of our study include its population-based design and the expertise of clinicians in and around Rochester. We evaluated each activation-related contribution to the yield of epileptiform abnormalities on sequential EEGs.
We analyzed each activation procedure independently of the others; however in many cases multiple procedures were performed during the same EEG, as this is routine clinical practice. The exception is when photic stimulation and/or hyperventilation was contraindicated. Because of the way our data were collected (through retrospective medical record and EEG report review), it was not possible to determine inter-rater reliability of EEG findings and activation methods.
In keeping with usual clinical practice, the presence and type of activation-related EEG abnormalities in the first six months after diagnosis were taken into account in the classification of seizure type and epilepsy syndrome. This diagnostic issue creates a circularity of reasoning, especially with regard to the yield of EEG abnormalities in primary generalized seizures and generalized epilepsy syndromes, where classification is greatly dependent on the identification of generalized epileptiform EEG abnormalities, and may have accounted in part for the greater yield we observed in generalized epilepsy (Figure 2).
We were unable to address information on sleep deprivation, use of sleep induction drugs, and sleep structure was not abstracted and information on sleep duration was not available in the EEG reports. Also, it is unclear whether sleep deprivation before an EEG recording is the stimulus that lowers the threshold for epileptiform abnormality (Mattson, Pratt et al. 1965, Pratt, Mattson et al. 1968, Rowan, Veldhuisen et al. 1982) or whether sleep itself is the stimulus, and is facilitated by sleep deprivation (Pratt, Mattson et al. 1968, Degen, Degen et al. 1987).
Among activation procedures, sleep is likely to be the most effective. After a first non-epileptiform EEG in which sleep was not recorded, a sleep-EEG should be performed, in all age groups. While clinically important when it occurs, the yield of photic stimulation and hyperventilation was low, especially in adults, even after serial studies.
Therefore for young patients, sleep, hyperventilation and photic stimulation should be performed if possible as the yield is greater than in adults. Given the low yield of hyperventilation and photic stimulation in adults, only a sleep EEG should be required, although photic stimulation and hyperventilation are easy to perform within the time allotted to a routine EEG.. The value of ordering multiple EEGs over time, including activation procedures, should be considered carefully, to avoid potentially unnecessary testing in adults.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supplementary Material
Supplemental Figure 1. Cumulative proportion with epileptiform abnormalities in incident epilepsy, during awake EEGs only, in the absence of epileptiform abnormalities due to photic stimulation or hyperventilation.
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
Supplemental Figure 2. Cumulative proportion with epileptiform abnormalities in incident epilepsy during awake EEGs only by seizure type in 1–19 year olds in the absence of epileptiform abnormalities due to photic stimulation or hyperventilation.
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
Supplemental Figure 3. Cumulative proportion with epileptiform abnormalities in incident epilepsy during awake EEGs only by syndrome in children aged 1–19 years in the absence of epileptiform abnormalities due to photic stimulation or hyperventilation.
GGE= genetic generalized epilepsies; Gen.not_GGE=Generalized epilepsy other than GGE; Focal =Focal epilepsy; Unknown=unknown whether focal or generalized, cause unknown/structural/metabolic.. The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
Acknowledgments
We thank Jane Emerson, RN, Melissa Petersen, RN, Diane Carlson, RN, Ann Van Oosten, and Thomas Bitz for assistance with data collection.
Funding Source
Dr. Hauser was a consultant Federal Aviation Administration; received funding for travel from the American Epilepsy Society and received research support from the CDC and from the NIH through #R01 NS043472 [Co-I]. He is on the editorial advisory board of Epilepsy Research, Acta Scandinavia Neurologica and Neuroepidemiology. He is a member of a data and safety monitoring board for Neuropace.
Dr. Buchhalter served as a consultant for Lundbeck, Inc., Eisai, Ltd, and Upsher-Smith; is on the editorial advisory board for Clinical Neurology News; received research support from the Alberta Children’s Hospital Foundation and from the NIH through #R01 NS043472 [Co-I]. He also provides clinical evaluations and neurophysiology services that will not be benefitted by this paper.
Dr. Hesdorffer serves as consultant at the Mount Sinai Medical Center, Injury Prevention Center and at NYU Epilepsy Center; she received research support from CDC, NINDS, Epilepsy Foundation of America, PCORI, CURE, and the Epilepsy Study Consortium; she serves on advisory boards for Upsher-Smith Laboratories, Acorda and Cyberonics, and has received funding for travel from the University of Bologna, Upsher-Smith Laboratories, Acorda, and Cyberonics. She serves as Associate Editor for Epilepsia, is on the Editorial Board of Epilepsy and Behavior and is a contributing editor for Epilepsy Currents.
Dr. Ottman serves on the scientific advisory board for and holds stock options in Trigeminal Solutions, Inc; received funding for travel from the École des Hautes Etudes en Santé Publique, the University of Bologna, the University of Calgary, Ettore Majorana Centre for Scientific Culture, the American Epilepsy Society, and the International League Against Epilepsy; and received research support from the NIH.
This research was supported by U.S. NIH grant R01 NS043472 from the NINDS. Study data were obtained from the Rochester Epidemiology Project, which is supported by the NIA of the NIH under R01 AG034676. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
Dr. Baldin reports no disclosures.
Presented as a poster at the XIV Congresso Nazionale di Neuroepidemiologia, Milan (Italy), 21st –22nd November 2014
References
- Baldin E, Hauser WA, Buchhalter JR, Hesdorffer DC, Ottman R. Yield of epileptiform electroencephalogram abnormalities in incident unprovoked seizures: a population-based study. Epilepsia. 2014;55(9):1389–1398. doi: 10.1111/epi.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykan B, Matur Z, Gurses C, Aykutlu E, Gokyigit A. Typical absence seizures triggered by photosensitivity. Epilepsia. 2005;46(1):159–163. doi: 10.1111/j.0013-9580.2005.67303.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Binnie CD, Stefan H. Modern electroencephalography: its role in epilepsy management. Clin Neurophysiol. 1999;110(10):1671–1697. doi: 10.1016/s1388-2457(99)00125-x. [DOI] [PubMed] [Google Scholar]
- Carpay JA, de Weerd AW, Schimsheimer RJ, Stroink H, Brouwer OF, Peters AC, van Donselaar CA, Geerts AT, Arts WF. The diagnostic yield of a second EEG after partial sleep deprivation: a prospective study in children with newly diagnosed seizures. Epilepsia. 1997;38(5):595–599. doi: 10.1111/j.1528-1157.1997.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Degen R. A study of the diagnostic value of waking and sleep EEGs after sleep deprivation in epileptic patients on anticonvulsive therapy. Electroencephalogr Clin Neurophysiol. 1980;49(5–6):577–584. doi: 10.1016/0013-4694(80)90398-3. [DOI] [PubMed] [Google Scholar]
- Degen R, Degen HE. The diagnostic value of the sleep EEG with and without sleep deprivation in patients with atypical absences. Epilepsia. 1983;24(5):557–566. doi: 10.1111/j.1528-1157.1983.tb03420.x. [DOI] [PubMed] [Google Scholar]
- Degen R, Degen HE, Reker M. Sleep EEG with or without sleep deprivation? Does sleep deprivation activate more epileptic activity in patients suffering from different types of epilepsy? Eur Neurol. 1987;26(1):51–59. doi: 10.1159/000116312. [DOI] [PubMed] [Google Scholar]
- el-Ad B, Neufeld MY, Korczyn AD. Should sleep EEG record always be performed after sleep deprivation? Electroencephalogr Clin Neurophysiol. 1994;90(4):313–315. doi: 10.1016/0013-4694(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Gabor AJ, Marsan CA. Co-existence of focal and bilateral diffuse paroxysmal discharges in epileptics. Clinical-electrographic study. Epilepsia. 1969;10(4):453–472. doi: 10.1111/j.1528-1157.1969.tb06141.x. [DOI] [PubMed] [Google Scholar]
- Gibbs E, Gibbs F. Diagnostic and Localizing Value of Electroencephalographic Studies in Sleep. A. Research Nerv., Ment. Dis., Proc. 1947;26(366) [Google Scholar]
- Guaranha MS, Garzon E, Buchpiguel CA, Tazima S, Yacubian EM, Sakamoto AC. Hyperventilation revisited: physiological effects and efficacy on focal seizure activation in the era of video-EEG monitoring. Epilepsia. 2005;46(1):69–75. doi: 10.1111/j.0013-9580.2005.11104.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71(6):576–586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- Herrlin KM. EEG with photic stimulation: a study of children with manifest or suspected epilepsy. Electroencephalogr Clin Neurophysiol. 1954;6(4):573–589. doi: 10.1016/0013-4694(54)90085-1. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Dewaraja AS, Vanhatalo S. Does hyperventilation elicit epileptic seizures? Epilepsia. 2004;45(6):618–620. doi: 10.1111/j.0013-9580.2004.63803.x. [DOI] [PubMed] [Google Scholar]
- King MA, Newton MR, Jackson GD, Fitt GJ, Mitchell LA, Silvapulle MJ, Berkovic SF. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352(9133):1007–1011. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- Mattson RH, Pratt KL, Calverley JR. Electroencephalograms of epileptics following sleep deprivation. Arch Neurol. 1965;13(3):310–315. doi: 10.1001/archneur.1965.00470030090009. [DOI] [PubMed] [Google Scholar]
- Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- Mendez OE, Brenner RP. Increasing the yield of EEG. J Clin Neurophysiol. 2006;23(4):282–293. doi: 10.1097/01.wnp.0000228514.40227.12. [DOI] [PubMed] [Google Scholar]
- Miley CE, Forster FM. Activation of partial complex seizures by hyperventilation. Arch Neurol. 1977;34(6):371–373. doi: 10.1001/archneur.1977.00500180065014. [DOI] [PubMed] [Google Scholar]
- Ottman R, Barker-Cummings C, Leibson CL, Vasoli VM, Hauser WA, Buchhalter JR. Validation of a brief screening instrument for the ascertainment of epilepsy. Epilepsia. 2010;51(2):191–197. doi: 10.1111/j.1528-1167.2009.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman R, Barker-Cummings C, Leibson CL, Vasoli VM, Hauser WA, Buchhalter JR. Accuracy of family history information on epilepsy and other seizure disorders. Neurology. 2011;76(4):390–396. doi: 10.1212/WNL.0b013e3182088286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto AL, Barker-Cummings C, Vasoli VM, Leibson CL, Hauser WA, Buchhalter JR, Ottman R. Familial risk of epilepsy: a population-based study. Brain. 2014;137(Pt 3):795–805. doi: 10.1093/brain/awt368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KL, Mattson RH, Weikers NJ, Williams R. EEG activation of epileptics following sleep deprivation: a prospective study of 114 cases. Electroencephalogr Clin Neurophysiol. 1968;24(1):11–15. doi: 10.1016/0013-4694(68)90061-8. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan AJ, Veldhuisen RJ, Nagelkerke NJ. Comparative evaluation of sleep deprivation and sedated sleep EEGs as diagnostic aids in epilepsy. Electroencephalogr Clin Neurophysiol. 1982;54(4):357–364. doi: 10.1016/0013-4694(82)90199-7. [DOI] [PubMed] [Google Scholar]
- Roy AK, Pinheiro L, Rajesh SV. Prevalence of photosensitivity--an Indian experience. Neurol India. 2003;51(2):241–243. [PubMed] [Google Scholar]
- Shinnar S, Kang H, Berg AT, Goldensohn ES, Hauser WA, Moshe SL. EEG abnormalities in children with a first unprovoked seizure. Epilepsia. 1994;35(3):471–476. doi: 10.1111/j.1528-1157.1994.tb02464.x. [DOI] [PubMed] [Google Scholar]
- St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Lin D, Weissfeld L. Regression-analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. [Google Scholar]
- Wolf P, Goosses R. Relation of photosensitivity to epileptic syndromes. J Neurol Neurosurg Psychiatry. 1986;49(12):1386–1391. doi: 10.1136/jnnp.49.12.1386. http://www.acns.org/pdf/guidelines/Guideline-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cumulative proportion with epileptiform abnormalities in incident epilepsy, during awake EEGs only, in the absence of epileptiform abnormalities due to photic stimulation or hyperventilation.
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
Supplemental Figure 2. Cumulative proportion with epileptiform abnormalities in incident epilepsy during awake EEGs only by seizure type in 1–19 year olds in the absence of epileptiform abnormalities due to photic stimulation or hyperventilation.
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
Supplemental Figure 3. Cumulative proportion with epileptiform abnormalities in incident epilepsy during awake EEGs only by syndrome in children aged 1–19 years in the absence of epileptiform abnormalities due to photic stimulation or hyperventilation.
GGE= genetic generalized epilepsies; Gen.not_GGE=Generalized epilepsy other than GGE; Focal =Focal epilepsy; Unknown=unknown whether focal or generalized, cause unknown/structural/metabolic.. The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.