Abstract
Objective
Early childhood development (ECD) programs typically combine healthy nutrition and cognitive stimulation in an integrated model. We separately delivered these two components in a cluster randomized controlled trial (RCT) to evaluate their comparative effectiveness in promoting healthy child development and caregiver mental health. This is the first study to do so for HIV-affected children and their infected mothers,.
Methods
221 HIV-exposed but uninfected (HEU) child (2 to 3 years old) and caregiver dyads in 18 geographic clusters in Eastern Uganda were randomized by cluster to receive biweekly individualized sessions of either 1) Mediational Intervention for Sensitizing Caregivers (MISC) training emphasizing cognitive stimulation, or 2) Uganda Community Based Association for Child Welfare program that delivered (UCOBAC) health and nutrition training. Children were evaluated at baseline, six months, one year (training conclusion), and one-year post-training with the Mullen Scales of Early Learning (MSEL), the Color-Object Association Test (COAT) for memory, the Early Childhood Vigilance Test (ECVT) of attention, and the Behavior Rating Inventory of Executive Function (BRIEF-parent). The Caldwell HOME was completed by observers to gauge caregiving quality after training. Caregiver depression/anxiety (Hopkins Symptom Checklist-25) and functionality (list of activities of daily living) were also evaluated. Data collectors were blinded to trial arm assignment.
Results
MISC resulted in significantly better quality of caregiving compared to UCOBAC mid-intervention with an adjusted mean difference (MadjDiff ) of 2.34 (95% CI: 1.54, 3.15, p<0.01), post intervention (MadjDiff=2.43, 95% CI: 1.61, 3.25, p<0.01) and at one year follow-up (MadjDiff=2.07, 95% CI: 1.23, 2.90, p<0.01). MISC caregivers reported more problems on the BRIEF for their child at one-year post-training only (p<0.01). Caregiving quality (HOME) was significantly correlated with MSEL composite performance one-year post training for both the MISC and the UCOBAC trial arms. Likewise, physical growth was significantly related to child development outcomes even though it did not differ between trial arms.
Conclusions
Even though MISC demonstrated an advantage of improving caregiving quality, it did not produce better child cognitive outcomes compared to health and nutrition training.
Keywords: HIV, Caregiving, Child Development, Mental Health, Attention, Memory, Behavior, Executive Function, Africa, Home Environment
INTRODUCTION
Exposure to poverty-related cumulative risk in early childhood negatively affects cognitive and emotional developmental trajectories due in part to limited cognitive stimulation and poor nutrition.1–3 The Lancet published a seminal series on this topic in 2007.3, 4,5 In a second series in 2016, “Advancing Early Childhood Development: From Science to Scale”,1, 6–10 45 experts from dozens of global institutions were brought together to review the latest early childhood development intervention strategies in low and middle income countries (LMICs).7, 8 From this literature, the WHO/UNICEF sponsored Care for Child Development (CCD) package emerges as the most widely used manualized program for early childhood development (ECD) intervention in LMICs (https://www.unicef.org/earlychildhood/index_68195.html,).1, 6, 9 A core feature of CCD, and other similar integrated ECD intervention packages, is the use of caregiver training that focuses on promoting both child nutrition and cognitive stimulation typically via play interactions with home-made toys and learning materials.1, 11–13
Despite a high incidence of HIV infection among impoverished communities in some LMIC contexts and the developmental risks associated with exposure to HIV,14 this factor is often overlooked in evaluations of ECD program effectiveness.15 HIV exposed-uninfected children (HEU) have been found to be at increased risk of neurocognitive disturbances; 40% of HEU children in sub-Saharan Africa were identified as having general cognitive impairment. In addition, HEU children were found to be more likely to experience motor impairment (14%), delays in expressive language, and fine motor problems.16 These impairments could be due to perinatal exposure to the HIV virus or antiretroviral therapy (ART),17, 18 For instance antenatal HIV-exposure (without infection) may compromise HEU children’s cognitive development, when maternal immune activation negatively affects the developing fetal brain.19
Another possible mechanism of the neurocognitive deficits observed in HEU children is exposure to environmental factors already present in low-resource settings that can be exacerbated by maternal HIV infection. These include maternal psychological factors (e.g. depression), behavioral factors (e.g. compromised caregiving), and socioeconomic factors (e.g. poverty and orphanhood).20 Compromised quality of caregiving in particular can compound the already serious neurodevelopmental effects of HIV infection and exposure for these children, irrespective of the availability of medical treatment and care.1, 11–13 In western Africa, children of depressed mothers have been found to have elevated risk of morbidity and mortality as compared to those of non-depressed mothers.21, 22 Despite this, most studies have not evaluated the effects of ECD interventions on the mental health of the child’s mother or caregiver.6
The present study seeks to address these gaps in knowledge by comparing caregiver training comprised of a health and nutrition curriculum to a specific cognitive-stimulation strategy in a cluster-randomized controlled trial (RCT) on child cognitive and emotional development outcomes, as well as caregiver mental health and functional impairment. The population of focus for this RCT is young (i.e. under three years of age) HEU children born to HIV-infected mothers (i.e. exposed to HIV in utero) in a rural district of Uganda. To the authors’ knowledge, this is one of the few studies to attempt to disentangle the comparative child development and caregiver mental health benefits of the typically integrated components of CCD and ECD programs. Furthermore, the present study is the only one to do so while providing a nutritional supplement to all study children throughout the study. This enables us to compare the two training arms apart from the developmental benefits of better nutrition per se in an often overlooked yet highly at-risk population of young HEU children.23, 24
METHODS
Randomization and Masking
Perinatally HIV exposed but uninfected (HEU) children between two and three years of age having completed a malaria chemoprevention study25 (n=175) or prevention of mother-to-child transmission of HIV trial (n=46)26 were recruited successively to the present study from the Infectious Disease Research Collaboration (IDRC) at a District Hospital in Eastern Uganda. The study was designed as a cluster-randomized controlled trial; households from eighteen geographic clusters in Eastern Uganda in which participating dyads were grouped in distinct village or community settings were mapped using GPS technology. These clusters were subsequently randomized to one of two caregiver training treatment arms described below. Staff conducting the child assessments (blinded to cluster allocation) enrolled study participants across the sub-counties. The Institutional Review Boards (IRB) in Uganda and a US-based University approved this study.
Sample
A total of 221 eligible HEU children and their caregivers were enrolled between March 2012 and April 2014 and caregivers provided written informed consent for themselves and their child. Child eligibility was based on confirmed birth to an HIV-positive mother with the child confirmed as non-infected with enzyme-linked immunosorbent assay (ELISA) by the study medical officers. They also confirmed that the child had no history of neurological insult by reviewing IDRC medical records, conducting a physical exam, and administering the widely/used Ten-Question Questionnaire (TQQ) screening measure for neurodevelopmental disability.27 The caregiver exclusion criterion was having a serious mental health problem that would prevent engagement in the intervention.
Caregiver Training Interventions
Mediational Intervention for Sensitizing Caregivers (MISC)
MISC is a model for training caregivers to enhance their children’s cognitive development and is based on Feuerstein’s theory of cognitive modifiability.28–30 MISC was developed as a model for early intervention as a means for effecting flexibility or plasticity of the minds of young children.31 MISC was previously adapted to the Ugandan context by the authors and this study represents the first time it has been tested in a large trail with HEU children. Hour-long bi-weekly individual training sessions were conducted with caregivers over a period of one year. The trainings were delivered by one of four Ugandan University Psychology or Social-Work graduates trained and certified by the intervention developer and her colleagues. Caregiver-child dyad interactions were video-taped monthly and used, with role-playing, to teach caregivers how to focus their child (gain child’s attention and direct him/her to the intended learning experience); provide meaning (name objects, people, experiences and convey emotional excitement, appreciation, and affection during the experience); expand (make the child aware of how a learning experience transcends the present situation and how to using comparisons, analogies, and grouping descriptions to expand understanding); encourage (transmit a message of satisfaction regarding the child’s accomplishment while explaining the reason for success); and regulate (constructively direct and shape the child’s behavior in relation to specific task requirements ).28–30 MISC does not rely on outside resources, equipment, toys, or other educational materials. Trainings alternated between the caregiver’s home and the research office to provide opportunities for in-situ training (home) and videotape reviewing (office).
Uganda Community Based Association for Child Welfare program (UCOBAC)
The comparison caregiver training arm was a manualized nutrition and hygiene information program designed and originally implemented by the Uganda Community Based Association for Women & Child Welfare (UCOBAC; http://ucobac.org/) that met the minimum standard of care for families affected by HIV in Uganda. The UCOBAC curriculum is information-based and structured around 13-topics related to hygiene and sanitation practices, child nutrition and growth, communication and listening skills, family planning, sexually transmitted infections, and living with HIV/AIDS. The UCOBAC intervention had previously been implemented in Ugandan-based studies and the 4 field staff in the present study had comparable educational backgrounds to MISC trainers.32, 33 UCOBAC caregiver training was structured in a similar manner to MISC, with bi-weekly sessions for one year alternating between the dyad’s home and the study office.
All intervention providers received an initial two-week training in their respective intervention, followed by a weeklong refresher training held mid-way through the study. An intervention log was kept for both trial arms, noting the number of sessions and the material covered. In addition, MISC and UCOBAC caregivers received a bi-weekly nutritional support package for their family in the form of a locally-produced enriched porridge of millet, soya, sesame, peanuts, rice and sugar.
Measures and Outcomes
Study measures were administered at home (all caregiver outcomes and assessment of the quality of child and caregiver interactions) and at the study office (all child outcomes) in one of three local languages (Japhadola, Ateso, or Luganda) preferred by the participants. The translation process for these measures included independent back translation with reconciliation of differences made by group consensus. A team of 7 assessors with college degree education and fluent in at least 2 of the 3 local languages received two weeks of training in their respective tests, and were blinded to study intervention arm. Testing sessions lasted between 30–90 minutes (depending on child age) and children were provided breaks and snacks as needed.
Data were collected at baseline, 6 months (midway through training), 1 year (post-training), and at a 12-month follow-up (2 years after baseline). Measures of caregiver mental health and all child outcomes were previously used in Uganda to assess the developmental benefit of the present caregiver training interventions.32, 33 They have also provided measures of child development related to quality of caregiving at baseline in the present study cohort.34 The present child development assessments have also proven sensitive to the effects of severe malaria in Ugandan children.35, 36
Demographics: child demographics were recorded at baseline and included age, sex, and physical growth (weight, height, upper-arm circumference). Caregivers reported on their age, marital status (married/unmarried), education (any/none), and relationship to the study child (mother/other).
Mullen Scales of Early Learning (MSEL)37 is a comprehensive test assessing specific developmental domains: visual reception, gross motor skills, fine motor skills, receptive language, and expressive language. A composite score derived from standardized t-scores of the four domains (excluding gross motor) provides a measure of g, the general measure of fluid intelligence thought to underlie general cognitive ability. The Mullen scales have previously been adapted for use with young children in rural Uganda and demonstrated high sensitivity when used in this population.38
Color Object Association Test (COAT)39 uses the placement of 4-inch square color-coded boxes with pictures and small, colorful familiar toys (e.g., ball, doll, book) to test associative memory. The principal outcomes are immediate memory (assessed by number of recalled items) and overall total recall (assessed by number of correctly placed items). The COAT has been previously adapted and used as a valid measure of memory for Ugandan children.32, 33
Early Childhood Vigilance Test (ECVT)40, 41 is an experimental measure of vigilance used in preschool children to evaluate sustained attention.24 Children are required to monitor a colorful computer screen on which active cartoon animal characters appear unpredictably at 5-to-15 second intervals. The principal outcome is the proportion of total time spent looking at the animation as scored from a video recorded from a computer-mounted webcam.
Behavior Rating Inventory of Executive Function–Preschool version (BRIEF-P)42 is a questionnaire assessing behavior, attention and cognitive problems related to disruption of executive functions as reported by the principal caregiver. Indices used in this trial include Inhibitory Self-Control, Flexibility (a combination of Shift and Emotional Control) and Emergent Metacognition (a combination of Working Memory and Plan/Organize). All three indices are combined into a Global Executive Composite (GEC) score.25
Home Observation for the Measurement of the Environment (HOME)43 is a composite measure designed to assess the quality and quantity of stimulation that the child is exposed to in their home environment. The Infant/Toddler version includes 45 yes/no items. A total HOME score was generated by summing the number of ‘yes’ responses; higher HOME scores indicate higher quality interactions.
Hopkins Symptoms Checklist-25 (HSCL)44, 45 has previously been used to assess depression and anxiety symptoms in Ugandan adults living with HIV. The HSCL-25 consists of two subscales: anxiety (10 items) and depression (15 items) symptoms. Caregivers indicated how frequently they experienced each symptom in the last two weeks on a scale of 0 (not at all) to 3 (a lot). Subscale scores were calculated by averaging item responses. Cronbach’s alpha at baseline was 0.85 for each of the two subscales.
Caregiver functional impairment for activities of daily living was measured using daily tasks identified during a brief qualitative study with local women. The 12 items relate to tasks women regularly do to care for themselves, their family, their community and their young child. Caregivers indicated how much difficulty they had completing each task, with responses ranging from 0 (no difficulty at all) to 4 (cannot complete). An impairment scale was calculated by averaging item responses. Cronbach’s alpha was 0.82 at baseline.
Analysis
During the design phase of the trial, the target enrolment was informed by a sample size calculated using the magnitude of effects seen in a prior study, in order to obtain 80% power.32 In a post-hoc analysis of achieved power given the 112 (MISC) and 109 (UCOBAC) children enrolled, an unadjusted effect size of 0.38 was detectable with 0.80 power in two-sided tests at 0.05 level of significance. In adjusted analyses with 3 repeated measures correlated at 0.70 and covariance adjustment for baseline, the detectable adjusted effect size was 0.21 for the time-averaged differences between arms and 0.27 for differences at each time.
Baseline intervention arm comparisons were performed using t-, chi-square or Fisher’s exact tests as appropriate. To adjust for any differences found, the corresponding variables were included as covariates in subsequent analyses. Linear mixed effects (LME) models were employed to analyze outcomes at 6, 12 and 24 months while adjusting for baseline. With this strategy, those with at least one completed assessment after baseline were included (n=17 dyads excluded, 9 in the MISC arm, and 8 in the UCOBAC arm; see Figure 1). Correlations arising from repeated measures were accounted for by specifying an autoregressive covariance structure. Inclusion of a random effect for sub-county (unit of randomization) was explored, but the resulting intra-class correlation coefficients were virtually zero across outcomes indicating that the cluster randomization did not result in an appreciable dependence of outcomes within clusters. Primary outcomes were pre-specified in the trial design and included MSEL and caregiver mental health symptoms and functional problems as they pertained to daily activities of caregiving for their families.
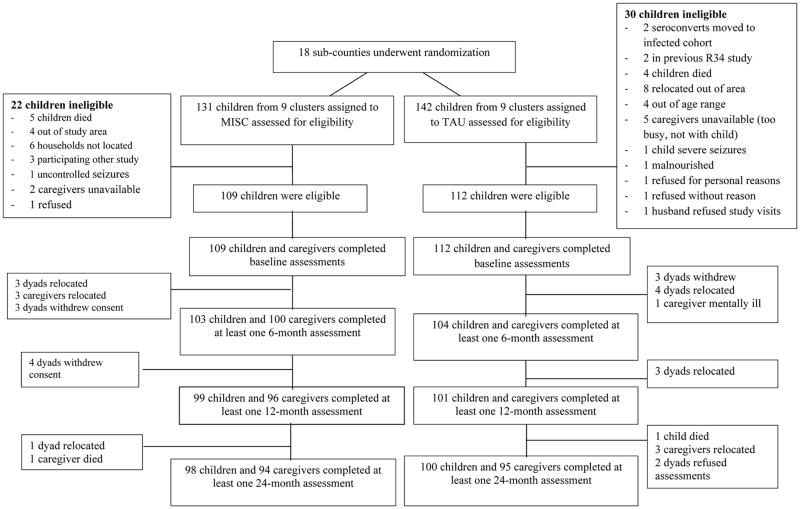
Figure 1.
This is the CONSORT diagram for the cluster randomization of dyads to either the Mediational Intervention for Sensitizing Caregivers (MISC) caregiver training intervention for child cognitive stimulation, and the UCOBAC manualized healthy nutrition caregiver training curriculum.
Each outcome was analyzed separately using an LME model with common covariates selected due to association with the outcomes in prior study (child’s age, sex, and outcome value at baseline)12 or trial-arm difference in the variable at baseline despite randomization. To model potentially non-linear longitudinal patterns, follow-up time point was entered as a categorical variable. Time-by-intervention interactions were included to capture potential changes in differences by intervention arm over time. The least squares (adjusted) means for each time point and trial arm were output from the LME models, and difference. This included an exploratory investigation of moderation of intervention effects by baseline values of the outcome of interest. An example of this would be whether a child with a great deficit in receptive language was more or less likely to experience improvements in language relative to a child with less of a deficit at baseline. A trial arm by baseline value interaction was added to the LME model and differences in slopes by trial arm were evaluated. SAS 9.4 was used for all analyses.
RESULTS
Of the 273 caregiver-child dyads that underwent eligibility screening, 52 were excluded. Reasons for exclusion included the child in the dyad dying before study initiation (n=9), having uncontrolled seizures (n=2), being confirmed as living with HIV (n=2), experiencing severe malnutrition (n=1), and falling outside the study age range (n=4). In addition, 12 dyads were found to live outside the study catchment area; 6 dyads’ homes could not be located; 5 caregivers were participating in another study; 5 caregivers were unavailable; and 4 refused to participate. Of the 221 child-caregiver dyads who began the interventions, 207 children (94%) and 204 caregivers (92%) completed the mid-program assessment (6-months after baseline); 200 children (90%) and 197 caregivers (89%) completed the post-program assessment (12-months after baseline); and 198 children (90%) and 189 caregivers (85%) completed the follow-up assessment (24-months after baseline) (Figure 1). Reasons for study attrition were similar across the two trial arms, with relocation and study withdrawal being the most frequent due to loss of interest in the caregiver training program. The characteristics of dyads’ that dropped out were not significantly different by trial arm.
MISC and UCOBAC child-caregiver dyads were demographically similar in outcome scale scores at baseline (Table 1); only for the BRIEF scales did MISC children score worse than UCOBAC children. Study caregivers were on average 35 years of age, predominantly married (71%), the study child’s biological mother (89%), and had at least primary education (79%). At baseline, caregivers reported moderate mental health problems. Functional impairment was greater in the UCOBAC arm compared to MISC (Table 1).
Table 1.
Child and caregiver characteristics and outcomes at baseline by trial arm
| Characteristic | Mediational Intervention for Sensitizing Caregivers (MISC) N= 112 |
UCOBAC Health/Nutrition Curriculum N= 109 |
P-value for comparison by arm |
|---|---|---|---|
| Child | |||
| N (%) | N (%) | ||
| Sex | |||
| Male | 59 (54%) | 66 (58%) | |
| Female | 50 (46%) | 46 (42%) | 0.47 |
| Mean (St Dev) | Mean (St Dev) | ||
| Age in years | 2.87 (0.44) | 2.87 (0.40) | 0.99 |
| Height (cm) | 89.77 (6.88) | 90.12 (5.51) | 0.63 |
| Weight (kg) | 12.00 (1.90) | 12.89 (1.85) | 0.21 |
| Home score | 20.51 (3.55) | 20.63 (2.78) | 0.79 |
| Mullen composite | 70.50 (11.20) | 71.13 (11.20) | 0.68 |
| Mullen gross motor | 26.41 (3.41) | 26.55 (3.17) | 0.75 |
| Mullen fine motor | 33.88 (8.84) | 33.92 (8.78) | 0.97 |
| Mullen visual reception | 29.84 (8.74) | 29.42 (9.25) | 0.73 |
| Mullen receptive language | 38.02 (8.68) | 38.25 (7.97) | 0.84 |
| Mullen expressive language | 34.36 (9.58) | 36.87 (10.21) | 0.26 |
| BRIEF emergent metacognition | 66.45 (13.88) | 61.67 (12.50) | 0.01 |
| BRIEF inhibitory self-control | 67.58 (12.27) | 63.12 (11.47) | <0.01 |
| BRIEF flexibility | 60.45 (12.47) | 57.39 (10.94) | 0.05 |
| BRIEF global executive function | 67.87 (13.70) | 63.00 (12.45) | <0.01 |
| COAT immediate recall | 3.46 (3.18) | 3.60 (3.14) | 0.75 |
| COAT total recall | 7.30 (8.57) | 7.22 (7.79) | 0.94 |
| ECVT percent of time looking | 68.55 (17.36) | 68.66 (19.08) | 0.90 |
| Caregiver | |||
| N(%) | N(%) | ||
| Relationship to child* | 0.06 | ||
| Mother | 94 (87%) | 101 (94%) | |
| Other | 14 (13%) | 6 (6%) | |
| Marital status* | 0.05 | ||
| Married | 74 (69%) | 78 (73%) | |
| Other | 34 (31%) | 29 (27%) | |
| Education level* | 0.77 | ||
| No education | 24 (22%) | 21 (19%) | |
| Some education | 83 (78%) | 87 (81%) | |
| Caregiver age in years | 35.84 (8.90) | 34.28 (7.38) | 0.16 |
| Caregiver depression symptoms | 1.04 (0.51) | 0.94 (0.55) | 0.17 |
| Caregiver anxiety symptoms | 0.96 (0.71) | 0.85 (0.63) | 0.21 |
| Caregiver functional impairment | 0.25 (0.30) | 0.42 (0.47) | <0.01 |
some data are missing
Child Outcomes
Children in both interventions experienced positive developmental and cognitive development changes (adjusted outcome findings presented in Table 2, unadjusted findings presented in Supplemental Figures 1 and 2). There were no growth differences (height or weight adjusted for age and gender) between MISC and UCOBAC children across the four assessment points (baseline, 6 months, 12 months, 24 months), indicating that the nutritional supplement provided to all study children across both caregiver training treatment arms had a comparable benefit on physical growth throughout the two-year study period. MSEL scores are presented age-standardized, so a decrease is interpreted as study children on average not making development gains on a similar trajectory as children from high-income countries, on which standardized scores are based (Supplemental Figure 1). There were no differences between-trial arms on MSEL scores during follow up. MISC children had gains on receptive language scores mid- and post-intervention (Supplemental Figure 1, upper right graph), but this difference was not statistically significant at either time point (p=0.10) and was not sustained at 12-month follow-up (Table 2). At post-program assessment, MISC children had significantly worse (higher) BRIEF flexibility, inhibitory self-control subscales, and global executive function scores than UCOBAC (Table 2). All of these differences were maintained at the final follow up. There were no other statistically significant effects of MISC on child outcomes relative to UCOBAC (Table 2). However, it is very important to note that both weight and height (adjusted for age and gender) were significantly related to all of the principal child development outcomes in the adjusted multiple regression models presented in Table 2, although they did not differ significantly between MISC versus UCOBAC caregiver training arms (Table 1) since all of the children in both treatment arms received the monthly nutritional supplement.
Table 2.
Child outcomes: Least square (LS) means, their standard errors and 95% confidence intervals for their differences at mid-program, immediately post program, and at 12 month follow-up adjusted for child’s age, sex, outcome score at baseline, BRIEF global executive function at baseline, and caregiver lack of functionality at baseline
| MISC LS Mean (SE) | UCOBAC LS Mean (SE) | Adjusted mean difference (95% confidence interval) | P-value for comparison by arm | |
|---|---|---|---|---|
| Home score | ||||
| Mid program | 22.99 (0.29) | 20.65 (0.20) | 2.34 (1.54, 3.15) | <0.01 |
| Immediately post program | 23.84 (0.29) | 21.40 (0.29) | 2.43 (1.61, 3.25) | <0.01 |
| 12 month follow up | 23.40 (0.30) | 21.24 (0.29) | 2.07 (1.23, 2.90) | <0.01 |
| Mullen composite | ||||
| Mid program | 70.47 (0.97) | 69.04 (0.96) | 1.43 (−1.28, 4.14) | 0.30 |
| Immediately post program | 71.14 (1.00) | 68.97 (0.97) | 2.17 (−0.59, 4.93) | 0.12 |
| 12 month follow up | 65.24 (1.00) | 65.20 (0.98) | 0.04 (−2.73, 2.82) | 0.97 |
| Mullen gross motor | ||||
| Mid program | 28.80 (0.31) | 29.11 (0.31) | −0.31 (−1.17, 0.55) | 0.48 |
| Immediately post program | 31.01 (0.32) | 31.13 (0.31) | −0.12 (−0.99, 0.76) | 0.80 |
| 12 month follow up | 33.37 (0.32) | 33.61 (0.31) | −0.24 (−1.12, 0.64) | 0.59 |
| Mullen fine motor | ||||
| Mid program | 32.68 (0.88) | 31.15 (0.87) | 1.53 (−0.93, 3.99) | 0.22 |
| Immediately post program | 31.86 (0.91) | 31.08 (0.88) | 0.78 (−1.73, 3.29) | 0.54 |
| 12 month follow up | 29.87 (0.90) | 30.49 (0.89) | −0.61 (−3.14, 1.91) | 0.63 |
| Mullen visual reception | ||||
| Mid program | 30.65 (0.83) | 29.30 (0.82) | 1.35 (−0.96, 3.66) | 0.25 |
| Immediately post program | 32.18 (0.85) | 30.70 (0.83) | 1.48 (−0.88, 3.84) | 0.21 |
| 12 month follow up | 27.29 (0.85) | 27.07 (0.84) | 0.22 (−2.15, 2.59) | 0.85 |
| Mullen receptive language | ||||
| Mid program | 37.99 (0.68) | 36.40 (0.67) | 1.59 (−0.31, 3.49) | 0.10 |
| Immediately post program | 35.67 (0.70) | 34.04 (0.68) | 1.63 (−0.31, 3.57) | 0.10 |
| 12 month follow up | 28.75 (0.70) | 28.97 (0.69) | −0.23 (−2.17, 1.72) | 0.82 |
| Mullen expressive language | ||||
| Mid program | 34.62 (0.93) | 35.54 (0.92) | −0.93 (−3.52, 1.67) | 0.48 |
| Immediately post program | 38.07 (0.96) | 36.48 (0.94) | 1.59 (−1.07, 4.25) | 0.24 |
| 12 month follow up | 37.60 (0.96) | 36.22 (0.95) | 1.38 (−1.29, 4.05) | 0.31 |
| BRIEF emergent metacognition | ||||
| Mid program | 61.27 (1.16) | 58.49 (1.15) | 2.79 (−0.45, 6.03) | 0.09 |
| Immediately post program | 60.50 (1.29) | 56.16 (1.16) | 4.33 (1.03, 7.64) | 0.01 |
| 12 month follow up | 55.46 (1.19) | 53.19 (1.17) | 2.27 (−1.05, 5.59) | 0.18 |
| BRIEF inhibitory self-control | ||||
| Mid program | 62.30 (1.07) | 58.67 (1.06) | 3.63 (0.64, 6.62) | 0.02 |
| Immediately post program | 61.11 (1.10) | 55.20 (1.07) | 5.91 (2.86, 8.96) | <0.01 |
| 12 month follow up | 58.17 (1.10) | 53.41 (1.08) | 4.76 (1.69, 7.82) | <0.01 |
| BRIEF flexibility | ||||
| Mid program | 56.79 (1.04) | 53.88 (1.03) | 2.91 (0.00, 5.82) | 0.05 |
| Immediately post program | 56.16 (1.07) | 51.94 (1.04) | 4.22 (1.26, 7.19) | 0.01 |
| 12 month follow up | 53.84 (1.07) | 49.19 (1.05) | 4.65 (1.67, 7.63) | <0.01 |
| BRIEF global executive function | ||||
| Mid program | 62.46 (1.15) | 58.84 (1.14) | 3.62 (0.41, 6.84) | 0.03 |
| Immediately post program | 61.16 (1.18) | 55.68 (1.15) | 5.47 (2.20, 8.75) | <0.01 |
| 12 month follow up | 56.82 (1.18) | 52.71 (1.16) | 4.11 (0.82, 7.40) | 0.01 |
| COAT immediate recall | ||||
| Mid program | 4.68 (0.34) | 4.27 (0.34) | 0.41 (−0.54, 1.36) | 0.39 |
| Immediately post program | 5.49 (0.35) | 5.38 (0.34) | 0.11 (−0.86, 1.08) | 0.82 |
| 12 month follow up | 5.78 (0.35) | 6.14 (0.34) | −0.37 (−1.34, 0.61) | 0.46 |
| COAT total recall | ||||
| Mid program | 9.00 (1.00) | 8.40 (0.99) | 0.60 (−2.19, 3.38) | 0.67 |
| Immediately post program | 10.72 (1.03) | 11.76 (1.00) | −1.04 (−3.89, 1.82) | 0.48 |
| 12 month follow up | 12.68 (1.03) | 14.57 (1.01) | −1.88 (−4.74, 0.97) | 0.20 |
| ECVT percent looking | ||||
| Mid program | 64.45 (1.89) | 63.84 (1.82) | 0.61 (−4.57, 5.79) | 0.82 |
| Immediately post program | 69.29 (1.89) | 73.29 (1.85) | −4.00 (−9.25, 1.24) | 0.13 |
| 12 month follow up | 75.03 (1.92) | 78.53 (1.87) | −3.50 (−8.81, 1.82) | 0.20 |
Caregiver Outcomes
MISC caregivers reported non-significantly lower levels of functional impairment at 12 month follow up with an adjusted mean difference of −0.08 (95% CI: −0.18, 0.01, p=0.10) (Table 3; Supplemental Figure 3, upper left graph). Caregiving quality, as reflected by HOME score, was greater among women in MISC throughout follow up (Table 3; Supplemental Figure 3, lower right graph). HOME score was significantly associated with MSEL composite score regardless of the trial arm, with an estimated 0.37 (standard error 0.14) increase in MSEL composite score per one unit increase in HOME score (p<0.01). However, unadjusted caregiver depression and anxiety scores were higher for MISC than for UCOBAC caregivers at all assessment points, with UCOBAC caregivers reporting less depressive symptoms throughout training and at one-year follow-up (F(1,187)=5.32;p<0.022) (Supplemental Figure 3, upper left and lower right graphs).
Table 3.
Caregiver outcomes: Least square (LS) means, their standard errors and 95% confidence intervals for their differences at mid-program, immediately post program, and at 12 month follow-up adjusted for child’s age, sex, outcome score at baseline, BRIEF global executive function at baseline, and caregiver functional impairment at baseline
| MISC LS Mean (SE) | UCOBAC LS Mean (SE) | Adjusted mean difference (95% confidence interval) | P-value for comparison by arm | |
|---|---|---|---|---|
| Caregiver depression symptoms | ||||
| Mid program | 1.01 (0.05) | 0.90 (0.05) | 0.11 (−0.04, 0.25) | 0.17 |
| Immediately post program | 1.00 (0.05) | 0.89 (0.05) | 0.11 (−0.04, 0.26) | 0.15 |
| 12 month follow up | 0.98 (0.05) | 0.85 (0.05) | 0.14 (−0.01, 0.29) | 0.08 |
| Caregiver anxiety symptoms | ||||
| Mid program | 0.85 (0.06) | 0.83 (0.06) | 0.03 (−0.15, 0.20) | 0.77 |
| Immediately post program | 0.89 (0.06) | 0.88 (0.06) | 0.94 (−0.17, 0.19) | 0.94 |
| 12 month follow up | 0.95 (0.07) | 0.82 (0.06) | 0.13 (−0.05, 0.31) | 0.17 |
| Caregiver functional impairment | ||||
| Mid program | 0.25 (0.04) | 0.27 (0.03) | −0.02 (−0.12, 0.07) | 0.56 |
| Immediately post program | 0.27 (0.03) | 0.27 (0.04) | 0.00 (−0.09, 0.09) | 0.94 |
| 12 month follow up | 0.18 (0.03) | 0.26 (0.03) | −0.08 (−0.18, 0.01) | 0.10 |
Exploratory Moderation Analyses
No significant interactions were found between values of study outcomes at baseline and trial arm for any child or caregiver outcome (data not presented in tables). For the MSEL composite, a difference in slopes by trial arm of 0.16 (standard error 0.09, p=0.06,) indicated that the effect of MISC on improving MSEL composite score was greater for children who entered with a relatively higher MSEL composite score. However, HOME caregiving quality was significantly related to MSEL composite score at one-year follow-up post training for both the MISC (r(91)=0.24, p=0.02) and UCOBAC (r(95)=0.27, p=0.008) trial arms. The significant correlations between HOME caregiving quality and MSEL composite at one-year follow-up for both trial arms did not seem to be mediated at all by caregiver functionality.
DISCUSSION
This randomized controlled trial compared the child neurodevelopmental and caregiver mental health and functionality benefits of two caregiver interventions; a health and nutrition (UCOBAC) curriculum versus a childhood cognitive stimulation (MISC) model. Compared to children in UCOBAC, MISC children generally scored better across measures of child neurocognitive development, but differences by trial arm were not statistically significant over the course of the one-year caregiver training period or at a 12-month post training follow up. These findings suggest that both of types of caregiver training (health/nutrition and cognitive stimulation) may be beneficial and should be retained along with nutritional supplementation in standard integrated CCD intervention packages.
MISC training did not result in significantly greater gains compared to the UCOBAC training arm on the MSEL cognitive performance composite or COAT object-placement learning outcomes post-training, as was the case with both HIV-infected and HIV-exposed Ugandan children in previous studies.32, 33 The greatest differences between children in the MISC and UCOBAC arms were observed in the domain of child language acquisition, particularly receptive language. Along with findings from a similar MISC cluster RCT study with HIV-infected children and caregivers, these results demonstrate that MISC can enhance child language by promoting everyday interactions in the home. Studies from high-income countries have shown that poor language skills in early grades may lead to frustration, avoidance and a negative attitude towards school and literacy.46 Oral language development, along with the ability to hear and record sounds, has also been demonstrated as a strong predictor of writing development, which in turn is important for success at school in general. With evidence from high-income countries suggesting that language and literacy skills are instrumental to success in the first years of, future research should assess if enhanced language development due to MISC results in better preparing HEU children for school.
An interesting exception to the trend of non-statistically significant differences in neurocognitive development outcomes between trial arms was greater report of child problems by MISC caregivers at one-year follow-up on the BRIEF-P scale. We have previously found that HIV-affected depressed mothers in this setting are more likely to report child emotional and behavioral problems.47, 48 In the present study, significantly higher reported behavior problems on the BRIEF-P for MISC children at one-year follow-up could have been due to MISC training resulting in increased caregiver sensitivity to their children’s behavior and their own behavioral expectations of their children compared to the health and nutrition focused curriculum.
We found a positive association between child weight- and height-for age with all of the principal neurodevelopmental outcomes assessed at endpoint, and these measures were significantly below WHO normative means for the present cohort of children. Previous studies also found that wasting and stunting were independently associated with poorer psychomotor and neurodevelopmental outcomes.32, 33, 49 Treating these nutritional deficiencies through the year of caregiver training and subsequent one-year follow-up, likely provided a significant developmental benefit to children in both MISC/UCOBAC treatment arms. This suggests that providing for the nutritional needs of HIV-affected children is paramount in optimizing the benefits of ECD intervention in HIV-affected populations in sub-Saharan Africa.
MISC caregivers reported significantly more depression symptoms compared to those who participated in the UCOBAC health and nutrition training. This finding is in contrast with findings from previous studies of MISC training for caregivers of children living with HIV.33 It also is not consistent with an independent evaluation of a parent-directed intervention in northern Uganda15 where caregivers in a parent training compared to those in control conditions reported less depression. Caregivers in MISC did however report less functional impairment than those in UCOBAC. Some of the non-significant but better developmental outcomes observed among MISC children or the improvements caregivers experienced in the quality of their interactions with their children could have led to less functional impairment. Caregivers in this context have described a complex interrelationship between their well-being and their children’s, in which caring for children who are unwell was seen as interfering with one’s ability to provide for the child and their family.34, 50, 51 For mothers whose caregiving duties are made complicated by living with the physical, economic, and social effects of HIV, this is an especially important benefit of an ECD intervention.
In the present study, improvements in caregiver mental health were observed as related to improved caregiver functionality. Improved functionality, especially for mothers coping with HIV disease, can mediate the benefits of improved caregiving on child development, as has been documented in an earlier report.34, 50, 51 Therefore, it is important to monitor the mental health benefits of caregiver training interventions as important potential mediators of how such training might enhance the development of at-risk children.
The main limitation of the present study was absence of a true control condition. The choice to compare two active interventions was made because we did not feel we could ethically withhold both the MISC and UCOBAC interventions to the present study dyads, given the developmental risks experienced by HIV-affected children in previous reported findings of effectiveness of ECD programs for children in such settings.11, 15 Given the study period, substantial intervention duration, and the importance of assessing sustainment of any immediate intervention effects, dyads randomized to a wait-list control would be denied support services for a substantial period of time during a particularly sensitive window for child development. The present cluster RCT study, however, is a rigorous comparison of two different caregiver training interventions in which (1) a nutritional supplement was provided to all study children throughout the study; and, (2) the interventions in both study arms were of comparable structure (in terms of the quality of training sessions and the psychosocial support dimensions provided to the caregivers) and duration. Therefore, any developmental differences observed by treatment arm in this study were likely due to the content of the training, rather than improved child nutrition or interaction with support services per se.
A second limitation is that, due to the standardization of measures of cognitive development being completed among children in high-income settings, it is difficult to interpret the absolute scores of children in both study arms. In addition, the lack of a long-term post-intervention follow up assessments limits our inferences about the relative effects of these two interventions to the early childhood period. Given prior findings in this setting on the association of poor caregiving quality with neurocognitive developmental deficits among HIV-affected children,34 an important avenue of further research is whether or not the significant effect of MISC on caregiving quality in the short term may mediate longer-term improvements in child developmental trajectories.
Conclusion
In high-income countries, early childhood programming often brings together children, parents and early childhood educators in school-based settings to provide formal pre-school services. In many low- and middle-income countries (LMIC), formal pre-school programming is lacking, resulting in a lack of opportunities for children to gain skills for entering school ready to learn. Parenting programs that are delivered in the community may be a more appropriate delivery opportunity for supporting early childhood development in these contexts. With the most substantial but non-significant effects of MISC observed for language skills, the present study’s findings suggest that interaction-intensive caregiver training programs may be a useful community based approach for enhancing this domain of early child development for HIV-affected impoverished children. Diminished language skills is an especially potent risk factor for long-term success of HIV exposed children, as entering school at a learning disadvantage may persist through middle childhood and lead to reduced opportunities for future education and being able to prosper and give back to their communities. With this in mind, more studies of this sort are needed within dissemination and implementation science so that we might better understand the contributions of good nutrition, good parenting, good stimulation, and good caregiver mental health and functionality – to overall better child development in resource-constrained settings.
Supplementary Material
Acknowledgments
Funding: NIH grant R01 HD070723
This research was supported by NIH grant RO1 HD070723 (PIs: Boivin, Bass). None of the authors have any conflicts of interest or financial interests to disclose. S.M. Murray was supported by NIMH Global Mental Health training grant (MH103210) The study sponsor had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The University of Michigan Medical School Global Reach program and the Michigan State University College of Human Medicine and College of Osteopathic Medicine provided summer stipends to support summer research internships for a number of students during the study period, and the support of these programs and participation of these medical students are greatly appreciated. This effort is dedicated to Professor Pnina Klein (1945 – 2014), who dedicated her professional life to the development and promotion of the Mediational Intervention for Sensitizing Caregivers (MISC), and without whose efforts this study would never have been possible.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT01640561
Disclosures: None of the authors have any conflicts of interest or financial interests to disclose.
Author Disclosures: None of the authors have any conflicts of interest or financial disclosures with respect to this work.
Author Contributions
Michael J. Boivin, PhD – (Corresponding author) Study PI, shared oversight over all phases of study design and implementation and analyses, wrote the first draft of the manuscript, and approved the manuscript as submitted.
Noeline Nakasujja, PhD – Ugandan Co-PI, responsible for psychiatric care and referral, Ugandan IRB submissions, and approved the manuscript as submitted.
Itziar Familiar-Lopez, PhD – On-site scientific director of study, contributed to the writing of the manuscript, and approved the manuscript as submitted.
Sarah M. Murray, PhD – Responsible for finalization of all study protocols and standard operating procedures, caregiver assessment validations, participated in writing the manuscript, and approved the manuscript as submitted.
Alla Sikorskii, PhD – Responsible for all statistical analyses and tables and approved the manuscript as submitted.
Jorem Awadu, MS -- Led the on-site and clinic assessment teams and outcomes scoring, supervised quality assurance for the developmental assessment and database for the caregiver training logs, supported data management, and approved the manuscript as submitted.
Cilly Shohet, PhD -- Responsible for adaptation of MISC to study context and training and certification of MISC research assistants, support of MISC training team, and approved the manuscript as submitted.
Deborah Givon, MS -- Responsible for adaptation of MISC to study context and training and certification of MISC research assistants, support of MISC training team, and approved the manuscript as submitted.
Horacio Ruiseñor-Escudero, PhD -- Provided the UCOBAC training and re-worked the manual for this training curriculum, supported study supervision and data management and helped organize the district cluster map for randomization to treatment arms, and approved the manuscript as submitted.
Elizabeth E. Schut, MD – Contributed to medical care of children, support of study medical care staff, data management and support, and approved the manuscript as submitted.
Robert O. Opoka, MMed – Negotiated use of facilities at Tororo District Hospital, responsible for management and oversight of Tororo-based research staff and intervention providers, and approved the manuscript as submitted.
Judith K. Bass, PhD – Study co-PI, shared oversight over all aspects and phases of study design and implementation, participated in the initial drafting and further revisions of manuscript, and approved the manuscript as submitted.
References
- 1.Black MM, Walker SP, Fernald LCH, et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389:77–90. doi: 10.1016/S0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grantham-McGregor S. What is the best design for early childhood interventions? Dev Med Child Neurol. 2016;58:222–223. doi: 10.1111/dmcn.12951. [DOI] [PubMed] [Google Scholar]
- 3.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 5.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378:1325–1338. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 6.Britto PR, Lye SJ, Proulx K, et al. Nurturing care: promoting early childhood development. Lancet. 2017;389:91–102. doi: 10.1016/S0140-6736(16)31390-3. [DOI] [PubMed] [Google Scholar]
- 7.Daelmans B, Darmstadt GL, Lombardi J, et al. Early childhood development: the foundation of sustainable development. Lancet. 2017;389:9–11. doi: 10.1016/S0140-6736(16)31659-2. [DOI] [PubMed] [Google Scholar]
- 8.Lo S, Das P, Horton R. A good start in life will ensure a sustainable future for all. Lancet. 2017;389:8–9. doi: 10.1016/S0140-6736(16)31774-3. [DOI] [PubMed] [Google Scholar]
- 9.Richter LM, Daelmans B, Lombardi J, et al. Investing in the foundation of sustainable development: pathways to scale up for early childhood development. Lancet. 2017;389:103–118. doi: 10.1016/S0140-6736(16)31698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shawar YR, Shiffman J. Generation of global political priority for early childhood development: the challenges of framing and governance. Lancet. 2017;389:119–124. doi: 10.1016/S0140-6736(16)31574-4. [DOI] [PubMed] [Google Scholar]
- 11.Grantham-McGregor SM, Fernald LC, Kagawa RM, Walker S. Effects of integrated child development and nutrition interventions on child development and nutritional status. Ann N Y Acad Sci. 2014;1308:11–32. doi: 10.1111/nyas.12284. [DOI] [PubMed] [Google Scholar]
- 12.Yousafzai AK, Aboud F. Review of implementation processes for integrated nutrition and psychosocial stimulation interventions. Ann N Y Acad Sci. 2014;1308:33–45. doi: 10.1111/nyas.12313. [DOI] [PubMed] [Google Scholar]
- 13.Yousafzai AK, Rasheed MA, Rizvi A, et al. Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster-randomised factorial effectiveness trial. Lancet. 2014;384:1282–1293. doi: 10.1016/S0140-6736(14)60455-4. [DOI] [PubMed] [Google Scholar]
- 14.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singla DR, Kumbakumba E, Aboud FE. Effects of a parenting intervention to address maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster randomised trial. Lancet Glob Health. 2015;3:e458–469. doi: 10.1016/S2214-109X(15)00099-6. [DOI] [PubMed] [Google Scholar]
- 16.Alcock KJ, Abubakar A, Newton CR, Holding P. The effects of prenatal HIV exposure on language functioning in Kenyan children: establishing an evaluative framework. BMC Res Notes. 2016;9:463. doi: 10.1186/s13104-016-2264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dyke RB, Chadwick EG, Hazra R, et al. The PHACS SMARTT Study: Assessment of the Safety of In Utero Exposure to Antiretroviral Drugs. Front Immunol. 2016;7:199. doi: 10.3389/fimmu.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr SJ, Puthanakit T, Vibol U, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. 2014;26:1327–1335. doi: 10.1080/09540121.2014.920949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirois PA, Huo Y, Williams PL, et al. Safety of perinatal exposure to antiretroviral medications: developmental outcomes in infants. Pediatr Infect Dis J. 2013;32:648–655. doi: 10.1097/INF.0b013e318284129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ. 2011;89:608–615. doi: 10.2471/BLT.11.088187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bindt C, Appiah-Poku J, Te Bonle M, et al. Antepartum depression and anxiety associated with disability in African women: cross-sectional results from the CDS study in Ghana and Côte d’Ivoire. PLoS ONE. 2012;7:e48396. doi: 10.1371/journal.pone.0048396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weobong B, ten Asbroek AHA, Soremekun S, et al. Association between probable postnatal depression and increased infant mortality and morbidity: findings from the DON population-based cohort study in rural Ghana. BMJ Open. 2015;5:e006509. doi: 10.1136/bmjopen-2014-006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellins CA, Elkington KS, Leu CS, et al. Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care. 2012;24:953–962. doi: 10.1080/09540121.2012.668174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellins CA, Elkington KS, Leu CS, et al. Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care. 2013;24:953–962. doi: 10.1080/09540121.2012.668174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamya MR, Kapisi J, Bigira V, et al. Efficacy and safety of three regimens for the prevention of malaria in young HIV-exposed Ugandan children: a randomized controlled trial. AIDS. 2014;28:2701–2709. doi: 10.1097/QAD.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquez C, Okiring J, Chamie G, et al. Increased morbidity in early childhood among HIV-exposed uninfected children in Uganda is associated with breastfeeding duration. J Trop Pediatr. 2014;60:434–441. doi: 10.1093/tropej/fmu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durkin MS, Gottlieb CA, Maenner MJ, et al. Monitoring child disability in developing countries: results from the Multiple Indicator Cluster Surveys. New York: UNICEF; 2008. [Google Scholar]
- 28.Klein P. More Intelligent and Sensitive Child. Ramat-Gan, Israel: Bar-Ilan University; 1985. [Google Scholar]
- 29.Klein P. Improving the quality of parental interaction with very low birth weight of children: a longitudinal study using mediated experience model. Infant Mental Health Journal. 1991;12:321–337. [Google Scholar]
- 30.Klein P. Early Intervention: Cross-cultural experiences with a mediational approach. New York, NY: Garland Press; 1996. [Google Scholar]
- 31.Klein P, editor. Seeds of hope: twelve years of early intervention in Africa. Oslo, Norway: unipub forlag; 2001. [Google Scholar]
- 32.Boivin MJ, Bangirana P, Nakasuja N, et al. A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr. 2013;34:269–278. doi: 10.1097/DBP.0b013e318285fba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boivin MJ, Bangirana P, Nakasujja N, et al. A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J Pediatr. 2013;163:1409–1416. doi: 10.1016/j.jpeds.2013.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bass JK, Nakasujja N, Familiar-Lopez I, et al. Association of caregiver quality of care with neurocognitive outcomes in HIV-affected children aged 2–5 years in Uganda. AIDS Care. 2016;28(Suppl 1):76–83. doi: 10.1080/09540121.2016.1146215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bangirana P, Opoka RO, Boivin MJ, et al. Neurocognitive domains affected by cerebral malaria and severe malarial anemia in children. Learning and Individual Differences. 2015 doi: 10.1016/j.lindif.2015.01.010. http://dx.doi.org/10.1016/j.lindif.2015.01.010. [DOI] [PMC free article] [PubMed]
- 36.Bangirana P, Opoka RO, Boivin MJ, et al. Severe malarial anemia is associated with longterm neurocognitive impairment. Clinical Infectious Diseases. 2014;59:336–344. doi: 10.1093/cid/ciu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullen EM. Mullen Scales of Early Learning: AGS Edition. Minneapolis, MN: American Guidance Services; 1995. [Google Scholar]
- 38.Busman RA, Page C, Oka E, et al. Factors contributing to the psychosocial adjustment of Ugandan preschool children with HIV/AIDS. In: Boivin MJ, Giordani B, editors. Neuropsychology of Children in Africa: Perspectives on Risk & Resilience. New York, NY: Springer Media & Business Publishing; 2013. pp. 95–115. [Google Scholar]
- 39.Jordan CM, Johnson AL, Hughes SJ, Shapiro EG. The Color Object Association Test (COAT): the development of a new measure of declarative memory for 18- to 36-month-old toddlers. Child Neuropsychology. 2008;14:21–41. doi: 10.1080/09297040601100430. [DOI] [PubMed] [Google Scholar]
- 40.Goldman DZ, Shapiro EG, Nelson CA. Measurement of vigilance in 2-year-old children. Developmental Neuropsychology. 2004;25:227–250. doi: 10.1207/s15326942dn2503_1. [DOI] [PubMed] [Google Scholar]
- 41.Ruff HA, Capozzoli M, Dubiner K, Parrinello R. A measure of vigilance in infancy. Infant Behavior & Development. 1990;13:1–20. [Google Scholar]
- 42.Gioia GA, Espy KA, Isquith PK. BRIEF-P Behavior Rating Inventory of Executive Function - Preschool Version: Professional Manual. Lutz, FL: Psychological Assessment Resources (PAR); 2003. [Google Scholar]
- 43.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas Press; 1979. [Google Scholar]
- 44.Bass JK, Neugebauer R, Clougherty KF, et al. Group interpersonal psychotherapy for depression in rural Uganda: 6-month outcomes: randomised controlled trial. Br J Psychiatry. 2006;188:567–573. doi: 10.1192/bjp.188.6.567. [DOI] [PubMed] [Google Scholar]
- 45.Bolton P, Bass J, Betancourt T, et al. Interventions for depression symptoms among adolescent survivors of war and displacement in northern Uganda: a randomized controlled trial. JAMA. 2007;298:519–527. doi: 10.1001/jama.298.5.519. [DOI] [PubMed] [Google Scholar]
- 46.Beyer T, Postert C, Muller JM, Furniss T. Prognosis and continuity of child mental health problems from preschool to primary school: results of a four-year longitudinal study. Child Psychiatry Hum Dev. 2012;43:533–543. doi: 10.1007/s10578-012-0282-5. [DOI] [PubMed] [Google Scholar]
- 47.Familiar I, Nakasujja N, Bass J, et al. Caregivers’ depressive symptoms and parent-report of child executive function among young children in Uganda. Learning and Individual Differences. 2014 doi: 10.1016/j.lindif.2015.01.012. http://dx.doi.org/10.1016/j.lindif.2015.01.012. [DOI] [PMC free article] [PubMed]
- 48.Familiar I, Ruisenor-Escudero H, Giordani B, et al. Use of the Behavior Rating Inventory of Executive Function and Child Behavior Checklist in Ugandan children with HIV or a history of severe malaria. J Dev Behav Pediatr. 2015;36:277–284. doi: 10.1097/DBP.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruiseñor-Escudero H, Familiar I, Nakasujja N, et al. Immunological correlates of behavioral problems in school-aged children living with HIV in Kayunga, Uganda. Global Mental Health. 2015;2:1–9. doi: 10.1017/gmh.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bass JK, Opoka R, Familiar I, et al. Randomized controlled trial of caregiver training for HIV-infected child neurodevelopment and caregiver well-being. AIDS. 2017 doi: 10.1097/QAD.0000000000001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray SM, Familiar I, Nakasujja N, et al. Caregiver mental health and HIV-infected child wellness: perspectives from Ugandan caregivers. AIDS Care. 2017;29:793–799. doi: 10.1080/09540121.2016.1263722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.