Abstract
Curative treatment for metastatic solid cancers remains elusive. The liver, which is nourished by a rich blood supply from both the arterial and portal venous systems is the most common site of visceral metastases, particularly from cancers arising in the gastrointestinal (GI) tract, with colorectal cancer (CRC) being the predominant primary site in Western countries. A mounting body of evidence suggests that the liver microenvironment (LME) provides autocrine and paracrine signals originating from both parenchymal and non-parenchymal cells, that collectively create both pre-and pro-metastatic niches for the development of hepatic metastases. These resident cells and their molecular mediators represent potential therapeutic targets for the prevention and/or treatment of liver metastases (LM). This review summarizes: 1) the current therapeutic options for treating LM with a particular focus on CRC LM (CRCLM); 2) the role of the LME in LM at each of its phases 3) potential targets in the LME identified through pre-clinical and clinical investigations and 4) potential therapeutic approaches for targeting elements of the LME before and/or after the onset of LM, as the basis for future clinical trials.
Keywords: hepatic metastasis, tumor microenvironment, colon cancer, colorectal cancer, colorectal liver metastases, immunosuppression
A. BACKGROUND
Metastases remain the primary source of morbidity and mortality from solid tumors, and the liver is the dominant site of metastases from GI malignancies, such as CRC2. Systemic treatments directed at cancer cells have had limited success, in large part due to the presence of numerous malignant clones, which allow rapid selection of resistance in the face of cytotoxic and “targeted” therapies. Our recent recognition that the LME is also critical for facilitating access and fostering the growth of cancer cells within the liver have led to the concept of targeting both cells and molecules within the LME as a strategy for preventing and treating LM. This strategy has many potential advantages over targeting the cancer cells only, including the sheer number of potential targets and the potential to engage the immune system – an approach recently shown to be a highly effective and durable therapeutic modality. In this review, we utilize CRC as a paradigm to discuss the rationale for targeting the ME as a strategy for prevention and treatment of LM.
A.1 Origins of Liver Metastases
LM are tumors that have spread to the liver from other malignant sites. Secondary hepatic malignancies are reportedly 18–40 times more common than primary hepatic malignancies in Western countries (1). Approximately half of all patients afflicted with LM have primary CRC (mCRC) while other primary GI cancers such as esophageal (≈1–2%) and gastric carcinomas (≈5–9%), pancreatic and intestinal neuroendocrine tumors (≈1%), biliary tract cancers (≈5–10%), as well as pancreatic ductal adenocarcinomas (PDAC, ≈14%) and gastrointestinal stromal tumors (<1%) also give rise to LM. LM from non-GI cancers are less common, but include breast (<1–2%), lung (12–20%), kidney (1–2%) cancers and melanoma (<1%) (2, 3).
The liver has a dual blood supply with two-thirds to three-fourths of the blood supply derived from the portal vein and the remaining from the hepatic artery. Dissemination of tumors from the GI tract to the liver is thought to originate from cancer cells that have gained access to the portal venous circulation. On the other hand, dissemination of tumors from outside the GI tract may originate from cancer cells that have gained access to the systemic arterial circulation. For instance, lung cancer cells may enter via the pulmonary vein and then embolize the liver via the hepatic artery (4).
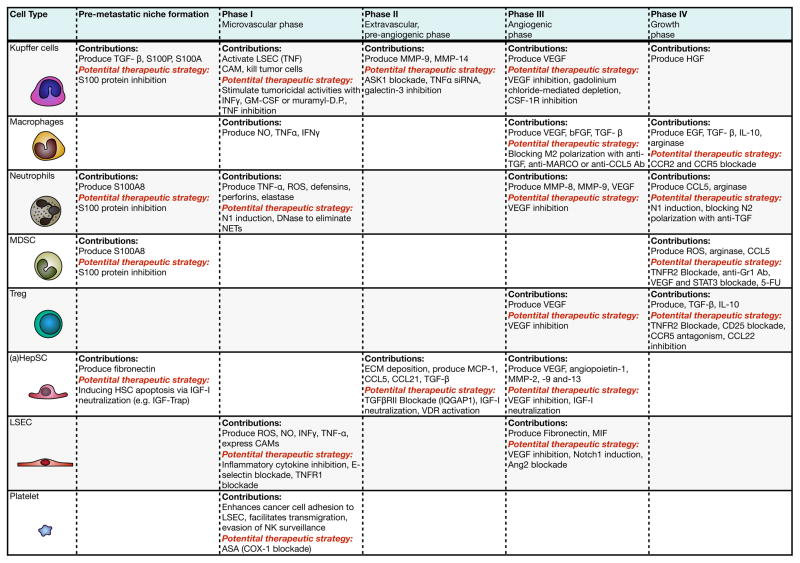
These processes of liver metastasis is facilitated by two critical niches, namely the pre-metastatic niche driven by factors secreted by the primary tumor that in turn, recruit non-parenchymal cells including Kupffer cells (KC), hepatic stellate cells (HepSC), myeloid-derived suppressor cells (MDSC) and neutrophils, and the post-tumor invasion niche, which develops following tumor cell entry into the liver and can be characterized by four key phases (i) a microvascular phase (ii) an extravascular pre-angiogenic phase (iii) an angiogenic phase and (iv) the growth phase (detailed below and reviewed extensively in (5–7)). With the exception of the angiogenic phase, the potential therapeutic benefit of targeting the ME at each of these phases, has not been adequately explored.
A.2 Traditional Systemic Therapy for Colorectal Liver Metastases
Approximately 20–34% of patients with CRC present with synchronous LM (8, 9) and up to 50–60% will develop LM at some point in their disease course (10, 11). At present, the estimated 5-year overall survival (OS) for all patients with Stage IV colorectal cancer is 13% (12). Treatment goals for patients with mCRC can be classified as: (1) curative or potentially curative; this identifies a group of patients where LM may be resectable; (2) non-curative with active treatment intent (most patients fall into this group); or (3) palliative intent (13). Cytotoxic systemic chemotherapy is the mainstay of treatment for most advanced malignancies, including colorectal cancer (Table 1). The National Comprehensive Cancer Network (NCCN) guidelines consider fluorouracil (5-FU) combined with leucovorin (LV) and oxaliplatin (i.e., FOLFOX) or irinotecan (i.e., FOLFIRI) to be standard of care (SOC), first-line chemotherapy regimens for patients with unresectable CRCLM (Table 1) (14, 15). These recommendations are based on the results of Phase II and III trials that demonstrated improved median OS and progression-free survival (PFS) with combination therapy versus 5-FU and LV alone. A recent meta-analysis found however, that the response rates in these trials averaged only 68% (16). First line regimens may also include the combination of FOLFOX or FOLFIRI with: 1) Bevacizumab; 2) Cetuximab; or 3) Panitumumab. These three biologic agents are humanized, chimeric mouse/human, and human antibodies, respectively. Bevacizumab targets vascular endothelial growth factor-A (VEGF-A), while the latter two target the epidermal growth factor receptor (EGFR) and downstream signaling including the MAPK pathway (Table 1). At present, only Bevacizumab and three additional therapies (i.e., Ramucirumab, Regorafenib, Ziv-Aflibercept), which are approved for later lines of therapy, target angiogenesis (15). Perhaps the modest improvements in PFS achieved with these agents is due to their utilization too late in the disease course to affect outcome. Alternatively, VEGF-independent angiogenesis may occur that renders them ineffective, as shown and discussed elsewhere (6, 17, 18). Across the various regimens containing these agents, response rates are generally modest with improvements in OS ranging from only 1.4 to 2.5 months (19). Thus, for patients with CRCLM who are not resection candidates, the prognosis remains poor, highlighting the need for novel therapeutic approaches.
Table 1.
FDA-approved Drugs and Drug Combinations for Metastatic Colorectal Adenocarcinoma.
| FDA-Approved Drugs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generic Name | Capecitabine | 5-FU | Leucovorin | Oxaliplatin | Irinotecan | Bevacizumab | Cetuximab | Panitumumab | Ramucirumab | Regorafenib | Ziv- Aflibercept |
Trifluridine + Tipiracil |
| Trade Name | Xeloda | Adrucil | Wellcovorin | Eloxatin | Camptosar | Avastin | Erbitux | Vectibix | Cyramza | Stivarga | Zaltrap | Lonsurf |
| Class | Antimetabolite; Pyrimidine analog | Antimetabolite; Pyrimidine analog | Vitamer of folic acid | Cytotoxic | Cytotoxic | Humanized antibody | Chimeric mouse/human antibody | Human antibody | Humanized antibody | Tyrosine kinase inhibitor | Recombinant fusion protein | Cytotoxic |
| Target | Thymidylate synthase (Pro-drug of 5-FU) | Thymidylate synthase | Purine/pyrimidine synthesis | DNA crosslinks | Topoisomerase 1 | VEGF-A | EGFR | EGFR | VEGFR2 | VEGFR2-TIE2 | VEGF | Nucleoside analog + Thymidine phosphorylase |
| Pathway | DNA replication | DNA replication | Preserves DNA replication (in normal cells) | DNA replication | DNA replication | Angiogenesis | EGFR/MAPK signaling | EGFR/MAPK signaling | Angiogenesis | Angiogenesis | Angiogenesis | DNA replication |
| Drug Combinations with FDA-Approved Drugs | ||||||||||||
| Capecitabine | 5-FU | Leucovorin | Oxaliplatin | Irinotecan | Bevacizumab | Cetuximab | Panitumumab | Ramucirumab | Regorafenib |
Ziv- Aflibercept |
Trifluridine + Tipiracil |
|
| FU-LV | ||||||||||||
| CAPOX (XELOX) * | ||||||||||||
| CAPOX+ Bevacizumab * | ||||||||||||
| FOLFOX * | ||||||||||||
| FOLFOX + Bevacizumab * | ||||||||||||
| FOLFOX + Cetuximab * | ||||||||||||
| FOLFOX + Panitumumab * | ||||||||||||
| XELIRI | ||||||||||||
| FOLFIRI * | ||||||||||||
| FOLFIRI + Bevacizumab * | ||||||||||||
| FOLFIRI + Cetuximab * | ||||||||||||
| FOLFIRI + Panitumumab * | ||||||||||||
| FOLFOXIRI | ||||||||||||
Recommended drug combinations according to 2016 Version 2 National Comprehensive Cancer Network (NCCN) Colon Cancer Practice Guidelines (74).
B. CLINICAL-TRANSLATIONAL ADVANCES
B.1. The role of the microenvironment in the different phases of liver metastasis
The process of liver metastasis has been divided into several phase based on the location of the cancer cells within the liver and the phase-specific interactions between the cancer cells and the LME. These phases were extensively reviewed elsewhere (5–7) and are briefly summarized here as background for subsequent sections.
B.1.1. The “pre-metastatic niche”
Although still contentious, accumulating evidence supports the concept that the ME of secondary organ sites can be rendered permissive to metastatic outgrowth in advance of tumor cell entry (5) For the liver, this was recently demonstrated in a murine model of aggressive PDAC, where tumor derived exosomes were shown to activate KC and set in motion a series of events leading to increased TGFβ production, HepSC activation and extracellular matrix (ECM) deposition (20). Macrophage migration inhibitory factor (MIF) was implicated in this process and intriguingly, exosomal MIF levels were associated with an increased risk of relapse in the liver among stage I PDAC patients. Collectively the data identified MIF and the level of circulating αv-bearing exosomes as potential early biomarkers of LM in this disease with possible therapeutic implications.
B.1.2. The microvascular phase
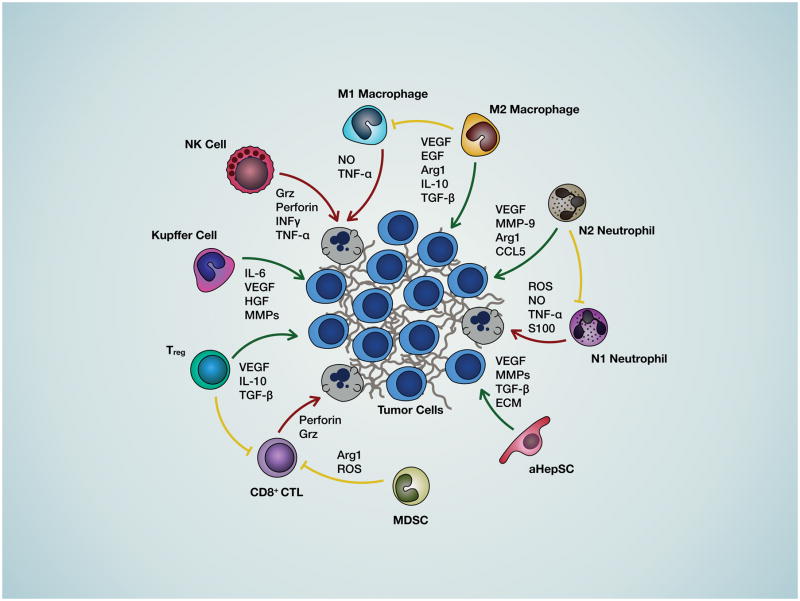
Once in the liver microvasculature, cancer cells encounter diverse cell types including liver sinusoid endothelial cells (LSEC), KC and hepatic natural killer cells (pit cells, NK) (21) (Figures 1 and 2). They may be rapidly eliminated through KC-mediated phagocytosis and NK-derived perforin and granzymes or through apoptosis induced by reactive oxygen species (ROS), nitric oxide (NO) interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα). However, cancer cells can escape these tumoricidal mechanisms by attaching to cytokine-induced endothelial CAM and transmigrating into the space of Disse, if they express the corresponding counter receptors (5). This may be facilitated via neutrophil extracellular (DNA) traps (NETs) (22). Cancer-LSEC adhesion alters gene expression in both cell types, triggering the process of diapedesis and extravasation (reviewed in (23) and see Fig. 1 for depiction of the different cell types and mediators of cell-cell communication in the LME).
Figure 1. Cell-cell interactions in the liver microenvironment.
Shown is a diagrammatic representation of the interactions between the cancer cells the various hepatic cell types that regulate progression of metastasis and the soluble factors mediating these interactions. Green arrows represent interactions that favor metastatic expansion, red arrows represent interactions that are detrimental to cancer cell growth and blunt-end yellow arrows indicate interactions that impede anti-tumor immunity.
Figure 2. Stromal and immune cells of the liver microenvironment and their contribution to progression of metastasis.
Listed are the cells constituting the hepatic microenvironment and their tumor-promoting contributions in each phase of the metastatic process. Also listed are potential therapeutic strategies aimed at inhibiting pro-tumorigenic stromal cell functions and inflammatory responses.
B.1.3. The pre-angiogenic phase
In response to pro-inflammatory cytokines unleashed during the microvascular stage, quiescent HepSC in the space of Disse are activated (aHepSC) and deposit type I and IV collagen and fibronectin, providing a scaffolding for endothelial cell migration, angiogenesis and the establishment of extravascular micrometastases (24, 25). TNFα and TGFβ are major drivers of this process and it can be accelerate by KC and neutrophil – derived matrix metalloproteinases MMP-9 and MMP-14 and neutrophil elastase that enhance tumor cell invasion into, and expansion within the hepatic parenchyma (5).
B.1.4. The angiogenic phase
Within the liver parenchyma metastatic cells can co-opt existing vessels to establish a blood supply. This is thought to result in a histological growth pattern (GP) termed the “replacement GP” (6, 18, 26). Alternatively, they can trigger a process of neovascularization driven by VEGF and basic FGF (bFGF). KC, newly recruited tumor associated macrophages (TAM) that are polarized to the M2 phenotype in response to TGFβ and IL-10, tumor associated neutrophils (TAN) that acquire the N2 phenotype in response to TGFβ (27, 28) and aHepSC also produce VEGF and therefore can contribute to neovascularization that is accelerated by MMPs produced by cancer and LME cells (29, 30).
B.1.5. The growth phase
Once cancer cells gain access to a blood supply, proliferation and expansion can ensue. However, their presence in the liver can activate specific, T-cell mediated immune responses that may curtail metastatic expansion through different cytolytic mechanisms (for review see (31)). Cancer cells can evade CD4+ T helper cell and CD8+ cytotoxic T lymphocyte (CTL)-mediated kill via co-inhibitory molecules such as death protein 1 (PD-1) that binds ligands PD-L1 or PD-L2 on the cancer cells, and the cytotoxic T lymphocyte-associated protein 4 (CTLA-4), resulting in inhibition of T effector cell functions. This state of immune tolerance can be further enhanced by the recruitment of immunosuppressive lymphoid and myeloid subsets including MDSC and regulatory T cells (Treg) to the liver. The MDSC, a heterogeneous population of granulocytic and monocytic precursors can be recruited by LSEC-, KC- and/or HepSC-derived chemokines such as CXCL1 and CXCL2 (32–34) and inhibit T cell activation by producing arginase, ROS and CCL5, a Treg chemoattractant. Naive T cells can also be polarized into inducible Treg (iTreg) in the presence of TGFβ and IL-2, and inhibit CD8+ T cell activation through the release of TGFβ, IL-10, perforin and granzymes (35) and the upregulation of the co-inhibitory receptor CTLA-4 (Figs 1 and 2). In the TGFβ-rich TME, TAM and TAN can acquire immunosuppressive (M2 and N2) phenotypes (reviewed in (36, 37)). Adding to the pro-tumorigenic ME at this phase, are growth factors such as the type I insulin-like growth factor (IGF-I), EGF and hepatocyte growth factor (HGF) produced by hepatocytes, M2 TAM and aHepSC, respectively, that activate survival and mitogenic signaling via their respective receptors on the cancer cells (5).
B.2. Targeting the tumor microenvironment to impede tumor progression: preclinical and clinical studies identify potential targets
Recent advances in cancer immunotherapy have heightened interest in targeting the TME as a strategy to prevent and treat metastatic disease, including LM (13). Targeting the TME alone or in combination with chemotherapy and/or tumor-directed biological therapy is appealing for several reasons: (i) cancer cells depend on a supportive ME for survival and growth; (ii) unlike cancer cells, the ME consists of cells that are genetically stable and their properties and responses are more predictable; (iii) targeting the ME may be beneficial across tumor types, particularly for tumors that metastasize primarily to the same secondary site, such as the liver. Indeed, anti-angiogenic drugs and immunotherapeutics that overcome T cell tolerance now form the SOC in several malignancies. Several lines of evidence based mainly on pre-clinical models, provide a strong rationale for also targeting pro-metastatic elements of the LME.
LSEC are the first barrier to cancer cell intravasation and become a source of immune cell-recruiting cytokines upon activation by invading cancer cells. Several studies suggest that modulation of endothelial CAM expression may provide a useful strategy to prevent LM. Targeting E-selectin by antibodies or by pretreatment with C-raf antisense oligonucleotides was shown to decrease tumor cell adhesion and reduce LM of lung and colon carcinoma cells, respectively (38–40). Inhibition of VCAM-1 expression by antibody mediated blockade of IL-1β, TNFα and IL-18 also impaired the retention of cancer cells in the liver sinusoids and reduced LM (41) and this was also seen in TNFR1-deficient mice (42). These studies identified LSEC CAM and their inducers as potential targets during the microvascular phase of LM. As discussed below, EC are also a source of blood supply for metastatic cells and anti-angiogenic drugs are already part of current SOC for mCRC.
Targeting KC may also represent an effective strategy to preventing the growth of incipient LM, as shown when gadolinium chloride was used to deplete KC, resulting in decreased liver tumor burden (43). This was associated with decreased VEGF production and increased iNOS expression and CD3+ lymphocyte infiltration. More recently, Ries and colleagues found that a monoclonal antibody (RG7155) that inhibits CSF-1 receptor (CSF-1R) activation reduced the numbers of F4/80+ TAM in animal models and this was associated with increased CD8+ to CD4+ T cell ratios. When administered into patients (NCT01494688, phase 1), the antibody caused marked reductions in CSF-1R+CD163+ macrophages in tumor tissues and had anti-tumor effects (44). It may therefore be effective in blocking TAM recruitment into sites of LM. Another promising approach currently under study is the use of IFNγ, GM-CSF, antibodies or muramyl dipeptide to increase the tumoricidal activities of KC. This approach may be relevant as a strategy to prevent development of CRCLM, if it succeeds in converting anti-inflammatory M2 macrophages to tumoricidal M1 macrophages (45). KC targeting strategies are also being assessed for indications other than LM but their success could have implications for the management of malignant disease. For example, KC play an important role in the inflammatory processes that lead to HepSC activation and fibrosis. Ongoing phase 3 clinical trials STELLAR 3 and STELLAR 4 (NCT03053050 and NCT03053063) are currently assessing Selonsertib, an inhibitor of the inflammatory signal transducer ASK1 expressed in macrophages and hepatocytes (46) as an anti-fibrotic agent in cirrhotic patients. This class of inhibitors could potentially be useful in blocking the transient fibrogenic response characteristic of the pre-angiogenic phase of LM (see review in (46)), if applied within the critical time window. However, KC inactivation by anti-inflammatory agents was also shown to accelerate collagen production in a rat model of fibrosis (47), highlighting the complexity of KC functions and the potential risks in KC targeting. Finally, the specific delivery of anti-inflammatory drugs to KC has been investigated as a strategy for blocking KC-driven inflammation. For instance, oral delivery of nanoparticles carrying TNFα siRNA was shown to inhibit TNFα production in macrophages in vivo, protecting mice from LPS/d-GalN-induced hepatic injury and lethality (48). Similarly, Galectin-3 inhibitors were used to limit KC-mediated inflammation and shown to resolve cirrhosis and portal inflammation in experimental models (49, 50). The utility of these approaches in the context of LM prevention has not been assessed.
As discussed, TGFβ is a major driver of the immunosuppressive and fibrogenic microenvironments essential for the angiogenic and growth phases of LM (reviewed in (51, 52)). Several phase II clinical trials based on targeting the TGFβ axis are, in fact, currently in progress. For example, the NCT01373164 trial (phase 1/2, completed) is assessing the effect of Galunisertib - an inhibitor of the TGFβ receptor I kinase with a favourable toxicity profile in humans (53) in combination with Gemcitabine on the OS of patients with unresectable metastatic PDAC. In the NCT02423343 trial (phase 1/2, recruiting), the effect of this inhibitor is being evaluated in combination with the PD-1 inhibitor Nivolumab in patients with recurrent hepatocellular carcinoma. Blockade of TGFβR signaling could render the LME less favorable to metastatic expansion by altering key elements in the pro-metastatic niche. For example, it could inhibit the polarization of tumoricidal M1 TAM to the M2 phenotype. In addition, TGFβ signaling blockade can also increase the cytotoxic activities of CD11b+Ly6G+ TAN by increasing expression of the pro-inflammatory cytokines TNFα, IFNγ, IL-12 and CCL5. This was shown in 3 different mice strains, two different tumor types (NSCLC and mesothelioma), and in both flank and orthotopic models of lung adenocarcinoma (27). Moreover, blockade of TGFβRII signaling can also prevent HepSC activation, thereby disrupting the angiogenic phase of LM, as was recently shown in a murine CRC model and confirmed in surgical specimens (54). Vitamin D receptor (VDR)-conveyed signals were also implicated in blockade of TGFβ-mediated HepSC activation. Calcipotriol, a low-calcemic analog of calcitriol and agonist of VDR was shown to restrict the fibrogenic response of aHepSC by reducing SMAD3 occupancy at profibrotic target genes via chromatin remodeling (55). Although validated in the context of experimental fibrosis, these, and other inhibitors that target the process of HepSC activation (56–59) could potentially have beneficial anti-metastatic effects by blocking early events in LM and their evaluation in this context is therefore warranted.
TGFβ blockade can also potentially inhibit CD4+ T cell differentiation into Treg. Several other Treg-targeting strategies are currently in development, including the use of daclizumab - a CD25 - neutralizing antibody and the blockade of CCL22. These strategies recently showed promise in preclinical models and in breast cancer clinical trials (60–62) but have not yet been tested in LM models. Clinical trials with combination checkpoint inhibitors Tremelimumab (anti-CTLA-4) and MEDI4736 (anti-PD-L1) have recently been initiated for patients with resectable CRCLM, aimed at reactivating immune surveillance in the TME (NCT02754856, phase 1, recruiting). However, the benefit of immunotherapy, either as monotherapy or in combination with other modalities for mCRC remains to be confirmed.
The MDSC are another potential target for immune modulation. Recently, we have shown that MDSC and Treg recruitment into CRCLM were TNFR2-dependent and that treatment of tumor-bearing mice with TNFR2-targeting antisense oligonucleotide significantly reduced experimental LM (63). Other potential strategies include; 1) induction of MDSC differentiation into mature, non-immunosuppressive myeloid cells, 2) prevention of their expansion from BM precursors and 3) impairment of their accumulation or function. STAT3 (64) and VEGF inhibitors (65), chemotherapeutic drugs (e.g. 5-FU, Gemcitabine) and chemokine receptor antagonists have already been shown to reduce the accumulation of CD11b+GR-1+ cells in peripheral immune organs and the tumor stroma (extensively reviewed in (66) and see Fig. 2), although their specific effects on MDSC recruitment to LM remain to be verified.
B.3. The case for targeting the pro-metastatic niche for therapeutic management of hepatic metastases
Our increased understanding of the biological mediators of the four phases of LM, suggests multiple opportunities to disrupt both incipient and established disease through a variety of therapeutic strategies. In fact, there is already proof of principle, albeit unappreciated, for the utility of this concept because an effective “chemoprevention” strategy for CRCLM already exists, namely the use of low dose aspirin. Aspirin (ASA), a negative regulator of prostaglandin E2 signaling via its inhibitory effects on the COX1 and 2 enzymes has now been shown to significantly reduce CRCLM in several epidemiological studies (67). While not completely understood, it is believed that the mechanism of action (MOA) relates to the effect of ASA on COX1 signaling in platelets (68). In the microvascular phase of LM, platelets may promote metastases by enhancing cancer cell adhesion to EC and leukocytes, thereby facilitating transmigration (67). Platelets may also aid in the evasion of NK surveillance. Given the demonstrated positive effects of ASA, significant efforts should be directed to more clearly decipher the underlying MOA, so that additional agents that target the same mechanism(s) can be developed and optimized.
As discussed above, numerous agents targeting the angiogenic phase of metastasis have already been approved for CRC including Bevazucimab, Ziv-aflibercept and Regorafenib, each with a distinct MOA against VEGF-mediated signaling. Additional agents targeting VEGF and other pro-angiogenic molecules are presently in clinical trials (e.g. NCT02350530, NCT00055692, NCT00767468). For example, the NCT00055692 trial (phase 2, completed) sought to determine the biologic effects of Bevacizumab in unresectable hepatocellular carcinoma. Although significant clinical and biologic activity was observed in the treated arm, grade 3 or higher hemorrhages occurred in 11% of patients. The MOA of these agents remains incompletely understood, however, and thus there is ample opportunity for optimization of therapies directed at this phase of the metastatic cascade.
Chemokine and growth factor receptors are among the most interesting putative targets within the ME because they are “druggable” by small molecule and antibody-based strategies and there is compelling preclinical data supporting their utility as targets for treatment of LM. Furthermore, chemokines have been shown to influence numerous phases of LM, the angiogenic and growth phases in particular. CCL5 is produced by CRC cells and by T cells at the margin of CRCLM (69). CCL5/CCR5 signaling has pleiotropic effects including recruitment of monocytes and M2 polarization, promoting the expansion of cancer-associated fibroblasts and enhancing TGFβ-mediated killing of CD8+ T cells by Treg (69, 70). A recent Phase 1 trial with a CCR5 antagonist demonstrated activity against advanced refractory CRCLM, identifying it as a target worthy of further clinical investigation (69). Although no CCR5-targeting drugs are currently approved for the management of liver diseases, repositioning the clinically approved CCR5 antagonist Maraviroc, used against CCR5-tropic HIV strains, may be of clinical interest in this context. In addition, a recent report demonstrated that the growth factor IGF-1 participates in recruitment and activation of HepSC to enhance the growth of CRCLM (71). IGF-1 was shown to prevent apoptosis in HepSC exposed to TNFα. Importantly, stromal cells from resected CRCLM expressed activated IGF-IR and an IGF-Trap markedly reduced IGF-IR activation on HepSC in a murine model of metastatic CRC (72). Together, these data identify IGF signaling as another rational target within the LME.
While there is compelling evidence that the immune response to primary CRC correlates with patient prognosis, until recently, there was little clinical evidence to suggest that the immune cell infiltrate within the metastatic niche was of similar clinical impact. A recent study examined gene expression profiles in CRCLM resections from 96 patients (73). Genes involved in T cell proliferation were significant predictors of overall survival, while genes involved in T cell proliferation and activation were predictive of relapse-free survival. Analysis of an independent set of tumors by IHC validated these findings, showing that an increased lymphocytic infiltrate and increased expression of the TNFSF14/LIGHT protein were associated with improved overall and relapse-free survival. Another recent report, demonstrated that MDSC expand within the LME of CRCLM and can inhibit responses to CAR T cell therapy (74). These findings demonstrate that the immune cell infiltrate within the LME may be highly relevant to patient outcomes and manipulating these responses may be of therapeutic benefit.
While collectively these studies suggest that LME targeting holds promise as a therapeutic strategy, this approach is not without its challenges. For example, targeting HepSC activation could inhibit metastatic expansion by reducing ECM deposition (54). However, HepSC-derived angiogenesis and ECM remodeling are essential to the liver response to injury (75) and this approach may therefore have deleterious effects in patients undergoing hepatic resections. Our recent understanding of the processes governing immunosuppression in the TME has greatly increased interest in targeting immune cell polarization to improve tumor cell surveillance and clearance. However, as alluded to earlier, both pro-inflammatory and anti-inflammatory factors contribute to liver colonization by cancer cells. Given that the process of LM is dynamic and the different phases may temporally overlap, the window of opportunity for administrating pro-or anti-inflammatory agents may be limited and difficult to define. TGF axis targeting is also problematic, because of its central physiological role in wound healing and tissue repair. Moreover, limiting downstream effects of TGFβ may potentially contribute to metastatic progression because TGFβ can also have potent tumor cytostatic effects (76). For example TGFβR signaling was shown to induce the expression of cyclin-dependent kinase (CDK) inhibitors, arresting cell cycle progression at the G1 phase (77). The SMAD2/3-SMAD4 complex was also shown to upregulate SH2 domain-containing inositol-5-phosphatase (SHIP) expression, an inhibitor of AKT (78). Further studies are therefore crucial to determine if the immunomodulatory effects of TGF axis blockers can override their potential pro-tumorigenic activities.
Finally, the use of angiogenesis inhibitors such as Bevacizumab may not benefit all patients. This was documented in a recent study showing that CRCLM with a ‘replacement’ GP resulting from vessel co-option are resistant to, and respond poorly to anti-angiogenic therapy (79). Patient stratification based on the histological GP of their LM may therefore be essential to optimize the benefit from anti-angiogenic therapies (18, 80). However, at present, biomarkers to predict either the vascular response or the type of immune microenvironment engendered by individual LM are lacking, limiting the potential to personalize the clinical management of liver metastatic disease.
C. Conclusion
The LME consists of a diverse group of cells that are coopted by cancer cells to enable the establishment and growth of metastases. These varying cell types, along with the cytokines/chemokines and growth factors they secrete, represent putative targets to prevent and treat LM. An increased understanding of drivers of the four phases of LM should improve our ability to rationally select and combine therapeutic approaches for clinical investigation. The demonstrated importance of immune modulation during the evolution of LM provides particularly attractive therapeutic opportunities given the recent revelations regarding the power of immunotherapy in different malignancies. It is now recognized that the dominant cytokines and chemokines that modulate immune function within a particular TME differ between tumor types and (we hypothesize) may even differ between patients afflicted by the same cancer (81). Thus, we propose that in the future, it will be optimal to develop personalized panels of immune biomarkers from a patient’s tumor to understand the dominant signals driving local immunosuppression and how combination therapies might best engender an anti-tumor immune response. Targeting the LME will no doubt present new and unique challenges, including the probability of unforeseen toxicities. In addition, the identification of these numerous putative targets and accompanying therapeutic agents engenders a new set of challenges, namely, how to efficiently move this vast array of agents through clinical trials. This requires more widespread integration of biomarkers, and adaptive trials that utilize accumulating outcome data to rapidly discard less active agents and rapidly integrate new treatment arms (82). Finally, studying these novel approaches earlier in patients’ disease course, rather than relegating the study of new agents to 2nd, 3rd and 4th lines may be an important step towards subverting issues of intra- and inter-tumoral heterogeneity and drug resistance.
Acknowledgments
Funding: Research reported in this review was supported by the National Cancer Institute of the National Institutes of Health under award number K08CA168999, P30 CA023100, and R21CA192072 (J.K. Sicklick)1 and the FRQS (S. Milette). This work was also made possible by support from a MOP-80201 grant from the Canadian Institute for Health Research (P. Brodt) and a PSR-SIIRI-843 grant from the Québec Ministère de l’Économie, de l’Innovation et des Exportations (P. Brodt).
Abbreviations
- ASA
Acetylsalicylic acid
- bFGF
basic FGF
- CAM
cell adhesion molecule
- CCL
chemokine (C-C motif) ligand
- CXCL
chemokine (C-X-C motif) ligand
- CRC
colorectal cancer
- CRCLM
colorectal cancer liver metastases (or metastasis)
- CSF-1R
CSF-1 receptor
- CDK
cyclin-dependent kinase
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T lymphocyte-associated protein 4
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ECM
extracellular matrix
- 5-FU
fluorouracil
- GI
gastrointestinal tract
- GP
growth pattern
- NK
natural killer cells
- HepSC
hepatic stellate cells
- HGF
hepatocyte growth factor
- iTreg
inducible Treg
- IFNγ
interferon-γ
- IL
interleukin
- KC
Kupffer cells
- LV
leucovorin
- LM
liver metastasis
- LME
liver microenvironment
- LSEC
liver sinusoid endothelial cells
- MIF
macrophage migration inhibitory factor
- MMP
matrix metalloproteinase
- MOA
mechanism of action
- mCRC
metastatic colorectal cancer
- MDSC
myeloid-derived suppressor cells
- NCCN
National Comprehensive Cancer Network
- NET
neutrophil extracellular trap
- NO
nitric oxide
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
- PD-1
programmed death protein 1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- ROS
reactive oxygen species
- Treg
regulatory T cell
- SHIP
SH2-domain-containing inositol-5-phosphatase
- SOC
standard of care
- TGFβ
transforming growth factor β
- TAM
tumor associated macrophages
- TAN
tumor associated neutrophil
- TNFR
tumor necrosis factor receptor
- TNFα
tumor necrosis factor-α
- IGF-I
type I insulin-like growth factor
- VCAM-1
vascular cell adhesion protein 1
- VEGF-A
vascular endothelial growth factor-A
- VDR
Vitamin D receptor
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All abbreviations are listed below
Disclosures: Jason Sicklick receives research funds from Foundation Medicine, Novartis, and Blueprint Medicines, as well as consultant fees from Sirtex. Andrew Lowy receives research funds from Syros Pharmaceuticals and Tanabe-Mitsubishi, and has received consulting fees from Merck and Halozyme Therapeutics. The other authors have nothing to disclose.
References
- 1.Namasivayam S, Martin DR, Saini S. Imaging of liver metastases: MRI. Cancer imaging : the official publication of the International Cancer Imaging Society. 2007;7:2–9. doi: 10.1102/1470-7330.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyer M, Erichsen R, Gandrup P, Norgaard M, Jacobsen JB. Survival in patients with synchronous liver metastases in central and northern Denmark, 1998 to 2009. Clin Epidemiol. 2011;3(Suppl 1):11–7. doi: 10.2147/CLEP.S20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turdean S, Gurzu S, Turcu M, Voidăzan S, Sin A. Liver Metastases: Incidence and Clinicopathological Data. Acta Medica Marisiensis. 2012;58(4):254–8. [Google Scholar]
- 4.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 5.Brodt P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin Cancer Res. 2016;22(24):5971–82. doi: 10.1158/1078-0432.CCR-16-0460. [DOI] [PubMed] [Google Scholar]
- 6.Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Hoyer-Hansen G, et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73(7):2031–43. doi: 10.1158/0008-5472.CAN-12-3931. [DOI] [PubMed] [Google Scholar]
- 7.Vidal-Vanaclocha F. The Prometastatic Microenvironment of the Liver. Cancer Microenvironment. 2008;1(1):113–29. doi: 10.1007/s12307-008-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muratore A, Zorzi D, Bouzari H, Amisano M, Massucco P, Sperti E, et al. Asymptomatic colorectal cancer with unresectable liver metastases: immediate colorectal resection or up-front systemic chemotherapy? Annals of surgical oncology. 2007;14(2):766–70. doi: 10.1245/s10434-006-9146-1. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Inoue Y, Komeda K, Shimizu T, Asakuma M, Hirokawa F, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC surgery. 2010;10:27. doi: 10.1186/1471-2482-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. European journal of cancer. 2006;42(14):2212–21. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clinical colorectal cancer. 2006;6(3):202–7. doi: 10.3816/CCC.2006.n.036. [DOI] [PubMed] [Google Scholar]
- 12.Society AC. Colorectal Cancer Facts & Figures 2014–2016. 2014. [Google Scholar]
- 13.Sag AA, Selcukbiricik F, Mandel NM. Evidence-based medical oncology and interventional radiology paradigms for liver-dominant colorectal cancer metastases. World J Gastroenterol. 2016;22(11):3127–49. doi: 10.3748/wjg.v22.i11.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benson AB, 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 3.2014. Journal of the National Comprehensive Cancer Network : JNCCN. 2014;12(7):1028–59. doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 15.Benson ABr, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon Cancer, Version 2. Journal of the National Comprehensive Cancer Network. 2016 doi: 10.6004/jnccn.2014.0099. In Press. [DOI] [PubMed]
- 16.Sabanathan D, Eslick GD, Shannon J. Use of Neoadjuvant Chemotherapy Plus Molecular Targeted Therapy in Colorectal Liver Metastases: A Systematic Review and Meta-analysis. Clinical colorectal cancer. 2016 doi: 10.1016/j.clcc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41(4):278–86. doi: 10.5483/bmbrep.2008.41.4.278. [DOI] [PubMed] [Google Scholar]
- 18.Van den Eynden GG, Bird NC, Dirix LY, Eefsen RL, Gao ZH, Hoyer-Hansen G, et al. Tumor stromal phenotypes define VEGF sensitivity--letter. Clin Cancer Res. 2014;20(19):5140. doi: 10.1158/1078-0432.CCR-14-0158. [DOI] [PubMed] [Google Scholar]
- 19.Benson ABr, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon Cancer, Version 2. Journal of the National Comprehensive Cancer Network. 2016 doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–26. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braet F, Nagatsuma K, Saito M, Soon L, Wisse E, Matsuura T. The hepatic sinusoidal endothelial lining and colorectal liver metastases. World journal of gastroenterology : WJG. 2007;13(6):821–5. doi: 10.3748/wjg.v13.i6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witz IP. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008;27(1):19–30. doi: 10.1007/s10555-007-9101-z. [DOI] [PubMed] [Google Scholar]
- 24.Gressner AM, Bachem MG. Molecular mechanisms of liver fibrogenesis--a homage to the role of activated fat-storing cells. Digestion. 1995;56(5):335–46. doi: 10.1159/000201257. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eefsen RL, Van den Eynden GG, Hoyer-Hansen G, Brodt P, Laerum OD, Vermeulen PB, et al. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J Oncol. 2012;2012:907971. doi: 10.1155/2012/907971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schouppe E, De Baetselier P, Van Ginderachter JA, Sarukhan A. Instruction of myeloid cells by the tumor microenvironment: Open questions on the dynamics and plasticity of different tumor-associated myeloid cell populations. Oncoimmunology. 2012;1(7):1135–45. doi: 10.4161/onci.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135(5):1729–38. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 30.Copple BL, Bai S, Burgoon LD, Moon JO. Hypoxia-inducible factor-1alpha regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver international : official journal of the International Association for the Study of the Liver. 2011;31(2):230–44. doi: 10.1111/j.1478-3231.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6(2):123–33. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24(5):631–44. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao W, Zhang L, Xu Y, Zhang Z, Ren G, Tang K, et al. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab Invest. 2014;94(2):182–91. doi: 10.1038/labinvest.2013.139. [DOI] [PubMed] [Google Scholar]
- 34.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–90. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bresalier RS, Byrd JC, Brodt P, Ogata S, Itzkowitz SH, Yunker CK. Liver metastasis and adhesion to the sinusoidal endothelium by human colon cancer cells is related to mucin carbohydrate chain length. Int J Cancer. 1998;76(4):556–62. doi: 10.1002/(sici)1097-0215(19980518)76:4<556::aid-ijc19>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71(4):612–9. doi: 10.1002/(sici)1097-0215(19970516)71:4<612::aid-ijc17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 40.Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, Brodt P. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 2002;62(19):5393–8. [PubMed] [Google Scholar]
- 41.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A. 2000;97(2):734–9. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitakata H, Nemoto-Sasaki Y, Takahashi Y, Kondo T, Mai M, Mukaida N. Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res. 2002;62(22):6682–7. [PubMed] [Google Scholar]
- 43.Wen SW, Ager EI, Christophi C. Bimodal role of Kupffer cells during colorectal cancer liver metastasis. Cancer Biology & Therapy. 2013;14(7):606–13. doi: 10.4161/cbt.24593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 45.van der Bij GJ, Oosterling SJ, Meijer S, Beelen RH, van Egmond M. Therapeutic potential of Kupffer cells in prevention of liver metastases outgrowth. Immunobiology. 2005;210(2–4):259–65. doi: 10.1016/j.imbio.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.02.026. In Press. [DOI] [PubMed] [Google Scholar]
- 47.Melgert BN, Olinga P, Van Der Laan JM, Weert B, Cho J, Schuppan D, et al. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34(4 Pt 1):719–28. doi: 10.1053/jhep.2001.27805. [DOI] [PubMed] [Google Scholar]
- 48.He C, Yin L, Tang C, Yin C. Multifunctional polymeric nanoparticles for oral delivery of TNF-alpha siRNA to macrophages. Biomaterials. 2013;34(11):2843–54. doi: 10.1016/j.biomaterials.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 49.Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8(10):e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8(12):e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong Z, Carroll KD, Policarpio D, Osborn C, Gregory M, Bassi R, et al. Anti-transforming growth factor beta receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res. 2010;16(4):1191–205. doi: 10.1158/1078-0432.CCR-09-1634. [DOI] [PubMed] [Google Scholar]
- 53.Rodon J, Carducci M, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs. 2015;33(2):357–70. doi: 10.1007/s10637-014-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C, Billadeau DD, Abdelhakim H, Leof E, Kaibuchi K, Bernabeu C, et al. IQGAP1 suppresses TbetaRII-mediated myofibroblastic activation and metastatic growth in liver. J Clin Invest. 2013;123(3):1138–56. doi: 10.1172/JCI63836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153(3):601–13. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennett RG, Heimann DG, Tuma DJ. Relaxin reduces fibrosis in models of progressive and established hepatic fibrosis. Ann N Y Acad Sci. 2009;1160:348–9. doi: 10.1111/j.1749-6632.2008.03783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao C, Xie Y, Peng M, Ma L, Zhou Y, Zhang Y, et al. Inhibition of connective tissue growth factor suppresses hepatic stellate cell activation in vitro and prevents liver fibrosis in vivo. Clin Exp Med. 2014;14(2):141–50. doi: 10.1007/s10238-013-0229-6. [DOI] [PubMed] [Google Scholar]
- 58.Son MK, Ryu YL, Jung KH, Lee H, Lee HS, Yan HH, et al. HS-173, a novel PI3K inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci Rep. 2013;3:3470. doi: 10.1038/srep03470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu F, Su L, Ji S, Zhang S, Yu P, Zheng Y, et al. Inhibition of hepatic stellate cell activation and liver fibrosis by fat-specific protein 27. Mol Cell Biochem. 2012;369(1–2):35–43. doi: 10.1007/s11010-012-1366-z. [DOI] [PubMed] [Google Scholar]
- 60.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 61.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4(134):134ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014;33(38):4623–31. doi: 10.1038/onc.2013.432. [DOI] [PubMed] [Google Scholar]
- 63.Ham B, Wang N, D’Costa Z, Fernandez MC, Bourdeau F, Auguste P, et al. TNF Receptor-2 Facilitates an Immunosuppressive Microenvironment in the Liver to Promote the Colonization and Growth of Hepatic Metastases. Cancer Res. 2015;75(24):5235–47. doi: 10.1158/0008-5472.CAN-14-3173. [DOI] [PubMed] [Google Scholar]
- 64.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30(9):1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 66.Wesolowski R, Markowitz J, Carson WE., 3rd Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J Immunother Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boutaud O, Sosa IR, Amin T, Oram D, Adler D, Hwang HS, et al. Inhibition of the Biosynthesis of Prostaglandin E2 By Low-Dose Aspirin: Implications for Adenocarcinoma Metastasis. Cancer Prev Res (Phila) 2016;9(11):855–65. doi: 10.1158/1940-6207.CAPR-16-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guillem-Llobat P, Dovizio M, Bruno A, Ricciotti E, Cufino V, Sacco A, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7(22):32462–77. doi: 10.18632/oncotarget.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell. 2016;29(4):587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Frankenberger C, Rabe D, Bainer R, Sankarasharma D, Chada K, Krausz T, et al. Metastasis Suppressors Regulate the Tumor Microenvironment by Blocking Recruitment of Prometastatic Tumor-Associated Macrophages. Cancer Res. 2015;75(19):4063–73. doi: 10.1158/0008-5472.CAN-14-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez MC, Rayes R, Ham B, Wang N, Bourdeau F, Milette S, et al. The type I insulin-like growth factor regulates the liver stromal response to metastatic colon carcinoma cells. Oncotarget. 2016 doi: 10.18632/oncotarget.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandez MC, Rayes R, Ham B, Wang N, Bourdeau F, Milette S, et al. The type I Insulin-like growth factor regulates the liver stromal response to metastatic colon carcinoma cells. Oncotarget. 2016 doi: 10.18632/oncotarget.12595. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thorn M, Guha P, Cunetta M, Espat NJ, Miller G, Junghans RP, et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther. 2016;23(6):188–98. doi: 10.1038/cgt.2016.19. [DOI] [PubMed] [Google Scholar]
- 74.Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother. 2015;64(7):817–29. doi: 10.1007/s00262-015-1692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123(5):1902–10. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3(11):807–21. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 77.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9(15):1831–45. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 78.Valderrama-Carvajal H, Cocolakis E, Lacerte A, Lee EH, Krystal G, Ali S, et al. Activin/TGF-beta induce apoptosis through Smad-dependent expression of the lipid phosphatase SHIP. Nat Cell Biol. 2002;4(12):963–9. doi: 10.1038/ncb885. [DOI] [PubMed] [Google Scholar]
- 79.Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22(11):1294–302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van den Eynden GG, van Dam PJ, Stroobants S, Dirix L, Vermeulen P Liver Metastasis Research N. Histopathological evaluation of resected colorectal cancer liver metastases: what should be done? Histopathology. 2014;64(2):315–6. doi: 10.1111/his.12259. [DOI] [PubMed] [Google Scholar]
- 81.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rugo HS, Olopade OI, DeMichele A, Yau C, van ‘t Veer LJ, Buxton MB, et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N Engl J Med. 2016;375(1):23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benson AB, 3rd, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 2.2016. Journal of the National Comprehensive Cancer Network : JNCCN. 2016 doi: 10.6004/jnccn.2014.0099. In Press. [DOI] [PubMed] [Google Scholar]