Abstract
Hypertension is a comorbidity that is present in the majority of end-stage renal disease patients on maintenance hemodialysis. This population is particularly unique because of the dynamic nature of blood pressure (BP) during dialysis. Modest BP decreases are expected in most hemodialysis patients, but intradialytic hypotension and intradialytic hypertension are two special situations that deviate from this as either an exaggerated or paradoxical response to the dialysis procedure. Both of these phenomena are particularly important because they are associated with increased mortality risk compared patients with modest decreases in BP during dialysis. While the detailed pathophysiology is complex, intradialytic hypotension occurs more often in patients prescribed fast ultrafiltration rates, and reducing this rate is recommended in patients that regularly exhibit this pattern. Patients with intradialytic hypertension have a poorly explained increase in vascular resistance during dialysis, but the consistent associations with extracellular volume overload point towards more aggressive fluid management as the initial management choices for these patients. This up to date review provides the most recent evidence supporting these recommendations as well as the most up to date epidemiologic and mechanistic research studies that have added to this area of dialysis management.
Introduction
Hypertension is common in chronic kidney disease patients, and it is nearly universal in those with end-stage renal disease (ESRD) on maintenance hemodialysis. BP variability both during and between dialysis treatments is expected in maintenance hemodialysis patients, and it has been established that the best BP metric to determine long term risk of complications including mortality is the average of BP measurements obtained between dialysis treatments1. The hemodialysis procedure provides an opportunity to improve BP in ESRD patients by offsetting extracellular volume increases encountered during the interdialytic period, but it introduces additional risks in the process. Even when asymptomatic, fluctuations in BP during dialysis can be either causative or reflective of both short term and long term complications. The pathophysiology of both intradialytic hypotension and intradialytic hypertension is complex, but there are some recommended strategies to minimize and ultimately manage these complications. The purpose of this review is to discuss the factors responsible for changing BP during dialysis and the implications of both intradialytic hypotension and intradialytic hypertension. Furthermore, this review will go into extensive detail on the pathophysiology and management of intradialytic hypertension.
General Hypertension Management in Hemodialysis Patients
The nearly universal presence of hypertension in hemodialysis patients extends from the predisposition of earlier stages of chronic kidney disease to have higher prevalence of hypertension than the general population2. The pathophysiology responsible for such a high prevalence in these patients is complex and includes perturbations in the renin-angiotensin-aldosterone system, endothelial cell dysfunction, arterial stiffness, and increased sympathetic nervous system activity. Hypertension becomes more common as kidney disease advances, and BP becomes more difficult to control. An additional aggravating factor associated with chronic kidney disease that contributes to hypertension is the development of extracellular volume expansion as glomerular filtration rate and salt/water excretion decline.
The recommended guidelines for hemodialysis patients to achieve a pre-dialysis systolic BP less than 140 mmHg or post-dialysis systolic BP less than 130mmHg3 are not based on evidence from mortality-driven clinical trials, but rather the fact that cardiovascular complications most commonly associated with increased mortality risk become more prevalent beyond these thresholds4. Both judicious fluid management and aggressive pharmacologic antihypertensive therapy play a role in managing hypertension. However, a major challenge in BP management in hemodialysis patients lies in frequent BP changes that occur between dialysis treatments as well as during the dialysis treatment itself. Because there are multiple phenotypes of hypertensive hemodialysis patients, the management approach should differ as well.
Intradialytic BP Patterns
From the end of one dialysis treatment until the beginning of the next dialysis treatment, there is typically an ongoing increase in systolic BP. While the degree of aortic stiffness strongly influences the how high BP is at any point during the interdialytic time period, the amount of interdialytic weight gain strongly influences the rate of rise of BP during this time5. Consequently, there is at least a modest association between the percentage of interdialytic weight gain between dialysis treatments and the pre-dialysis systolic BP6. High pre-dialysis BP, therefore, will frequently be reflective of acute extracellular volume expansion since the prior treatment. However, high post dialysis BP has been implicated as a reflection of chronic extracellular volume overload.
This premise was indirectly shown in retrospective analysis of the HEMO trial using intradialytic change in plasma protein concentration as a metric of plasma volume change7. In that study, smaller changes in plasma volume during dialysis were associated with high pre and high post dialysis BP. More recently, in one cross sectional study based on a single hemodialysis treatment per patient, the patients with the highest post-dialysis BP were shown to have the highest ratio of extracellular water/total body water measured before dialysis with bioimpedance spectroscopy8. Based on these studies, patients with high BP before dialysis need stricter fluid restriction, and patients with high post dialysis BP require a dry weight challenge. Depending on the given patient and overall intradialytic BP pattern, neither, either, or both of these conditions may exist.
Beyond the possible information that can be extrapolated from looking at either pre or post-dialysis BP measurements, there has been significant research conducted on the actual change in BP from pre to post dialysis. Most patients experience a decrease in BP during dialysis, and one moderately-sized cohort study showed average change in systolic BP from pre to post dialysis was a reduction of 13 mmHg9. That study further illustrated a dual slope decline in BP where the initial acute decrease in BP was relatively unaffected by ultrafiltration parameters, but the more prolonged decrease in BP for the remainder of the treatment was amplified in the context of more aggressive fluid removal. Despite this tendency, there were clearly patients whose BP increased during dialysis despite ultrafiltration taking place. Similarly, there were patients who had much more prominent decreases in BP during dialysis than the average 13 mmHg decrease. While further defined below, these patients with either intradialytic hypertension or intradialytic hypotension represent opposing ends of the spectrum of BP response to dialysis.
Studies individually comparing a rise in BP during dialysis or large decrease in BP during dialysis with more modest reductions have consistently shown both intradialytic hypertension and intradialytic hypotension to be associated with increased hospitalization and mortality10-12. More recently, a large cohort study including more than 100,000 hemodialysis patients established the lowest mortality risk was associated with intradialytic BP reductions around 15 mmHg13. A significant increase in mortality risk occurred with BP reductions exceeding 30 mmHg from pre to post dialysis or any increase in BP from pre to post dialysis. The recognition of the poor prognosis of both conditions has prompted further research so that we now have a better understanding of the likely responsible mechanisms and recommended management/prevention strategies.
Intradialytic Hypotension
The definition of intradialytic hypotension according to guidelines set forth by the National Kidney Foundation Kidney Disease Quality Outcomes Inititative is a reduction in systolic BP of 20 mmHg during dialysis or reduction in mean arterial pressure that occurs with common related symptoms (nausea, vomiting, lightheadedness, etc)14. The presentation can be quite dramatic in the dialysis unit with patients appearing acutely ill, but there are clearly important long term consequences of this as well. Shoji et al first demonstrated that among a cohort of dialysis patients followed for two years, those that died during follow up had significantly lower nadir systolic BP than the survivors during an single initial treatment at the beginning of the study12. In another cohort study, frequent episodes of intradialytic hypotension (more than 10 episodes over 10 months) were associated with significantly increased mortality risk compared to no intradialytic hypotension in unadjusted analyses; but, this failed to achieve statistical significance in fully adjusted models15. The aforementioned study by Park et al identified that the mortality risk began to significantly rise when systolic BP decreases exceeded 30 mmHg from pre to post dialysis13.
Following these studies a large observational study of a large cohort within a large dialysis provider along with retrospective analysis of patients in the HEMO study provided additional perspective on whether the nadir BP or the decrease in BP is more clinically significant16. This study specifically identified a nadir systolic BP of 90 mmHg as the primary mortality risk factor related to intradialytic hypotension, whereas as pre to post dialysis reductions of varying magnitude had no independent effect on mortality. Collectively, these studies highlight the negative impact a large decrease in BP has on patient outcomes. The absolute BP achieved is likely more clinically relevant than how much the BP decreases to get to that point.
A full discussion of all the mechanisms responsible for intradialytic hypotension are beyond the scope of this review, but summarized in Figure 1. It is critical, however, to include a discussion of how ultrafiltration and ultrafiltration rate specifically impact intradialytic hypotension because these variables can be easily modified. The Dinesh study9 showing a dual slope decrease in BP established that the slope of the acute decrease in BP was not significantly affected by different ultrafiltration volumes or ultrafiltration rates. One potential contributing factor to this observation is the overriding BP effect of osmolarity changes during the early portion of a hemodialysis treatment. At this point in time, the diffusion gradients of varying solutes in the blood and dialysate are high enough to cause rapid changes in the extracellular osmolarity as solutes such as urea are dialyzed off. Changing osmolarity may cause ultrafiltration-independent reductions in intravascular and extracellular volume as well as changes in release of the vasoconstrictor vasopressin17. The Dinesh study also showed that the latter BP slope during the majority of the dialysis treatment was influenced by fluid removal with larger ultrafiltration volumes and faster ultrafiltration rates inducing steeper declines in BP. This indicates that the initial reduction in BP may be unavoidable (and is likely beneficial in many cases), but the tendency for intradialytic hypotension later in the treatment can be minimized by prescribing ultrafiltration differently.
Figure 1.
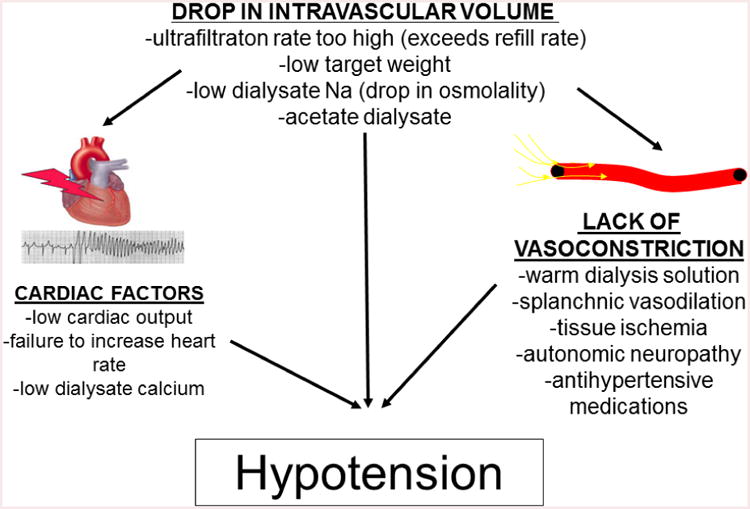
summarizes the important mechanisms contributing to intradialytic hypotension. The initial trigger for the decrease in blood pressure is a decrease in intravascular volume. This is achieved by the ultrafiltration rate exceeding the capacity for the interstitium to refill the intravascular space, and it is also achieved by the reduction in intravascular and extracellular osmolarity related to low dialysate sodium rapid reduction in urea the diffusion process of hemodialysis begins. Overt hypotension can occur if physiologic compensatory mechanisms to offset the decreased intravascular volume are inadequate. This includes are insufficient cardiac output or vascular resistance related to multiple host and comorbidity factors.
Ultrafiltration rates have emerged as an important and potentially modifiable risk factor in hemodialysis patients with evidence that high ultrafiltration rates are independently associated with poor outcomes. In retrospective analysis of the HEMO study, Flythe et al showed a significantly higher risk for all-cause and cardiovascular related mortality for ultrafiltration rates >13 mL/hr/kg compared to 10 mL/hr/kg18. High ultrafiltration rates correlate with more frequent cardiac regional wall motion abnormalities19, which are themselves independently associated with intradialytic hypotension and mortality20. Consequently, the initial approach to preventing intradialytic hypotension should include considering reduction of the ultrafiltration rate either by decreasing overall ultrafiltration volume or, more preferably, by lengthening the overall dialysis time.
Other plausible interventions to reduce intradialytic hypotension include lowering the dialysate temperature and/or the administration of a pre-dialysis dose of the alpha agonist midodrine. In one crossover study of patient's prone to intradialytic hypotension the use of either or both of these interventions resulted in higher nadir and post dialysis systolic BP21. Further investigation into dialysate cooling has shown potential long term benefits in minimizing cerebral injury22 and progression of cardiac dysfunction23. Other options include increasing dialysate sodium or calcium, but the short term benefits of these interventions must be carefully weighed against the long term risks which include the potential for chronic extracellular volume overload and positive calcium balance, respectively.
In summary, intradialytic hypotension occurs as an exaggerated decline in BP during dialysis. While large decreases in systolic BP are most frequently used to define intradialytic hypotension, recent evidence identifies the nadir systolic BP of 90 mmHg as having the strongest association with mortality. Excessive ultrafiltration rate is associated with both intradialytic hypotension and independently with mortality. The primary intervention to minimize intradialytic hypotension should involve a reduction in ultrafiltration rate, preferably by extending the overall weekly dialysis time. Dialysate cooling and/or midodrine also have objective benefits in not only stabilizing BP but also in slowing progression in end-organ damage.
Intradialytic Hypertension
The other side of the spectrum of intradialytic BP change is intradialytic hypertension. Intradialytic hypertension had previously been defined by arbitrary increases in systolic or mean arterial BP from pre to post dialysis. While BP decreases are the expected response, an increase in BP occurs in almost all hemodialysis patients from time to time. When considering a group of patients assessed during a single dialysis treatment or a cohort of patients followed for several months, an increase in systolic BP of at least 10 mmHg occurs in approximately 20% of all hemodialysis treatments8,24. However, the latter study24 established that these treatments are unequally distributed among a smaller subset of patients who experience this recurrently with 9% of all patients having an average change in systolic BP of +10 mmHg over the course of 6 months. Furthermore, 25% of patients in that study experienced a BP rise of this magnitude in almost 1/3 of all treatments. These findings establish that intradialytic hypertension is a chronic phenotype in a subset of hemodialysis patients.
The earliest studies showing the poor prognosis showed convincingly that an increase in systolic BP of at least 10 mmHg from pre to post dialysis was associated with increased hospitalization and/or mortality in both prevalent and incident hemodialysis patients10,11. Even when using the definition as an increase in systolic BP of at least 5 mmHg, another study confirmed the increase in both cardiac and all-cause mortality among patients with intradialytic hypertension25. The arbitrary nature of the exact definition of intradialytic hypertension is minimized by the findings from Park et al that any increase in BP during dialysis increases mortality risk13.
The large patient population from that study also provides an opportunity to better characterize the phenotype of an intradialytic hypertension patient. Table 1 provides a detailed assessment of these patients including older age, shorter dialysis vintage, increased cardiovascular comorbidities, lower body mass index, and lower amount of ultrafiltration during dialysis. Smaller case control studies had also identified the lower dry weight and smaller interdialytic weight gain in patients with intradialytic hypertension11. Taken along with the fact that these patients present to the dialysis unit with normal or only modestly elevated pre-dialysis BP, it is possible that these patients remain silently volume overloaded. Lower serum phosphorus levels in patients with intradialytic hypertension (likely as a reflection of decreased oral intake) may also mark them inappropriately as “low risk” patients if one does not pay particular attention to the intradialytic BP pattern.
Table 1. Characteristics of Patients With Intradialytic Hypertension (based on published data by Park13 et al).
|
In summary, the most recent studies on intradialytic BP patterns have brought to the spotlight a previously unrecognized phenotype of dialysis patient that carries an exceedingly high risk for morbidity and mortality. Further discussion will highlight the potential pathophysiology of this phenomenon and management strategies.
Proposed Pathophysiology of Intradialytic Hypertension
Extracellular Volume Status
Extracellular volume overload is a consistent finding among patients with intradialytic hypertension (Table 2). In one uncontrolled study, six hemodialysis patients underwent echocardiograms both before and during hemodialysis26. During the initial period of ultrafiltration, both BP and cardiac index were seen to rise. Following further fluid removal, BP normalized with a reduction in cardiac index back to baseline. The authors concluded that the BP increases were mediated by the effects of changing cardiac index in the context of volume overload26. While better controlled studies to be discussed later fail to identify the causal or associative role of cardiac output with intradialytic hypertension, most studies continue to support the association between extracellular volume overload with intradialytic hypertension.
Table 2. Controlled Studies Identifying Extracellular Volume Overload Among Patients With Intradialytic Hypertension.
| Author | Subjects | Extracellular Volume Assessment Tool | Intradialytic Hypertension Definition | Results | Conclusions |
|---|---|---|---|---|---|
| Van Buren et al.27 | n=36 | Bioimpedance Spectroscopy | SBP increase >10 mmHg in 4/6 treatments | Ratio of ECW/TBW Pre-HD: 0.478 (IH) vs. 0.453 (controls, p=0.05) Post-HD: 0.461 (IH) vs. 0.427 (controls, p=0.01) |
IH associated with pre and post-HD extracellular volume overload |
| Sebastian28 | n=190 | Bioimpedance Spectroscopy | SBP increase >10 mmHg in 4/6 treatments | Overhydration: Pre-HD: 2.6 L (IH) vs. 1.8 L (controls, p=0.06) Post-HD: 0.79 L (IH) vs. -0.17 L (controls, p=0.06) |
Trend for more overhydration pre and post HD in IH patients |
| Nongnuch8 | n=531 | Bioimpedance Spectroscopy | SBP increase >10 mmHg in single HD treatment compared to SBP decrease >20 mmHg or stable BP | ECW/TBW PreHD: 0.399 (IH) vs. 0.392 (stable) vs. 0.395 (decrease, p=0.006) Post-HD: 0.391 (IH) vs. 0.386 (stable) vs. 0.387 (decrease, p=0.03) |
IH occurring in single HD treatment is associated with pre and post-HD extracellular volume overload |
| Agarwal50 | n=150 | Intradialytic Blood Pressure Slopes | Change in slope of systolic blood pressure during dialysis in patients randomized to intensive ultrafiltration or standard care | Mixed linear regression model based relationship between SBP slope change and quartile of weight gain following intervention | Flat or increasing SBP slopes are associated with extracellular volume overload |
SBP= Systolic Blood Pressure, ECW=Extracellular Water, TBW=Total Body Water, IH=Intradialytic Hypertension HD=Hemodialysis
The use of bioimpedance spectroscopy to measure fluid volumes in body compartments has enhanced the ability to objectively recognize differences in extracellular volume between patients and has provided novel insights into intradialytic hypertension. One study defined recurrent intradialytic hypertension as an increase in systolic BP of at least 10 mmHg from pre to post dialysis in at least four out of the prior 6 treatments27. In that study patients with recurrent intradialytic hypertension had a larger ratio of extracellular water to total body water (using multifrequency bioimpedance spectroscopy) at the end of the dialysis treatment compared to patients whose BP routinely decreased by at least 10 mmHg from pre to post dialysis. The ratio of extracellular water to body weight was higher both before and after dialysis in the intradialytic hypertension patients. The residual volume overload did not occur in the context of failed ultrafiltration targets in that study, indicating that most likely extracellular volume overload was simply misdiagnosed in these patients.
While this study provides objective evidence of a greater ratio of extracellular volume to other body compartments, these metrics are not commonly utilized clinical variables. The Body Composition Monitor (Fresenius) measures extracellular, intracellular, and total body water; it also provides a calculation of relative “fluid overload” based on these measurements and comparisons to anthromorphically similar individuals. This unique feature provides data that can potentially be directly integrated into the clinical management of a patient. One study used a similar definition for intradialytic hypertension and found a trend for higher amount of “fluid overload” in the patients with intradialytic hypertension both before and after dialysis28. These two studies share very strict criteria for defining intradialytic hypertension, but such similar findings can be observed in larger populations when it is less strictly defined. Nongnuch defined intradialytic hypertension as a systolic BP increase of >10 mmHg from pre to post dialysis during a single hemodialysis treatment8. In this cross-sectional study, the amount of overhydration was higher both before and after dialysis in the intradialytic hypertension group compared to patients with different BP patterns.
Cardiovascular Findings In Intradialytic Hypertension
The association between intradialytic hypertension and extracellular volume overload is strong but does not sufficiently prove causality. The initial findings associating extracellular volume overload with intradialytic hypertension implicated intradialytic increases in cardiac output as the etiology for the BP rise26. This was later challenged by evidence from a case control study that increases in vascular resistance, not cardiac output, were associated with intradialytic hypertension using echocardiograms to measure the hemodynamic changes29. Another recent case control study using a non-invasive cardiac output monitor again found the change in cardiac output to be similar between patients with intradialytic hypertension and controls27, while the changes in total peripheral resistance were much different between the two groups. These studies support a vasoconstrictive component during dialysis as the likely etiology of the BP increase, but further mechanistic details remain elusive. For example, some studies have implicated an imbalance of vasoconstrictors or vasodilators during the dialysis treatment to explain the increases in vascular resistance, while others have failed to support this hypothesis30-33
The detailed mechanisms of how vasoconstriction increases disproportionately in intradialytic hypertension patients remains unclear, but other evidence supports an overwhelming burden of cardiovascular disease in these patients. This includes higher overall ambulatory BP34 and more pronounced endothelial cell dysfunction35. One study showed higher pulse wave velocity in patients with intradialytic hypertension compared to other dialysis patients36, while another study showed no difference between the two groups35.
Management of Intradialytic Hypertension
In most situations, the increase in BP with intradialytic hypertension does not reach dangerously high levels and does not require acute lowering. Patient with recurrent intradialytic hypertension do have higher ambulatory BP34, particularly in the initial 24 hours after dialysis33 than other hypertensive hemodialysis patients. The primary goal in management of intradialytic hypertension is to address the likely underlying etiology and reduce the patient's overall risk for longer term cardiovascular morbidity and mortality. Many patients with intradialytic hypertension may not appear overtly volume overloaded as they are older, smaller and have smaller interdialytic weight gains. Because of the strong association between extracellular volume overload and intradialytic hypertension from observational studies, dry weight reduction should be a primary consideration in the initial management. There is no clinical trial to support this approach specifically in patients with recurrent intradialytic hypertension. However, the Dry Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) trial37 showed in hypertensive hemodialysis patients who mostly had BP decreases during dialysis at baseline that reducing dry weight steepened the BP decrease in subsequent hemodialysis treatments. Extrapolating from this study would suggest that this intervention might succeed in minimizing the intradialytic BP increase and lower the overall ambulatory BP.
Modification of dialysate sodium is another option in patients with intradialytic hypertension. In general hypertensive hemodialysis patients, decreasing dialysate sodium concentration reduces interdialytic thirst and weight gain38. In one randomized crossover study of patients with recurrent intradialytic hypertension, BP decreased during treatments with low dialysate sodium (serum sodium minus 5) and increased during treatments with high dialysate sodium (serum sodium + 5)39. It remains unclear how effectively this intervention lowers ambulatory BP or overall mortality risk in patients with intradialytic hypertension with some epidemiologic data from large dialysis cohorts suggesting an association between lower dialysate sodium and increased mortality among patients with lower serum sodium levels40. Therefore, dialysate sodium modification should also be implemented cautiously to ensure that intradialytic hypotension does not occur.
Pharmacologic antihypertensive therapy is required in most hypertensive hemodialysis patients with no single drug or drug class standing out as superior to others in this population. In patients with recurrent intradialytic hypertension, one uncontrolled pilot study showed some benefits of administering carvedilol32. In these patients, there was lower ambulatory BP, improved endothelial cell dysfunction and reduction in the incidence of intradialytic hypertension after 8 weeks of carvedilol therapy. Its uncontrolled nature fails to establish carvedilol as a superior therapy to other antihypertensive drugs, however.
Chronic Hypertension
Intradialytic hypotension and intradialytic hypertension are extreme ends of a spectrum of BP changes during the course of dialysis. The preceding sections detail important information on the epidemiology, likely pathophysiology, prognosis, and recommended management for these conditions. More stable BP patterns may be associated with less mortality than intradialytic hypotension or hypertension, but hypertensive hemodialysis patients remain at much higher risk for cardiovascular morbidity and mortality than the general population. Just as in intradialytic hypotension or hypertension, attention to extracellular volume status is critical in all hypertensive hemodialysis patients. The Dry Weight Intervention in Hypertensive Hemodialysis Patients (DRIP) Study Identified that more intensive ultrafiltration could lower both dialysis unit BP as well as ambulatory BP in as little as 4 weeks37. The risks of overly aggressive ultrafiltration and ultrafiltration rate were explained in the intradialytic hypotension section and carry over to all hemodialysis patients.
Presently, there is no gold standard approach to identify patients that have the most extracellular volume overload and will benefit from the most aggressive ultrafiltration approach. Physical examination has proven to be fairly unreliable in identifying volume overload compared to other methods41. In the clinical research setting multifrequency bioimpedance spectroscopy has emerged as a potential tool to improve the assessment of extracellular volume. Several pilot studies have shown the ability to safely reduce volume overload or lower BP42,43, but there are numerous limitations to the use of this equipment for widespread use in clinical practice related to cost, availability, and patient exclusion criteria. As highlighted above, high post dialysis BP likely identifies the presence of chronic extracellular volume overload compared to pre dialysis BP and may be most useful for evaluating volume status in the absence of bioimpedance.
Finally, there remains a critical role for pharmacologic antihypertensive therapy in hypertensive hemodialysis patients. Meta-analyses have identified with mortality reduction association with prescribing of BP medications44,45. No single drug class has emerged as the first line therapy as observational studies and clinical trials have shown varying results of the benefits of inhibitors of the renin-angiotensin-aldosterone system and beta-adrenergic receptor antagonists46-49.
Conclusion
Hypertension is one of the most common cardiovascular complications seen in hemodialysis patients. Extracellular volume overload contributes to hypertension in almost all hemodialysis patients, and the dialysis procedure provides the ability to remove fluid and improve BP. Some patients experience excessive BP reduction during dialysis known as intradialytic hypotension or a paradoxical increase in BP, referred to as intradialytic hypertension. Both intradialytic hypotension and hypertension reflect an increased mortality risk compared to patients with more modest reductions in BP.
While the pathophysiology of both is complex, changes in extracellular volume between and during dialysis treatments play a large role in these phenomena. Judicious prescription of fluid removal is a key step in management of both, but further management strategies are emerging as more is learned about each.
Acknowledgments
Dr. Van Buren receives funding from NIH 1K23DK096007-01A1. He also has received institutional funding as a Dedman Family Scholar in Clinical Care at UT Southwestern Medical Center.
Funding Sources: Dr. Van Buren receives funding from NIH 1K23DK096007-01A1. He also has received institutional funding as a Dedman Family Scholar in Clinical Care at UT Southwestern Medical Center.
References
- 1.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 2.Kuznik A, Mardekian J, Tarasenko L. Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults wtih chronic kidney disease: an analysis of national health and nutritional examination survey data, 2001-2010. BMC Nephrol. 2013;14:132–142. doi: 10.1186/1471-2369-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foundation NK. KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Am J Kidney Dis. 2005;45:S1–S154. [PubMed] [Google Scholar]
- 4.Foley R, Parfrey P, Harnett J, Kent G, Murray D, Barre P. Impact of hypertension on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Kidney Int. 1996;49:1379–1385. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Light R. Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. American Journal of Renal Physiology. 2008;294:F303–308. doi: 10.1152/ajprenal.00575.2007. [DOI] [PubMed] [Google Scholar]
- 6.Inrig J, Patel U, Gillespie B, et al. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis. 2007;2007:108–118. doi: 10.1053/j.ajkd.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leypoldt J, Cheung A, Delmez J, et al. Relationship between volume status and blood pressure during chronic hemodialysis. Kidney Int. 2002;61:266–275. doi: 10.1046/j.1523-1755.2002.00099.x. [DOI] [PubMed] [Google Scholar]
- 8.Nongnuch A, Campbell N, Stern E, El-Kateb S, Fuentes L, Davenport A. Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int. 2015;87:452–457. doi: 10.1038/ki.2014.276. [DOI] [PubMed] [Google Scholar]
- 9.Dinesh K, Kunaparaju S, Cape K, Flythe J, Feldman H, Brunelli S. A model of systolic blood pressure during the course of dialysis and clinical factors associated with various blood pressure behaviors. Am J Kidney Dis. 2011;58:794–803. doi: 10.1053/j.ajkd.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Inrig J, Oddone EH, V, Gillespie B, et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71:454–461. doi: 10.1038/sj.ki.5002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inrig J, Patel U, Toto R, Szczech L. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis. 2009;54:881–890. doi: 10.1053/j.ajkd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 13.Park J, Rhee C, Sim J, et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 2013;84:795–802. doi: 10.1038/ki.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foundation NK. K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Am J Kidney Dis. 2005;45:S1–S154. suppl 153. [PubMed] [Google Scholar]
- 15.Tisler A, Akocsi K, Borbas B, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance hemodialysis. Nephrol Dial Transplant. 2003;18:2601–2605. doi: 10.1093/ndt/gfg450. [DOI] [PubMed] [Google Scholar]
- 16.Flythe J, Xue H, Lynch K, Curhan G, Brunelli S. Association of mortality risk with various definintions of intradialytic hypotension. J Am Soc Nephrol. 2015;2015:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ettema E, Kuipers J, Groen H, et al. Vasopressin release is enhanced by the hemocontrol biofeedback system and could contribute to better haemodynamic stability during haemodialysis. Nephrol Dial Transplant. 2012;27:3263–3270. doi: 10.1093/ndt/gfr793. [DOI] [PubMed] [Google Scholar]
- 18.Flythe J, Kimmel S, Brunelli S. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79:250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jefferies H, Virk B, Schiller B, Moran J, McIntyre C. Frequent hemodialysis schedules are associated with reduced levels of dialysis induced cardiac injury (myocardial stunning) Clin J Am Soc Nephrol. 2011;6:1326–1332. doi: 10.2215/CJN.05200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton J, Jefferies H, Selby N, McIntyre C. Hemodialysis-Induced Cardiac Injury: Determinants and Associated Outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz D, Mahnensmith R, Brickel H, Perazella M. Midodrine and Cool Dialysate Are Effective Therapies for Symptomatic Intradialytic Hypotension. Am J Kidney Dis. 1999;33:920–926. doi: 10.1016/s0272-6386(99)70427-0. [DOI] [PubMed] [Google Scholar]
- 22.Eldehni M, Odudu A, McIntyre C. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26:957–965. doi: 10.1681/ASN.2013101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odudu A, Eldehni M, McCann G, McIntyre C. Randomized Controlled Trial of Individualized Dialysate Cooling for Cardiac Protection in Hemodialysis Patients. Clin J Am Soc Nephrol. 2015;10:1408–1417. doi: 10.2215/CJN.00200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Buren P, Kim C, Toto R, Inrig J. The prevalence of persistent intradialytic hypertension in a hemodialysis population with extended follow-up. Int J Artif Organs. 2012;35:1031–1038. doi: 10.5301/ijao.5000126. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Yang W, Lin Y. Postdialysis blood pressure rise predicts long-term outcomes in chronic hemodialysis patients: a four year prospective observational cohort study. BMC Nephrol. 2012;13:12. doi: 10.1186/1471-2369-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunal A, Karaca I, Celiker H, Ilkay E, Duman S. Paradoxical rise in blood pressure during ultrafiltration is caused by increased cardiac output. J Nephrol. 2002;15:42–47. [PubMed] [Google Scholar]
- 27.Van Buren P, Zhou Y, Neyra J, et al. Extracellular Volume Overload and Increased Vasoconstriction in Patients With Recurrent Intradialytic Hypertension. Kidney Blood Press Res. 2016;41:802–814. doi: 10.1159/000450565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastian S, Filmalter C, Harvey J, Chothia M. Intradialytic hypertension during chronic hemodialysis and subclinical fluid overload assessed by bioimpedance spectroscopy. Clin Kidney J. 2016;9:636–643. doi: 10.1093/ckj/sfw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou K, Lee P, Chen C, et al. Physiologic changes during hemodialysis in patients with intradialysis hypertension. Kidney Int. 2006;69:1833–1838. doi: 10.1038/sj.ki.5000266. [DOI] [PubMed] [Google Scholar]
- 30.Raj D, Vincent B, Simpson K, et al. Hemodynamic changes during hemodialysis: Role of nitric oxide and endothelin. Kidney International. 2002;61:697–704. doi: 10.1046/j.1523-1755.2002.00150.x. [DOI] [PubMed] [Google Scholar]
- 31.El-Shafey E, El-Nagar G, Selim M, El-Sorogy H, Sabry A. Is there a role for endothelin-1 in the hemodynamic changes during hemodialysis? Clin Exp Nephrol. 2008;2008:370–375. doi: 10.1007/s10157-008-0065-2. [DOI] [PubMed] [Google Scholar]
- 32.Inrig J, Van BUren P, Kim C, Vongpatanasin W, Povsic T, Toto R. Probing the Mechanisms of Intradialytic Hypertension: A Pilot Study Targeting Endothelial Cell Dysfunction. Clin J Am Soc Nephrol. 2012;7:1300–1309. doi: 10.2215/CJN.10010911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hompesch C, Ma T, Neyra J, et al. Comparison of Ambulatory Blood Pressure Patterns in Patients With Intradialytic Hypertension and Hemodialysis Controls. Kidney Blood Press Res. 2016;41:240–249. doi: 10.1159/000443427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Buren P, Kim C, Toto R, Inrig J. Intradialytic Hypertension and the association with interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2011;6:1684–1691. doi: 10.2215/CJN.11041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inrig J, Van Buren P, Kim C, Vongpatanasin W, Povsic T, Toto R. Intradialytic Hypertension and its Association wtih Endothelial Cell Dysfunction. Clin J Am Soc Nephrol. 2011;6:2016–2024. doi: 10.2215/CJN.11351210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgianos P, Mpoutsioki F, Sabani E, et al. Hemodialysis patients with intradialytic rise in blood pressure display higher baseline aortic stiffness and neglible drop in augmentation index with dialysis. Int J Urol Nephrol. 2016;48:601–608. doi: 10.1007/s11255-015-1205-8. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal R, Alborzi P, Satyan S, Light R. Dry-Weight Reduction in Hypertensive Hemodialysis Patients (DRIP): A Randomized, Controlled Trial. Hypertens. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Paula F, Peixoto A, Pinto L, Dorigo D, Patricio P, Santos S. Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int. 2004;66:1232–1238. doi: 10.1111/j.1523-1755.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- 39.Inrig J, Molina C, D'Silva K, et al. Effect of low versus high dialysate sodium concentration on blood pressure and endothelial-derived vasoregulators during hemodialysis: a randomized crossover study. Am J Kidney Dis. 2015;65:464–473. doi: 10.1053/j.ajkd.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Hecking M, Karaboyas A, Saran R, et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2012;59:238–248. doi: 10.1053/j.ajkd.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal R, Andersen M, Pratt J. On the Importance of Pedal Edema in Hemodialysis Patients. Clin J Am Soc Nephrol. 2008;3:153–158. doi: 10.2215/CJN.03650807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hur E, Usta M, Toz H, et al. Effect of Fluid Management Guided by Bioimpedance Spectroscopy on Cardiovascular Parameters in Hemodialysis Patients: A Randomized Controlled Trial. Am J Kidney Dis. 2013;61:957–965. doi: 10.1053/j.ajkd.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Onofriescu M, Hogas S, Voroneanu L, et al. Bioimpedance-Guided Fluid Management in Maintenance Hemodialysis: A Pilot Randomized Controlled Trial. Am J Kidney Dis. 2014;64:111–118. doi: 10.1053/j.ajkd.2014.01.420. [DOI] [PubMed] [Google Scholar]
- 44.Heerspink H, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal R, Sinha A. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertens. 2009;53:860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal R, Sinha A, Pappas M, Abraham T, Tegegne G. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29:672–681. doi: 10.1093/ndt/gft515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi A, Takase H, Toriyama T, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients in chronic hemodialysis-a randomized study. Nephrol Dial Transplant. 2006;21:2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 48.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 49.Efrati S, Zaldenstein R, Dishy V, et al. ACE inhibitors and survival of dialysis patients. Am J Kidney Dis. 2002;40:1023–1029. doi: 10.1053/ajkd.2002.36340. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal R, Light R. Intradialytic hypertension is a marker of volume excess. Nephrol Dial Transplant. 2010;25:3355–3361. doi: 10.1093/ndt/gfq210. [DOI] [PMC free article] [PubMed] [Google Scholar]


